Dopamine (DA) was synthesized in 1910 (1) and during the 1950s it was reported to be present in brain tissue (2). Following the discoveries that DA is a neurotransmitter (3) and that DA receptors are drug targets (3), the role of the DA system has been explored in many neuropsychiatric disorders. In particular, the observation of a reduced DA content in postmortem brain tissue from Parkinson’s disease patients (4) spurred an intense interest in the role of DA in neurological and psychiatric disorders and other conditions. However, in comparison to the well-established role of DA deficiency in neurodegenerative diseases of the basal ganglia and rare neurometabolic syndromes affecting DA synthesis, transport or metabolism (5), research on dopamine’s role in most other traits has been challenging and subject to controversy.
Evidence linking the dopamine system and attention deficit hyperactivity disorderAttention deficit hyperactivity disorder (ADHD) is characterized by dominant symptoms of inattention (e.g. being sidetracked by external or unimportant stimuli) and/or hyperactivity (e.g. squirming or fidgeting while seated) and impulsiveness (e.g. difficulty waiting your turn). According to the current version of the Diagnostic and Statistical Manual for Mental Disorders (DSM-5), these symptoms need to persist for longer than six months, be observed in at least two settings, and negatively impact academic/social/occupational functioning to qualify for a diagnosis of ADHD (in addition to other diagnostic criteria) (6). It has long been speculated that ADHD symptoms may be related to some alterations in monoaminergic and in particular dopaminergic neurotransmission (7, 8). While it has been difficult to pinpoint exactly how a postulated dysfunction of DA occurs in ADHD, this hypothesis has gained much support in the scientific community and not the least in popular media (9). According to some “influencers”, popular books and other media intended for the lay audience, ADHD patients lack DA in their brain (10). Despite this widely held popular opinion, the scientific evidence supporting this version of the DA hypothesis is not necessarily present. Indeed, a simplistic portrayal of ADHD as a general DA deficiency syndrome might be one of the most common misconceptions regarding ADHD neurobiology. This is not the only misconception about ADHD that is widely distributed in social media. In a quantitative study of social media content quality, it was found that 52% of the 100 most popular videos on ADHD on TikTok were classified as misleading (11).
Scientifically, the DA hypothesis of ADHD has been based on several lines of evidence, including: 1) stimulant drugs target the monoamine systems, including DA, 2) animal models genetically engineered for altered DA homeostasis mimic some symptoms of ADHD, 3) brain imaging studies implicate the DA system, and 4) studies of genetic variants in the DA system in humans (9). However, ever since the DA hypothesis was first proposed about 40 years ago some researchers have also been skeptical of it. For example, when focusing on the effect of medications used for ADHD, the 1987 review by Zametkin and Rapoport (12) found evidence against the DA hypothesis when considering the limited effects of DA agonists and antagonists. They instead reported evidence primarily favoring the noradrenergic system.
Methylphenidate and amphetamines are stimulant drugs that are first-line pharmacotherapies for ADHD (13). These drugs block DA and norepinephrine (NE) transporters, inhibiting catecholamine reuptake into dopaminergic nerve terminals (Figure 1), increasing their availability at dopamine receptors. Stimulants can also interact with other target molecules and transmitter systems, e.g. amphetamines can increase DA release from synaptic vesicles, and inhibit monoamine metabolism, particularly at high doses (14). Stimulants have effects on ADHD symptoms, possibly by optimizing DA and NE modulated task-related brain networks that increase perceived saliency, reducing interference from the default mode network (15).
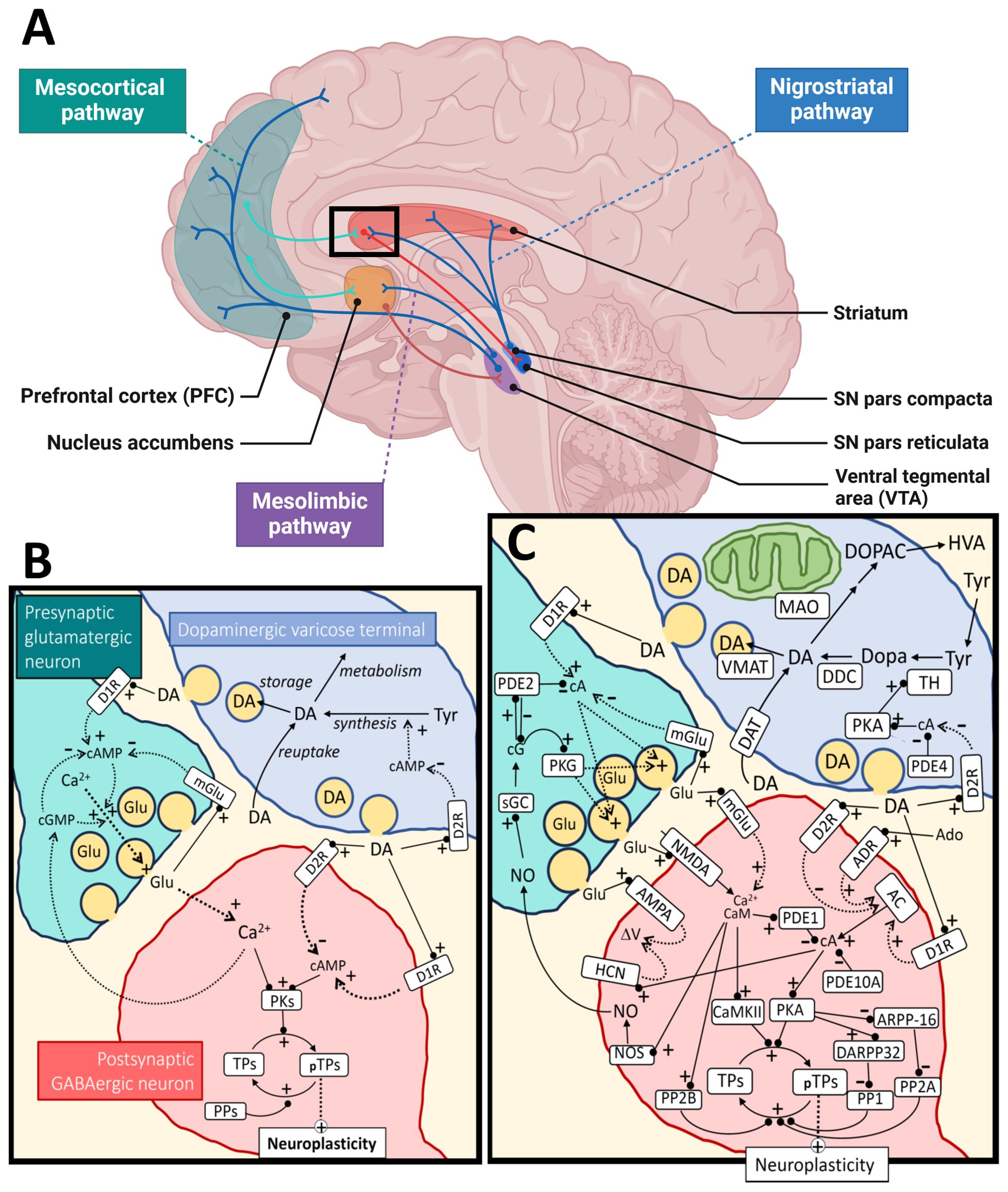
Figure 1. Dopamine metabolism and signaling. Panel (A) shows the major dopaminergic pathways in the human brain originating from the substantia nigra (SN) pars compacta for the nigrostriatal pathway and the ventral tegmental area (VTA) for the mesolimbic and mesocortical pathways. For simplicity, the panel shows only one of the output pathways (the direct pathway) from the striatal medium spiny neurons (of the D1R-type) to the SN pars reticulata (or globus pallidus internal). The modulation of cortical input to the striatum by dopamine (black square) is further illustrated at the synaptic and molecular level in panel (B), as a simplification, and in more detail in panel (C). The regulation of neuroplasticity by dopamine is shown for a cortico-striatal synapse under influence by a nearby dopaminergic varicose release site. Some of the relevant cyclic nucleotides [cAMP (cA), cGMP (cG)] and calcium signal interactions are shown for both the presynaptic cortical glutamatergic neuron and a postsynaptic GABAergic medium spiny neuron. For the dopaminergic terminal (upper right), synthesis and metabolism of dopamine are shown as well as a simplified regulation of TH activity through cAMP/PKA signaling, which is inhibited by D2 autoreceptors. The change in neuroplasticity is mediated by different target proteins (TPs; pTPs – phosphorylated TPs) of cAMP-dependent protein kinase (PKA) and calcium/calmodulin dependent protein kinase II (CaMKII). Examples of such TPs are CREB (cAMP-response element binding), STEP (striatal-enriched protein tyrosine phosphatase), proteins involved in cytoskeletal restructuring, AMPA receptors, etc. AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; AC, adenylate cyclase; Ado, adenosine; ADR, adenosine receptor; ARPP-16, cAMP-regulated phospho-protein 16; CaM, calmodulin; D1R, dopamine receptor type 1; D2R, dopamine receptor type 2; DA, dopamine; DAT, dopamine transporter; DARPP-32, dopamine and cAMP-regulated phospho-protein 32; DDC, dopa decarboxylase; Dopa, 3,4-dihydroxyphenylalanine; DOPAC, 3,4-dihydroxyphenylacetic acid; Glu, glutamate; HCN, hyperpolarization-activated cyclic nucleotide-gated ion channels; HVA, homovanillic acid; NMDA, N-methyl-D-aspartate receptor; NO, nitric oxide; NOS, nitric oxide synthase; MAO, monoamine oxidase; mGlu, metabotropic glutamate receptor; PDEx, phosphodiesterase type x; PPx, protein phosphatase type x; PKG, cGMP-dependent protein kinase; sGC, soluble guanylate cyclase; TH, tyrosine hydroxylase; Tyr, tyrosine; VMAT, vesicular monoamine transporter. Black arrows mean direct synthesis or transport, black lines with circled end mean direct positive (+) or negative (-) influence or degradation, dotted arrows mean indirect positive (+) or negative (-) influence. Panel (A) was created in BioRender.com and the rest of the figure with Microsoft PowerPoint.
Although specific NE transporter inhibitors and alpha-2a adrenergic agonists are also effective against ADHD symptoms (16), recent meta-analyses show that psychostimulants also acting on dopaminergic pathways, like methylphenidate and amphetamines, have greater effect sizes for ADHD symptom management compared to NE transporter inhibitors like atomoxetine or the alpha-2a agonist guanfacine (13). Some arguments against the mono-causal dopamine-deficit hypothesis such as that by Gonon (17) have cited evidence that psychostimulants improve behavior, including attention, in healthy children as well as those with ADHD (18, 19), the rodent models agreed to have the highest validity for ADHD (20) still cannot closely fit the neuropharmacology of ADHD (see (17)), and meta-analyses of brain imaging studies do not support the idea that ADHD symptoms can be explained by hypo-function within a few isolated brain regions (21). Some more recent skepticism of the hypothesis comes from highlighting the shortcomings of the dopamine-focused executive dysfunction theory in explaining hyperactivity and motor problems in ADHD (22). Conversely, recent meta-analyses continue to show that other dopamine-related cognitive dysfunctions, such as impaired inhibitory control, are robustly exhibited in ADHD [e.g. (23)].
Different versions of the DA hypothesis of ADHD have been around for decades and reviews dating as far back as 1991 (24) have collated studies arguing for and against the key role of DA in this condition. Since then, the link between DA and ADHD has been further investigated using a variety of approaches. By way of illustration, a literature search using the PubMed database in August 2024 retrieved 184,551 hits with the key word “dopamine”, 51,665 with “ADHD”, 2,990 with the search terms “dopamine”, “attention deficit hyperactivity disorder” and “ADHD”, including 39 meta-analyses. The main focus of these meta-analyses is on genetic studies, often exploring one or a few genetic markers in a single candidate gene. Across this extensive literature there is no clear consensus what the DA hypothesis of ADHD specifically refers to – either that DA plays a key role, there is a hypo-functioning DA system, there is a hypo-dopaminergic state in specific brain regions like the prefrontal cortex (PFC), there is a hypo-dopaminergic state throughout frontostriatal regions, or specifically that extracellular DA concentration/DA signaling/DA metabolism is lowered. For the purposes of this review, we refer to the DA hypothesis as: reduced synthesis, content, metabolism and rate of extracellular accumulation of the DA neurotransmitter within frontostriatal regions in people with ADHD.
Considering the large number of studies published on the connection between DA and ADHD, spanning >40 years of research using a diversity of methods - including different types of brain imaging, pharmacology, neurochemistry and genetic studies in ADHD patients, healthy humans and animal models - it is practically impossible to systematically explore and extensively review all the evidence collected during this period. The purpose of this review is rather to examine how far we have come in the past >40 years by evaluating different lines of evidence for the DA hypothesis. We take a multidisciplinary approach and extend our focus beyond genetics to also examine the DA hypothesis from a neurophysiological, neuropsychological, and neurometabolic perspective, attempting to cite representative and/or recent developments in these areas using publications entered into the PubMed database before September 2024 as the primary source of information. Major reviews and controversial reports have been discussed among the author group before being included. In this way we aim to provide an updated overview of the main aspects of the DA hypothesis in ADHD. Although we have strived to present a balanced and representative overview, we did not aim to include every possible perspective or all published reports in these fields. Inevitably, some aspects and data will unintentionally be overlooked or underrepresented in such a large and rapidly evolving research field.
We start by providing a brief overview of the dopaminergic system covering DA synthesis, receptors and downstream signaling mechanisms, while highlighting some points of complexity that may help to explain why the DA hypothesis is still so hotly debated.
An overview of the dopaminergic systemDA is a neurotransmitter/neuromodulator that plays crucial roles in several physiological processes in the brain and peripheral tissues. The brain functions include motor control, cognition, learning, food intake and reward (25, 26). Peripheral DA is implicated in the immune system, hormone release, gastrointestinal motility, regulation of blood pressure and sodium balance and probably other functions (27). The main components of the dopaminergic system include the biosynthetic machinery involved in DA synthesis and release, pre- and postsynaptic DA receptors, as well as downstream signaling mechanisms and enzymes and transporters involved in DA metabolism (25) (Figure 1). DA is synthesized from the amino acid tyrosine through two enzymatic reactions involving hydroxylation and decarboxylation of this precursor amino acid into a monoamine. Tyrosine hydroxylase (TH) catalyzes the first rate-limiting step in this process (28, 29) and dopa decarboxylase (aromatic amino acid decarboxylase, DDC) catalyzes the second step. Once synthesized, DA is stored in vesicles within the dopaminergic neurons. Upon neuronal activation, DA is released into the synaptic cleft or extrasynaptically (volume transmission) where it interacts with receptors on target cells.
Dopamine homeostasisAlthough neuronal communication is highly dynamic on a temporal scale, the concept of neurotransmitter homeostasis is still used. For rapidly acting transmitters this refers more to the signaling capability or the transmission process rather than the synaptic transmitter levels themselves. For more slowly acting neurotransmitters, like the neuromodulator DA, it makes more sense to also talk about the extracellular transmitter homeostasis – particularly for the volume transmission. Thus, neurotransmitter homeostasis is a result of transport processes between different compartments such as release, diffusion and reuptake, but also relies on the biosynthetic and metabolic fluxes acting locally in the region of interest. Presynaptic regulation is key to understanding these processes. However, the postsynaptic signaling responses are of course important for the neurotransmission homeostasis as a whole. Both signaling systems will be briefly discussed here.
Dopaminergic neurons of the central nervous system (CNS) have anatomically highly complex axons with extensive arborizations in their target areas of the brain – extending from their nuclear localization (substantia nigra pars compacta and ventral tegmental area) to large areas of the brain (30). There they form two general types of contacts; varicosities, which are enlargements of the axon that contain secretory vesicles and mediate diffuse modulatory stimuli on the surrounding neurons, and more defined synaptic contacts. For volume secretion from diffuse varicosities, reuptake is expected to be of little importance for temporal changes in the cytoplasmic dopamine levels as extracellular diffusion of dopamine is expected to be rapid (30). The frequency of release does not directly reflect the depolarization dynamics of the axon. These neurons are known to operate in different firing modes: low frequency tonic mode (~5 Hz) and transient phasic high frequency or bursting (< 30 Hz). The amount of DA released is much higher during phasic firing and is further modulated by local signaling inputs such as glutamine, gamma-aminobutyric acid (GABA), and acetylcholine. DA release and its temporal concentration profile has been extensively studied and measured quantitatively in many brain regions. Terminal release is modulated by DA-autoreceptors, which modulate the amount of DA released during depolarization. Somatodendritic release of DA modulates the activity of the neuron itself (31).
There are considerable differences between different brain regions in their turnover and reuptake of DA. High levels of the dopamine transporter (DAT) are found in the terminal regions of the nigrostriatal and mesolimbic pathways, whereas low levels are found in somatodendritic regions and the PFC (32). The different reuptake activities have a considerable impact on the volume to which DA spreads and the temporal profile of the transmission.
Dopamine receptorsDA exerts its effects by binding to specific G protein-coupled receptors (GPCRs) (Figure 1). There are five known human DA receptor subtypes: D1, D2, D3, D4, and D5 (33, 34). D1-like receptors (D1 and D5) activate adenylyl cyclase, leading to increased cyclic AMP (cAMP) levels. D2-like receptors (D2, D3, and D4) inhibit adenylyl cyclase and modulate cAMP levels (34). These receptors are widely expressed in both the CNS and peripheral tissues. In addition to regulating levels of cAMP, DA receptors can activate other signaling pathways, including the Gq/11 mediated activation of phospholipase C (PLC) (35), β-arrestin 2 induced activation of Akt, and transactivation of tyrosine receptor kinases such as TrkB (36).
Dopamine receptors (DRs) are found presynaptically on DA neurons and postsynaptically on different target cells (33) (Figure 1). DA signaling complexity is further increased by formation of oligomeric complexes between different types of DRs, as well as complexes with other GPCRs and other receptor molecules. Reported DR heteromers include D1/D2, D1/D3, D2/D4, D2/D3 and D2/D5 DR heterodimers, as well as D1/D2/D3 + adenosine A1 or A2 receptors, D2 + somatostatin SST5 receptors, D2 + 5HT2A receptors, D1 + metabotropic glutamate receptors (Glu5), D1 + Histamine H3 receptors, D2 + Histamine H3 receptors, D2 or D3 + glutamate NMDA receptors (NR2B) (37–39). Some of the heteromeric receptor complexes have been characterized, showing that their DA binding and effector coupling profiles are distinct from the respective homodimers or monomers (39). Still, the evidence for the in vivo formation and physiological relevance of most of the reported oligomeric complexes between DRs is limited and even considered controversial (38, 40).
Most D1 and D2 receptors are expressed on non-dopaminergic neurons and are involved in a diversity of brain functions, including learning, cognition, motivation and motor functions. D1-like receptors exert these functions partially by modulating ion channels, such as voltage gated sodium, potassium and calcium channels, as well as G-protein coupled inwardly rectifying potassium channels (GIRKs) (41, 42). D2-like receptors are coupled to Gi/o proteins and thereby inhibit GIRKs. Presynaptic D2 autoreceptors modulate DA functions via activation of potassium conductance, as well as by downregulating the activity of the plasma DA transporter and the activity of TH (43, 44) (Figure 1). As D2 receptors are the primary targets of antipsychotic drugs, the physiology and pharmacology of D2 subtype receptors have been extensively studied. However, due to the strong structural and functional interconnectivity of the dopaminergic system and other neurotransmitter systems, in particular other monoamines, it is virtually impossible to completely disentangle the “true” dopaminergic neurotransmission from other transmitter systems (37).
Dopamine during brain developmentDA production starts early during embryonic brain development, even before synapses are formed and DA exerts its functions at critical periods during development (45). For example, D2 receptors are involved in long term depression (LTD) and synaptic pruning during development of the rat anterior cingulate cortex (46). The hyperactive anterior cingulate cortex observed in Drd2+/- rats is believed to drive anxiety-like behaviors and perhaps also other behavioral symptoms (46, 47). Although different DRs are expressed during embryonic development, their patterns of expression are shifting during fetal and postnatal development, reaching a stable level at adulthood (45). This delayed maturation of DRs during development implies that the DA system also is subject to multiple genetic and environmental factors. Altered DA functions during neurogenesis have been linked to several neuropsychiatric/neurodevelopmental disorders including autism, schizophrenia and ADHD (45).
Dopamine transportersDAT is responsible for reuptake of DA from the extracellular space back into presynaptic neurons or other cell types. Together with the related monoamine transmitter transporters, such as the norepinephrine transporter (NET) and serotonin transporter (SERT), human DAT belongs to the family of neurotransmitter sodium symporter family of transporters, which are part of a large family of solute carrier 6 (SLC6) transporters (Figure 1). The human DAT, NET and SERT proteins are encoded by SLC6A3, SLC6A2 and SLC6A4, respectively (48). Inhibition of DAT by stimulant drugs like cocaine and amphetamines increases extracellular DA levels. Many natural monoamines and synthetic drugs bind with variable affinity to all these transporters, which can be predicted by their strong amino acid sequence conservation (49).
While DAT is responsible for transporting DA across the plasma membrane, another class of transporters, the vesicular monoamine transporters (VMATs) are responsible for the accumulation of DA and other monoamines (e.g. serotonin, NE and histamine) in synaptic vesicles (50) (Figure 1). VMAT2 (encoded by SLC18A2 in humans) is the major vesicular monoamine transporter for DA in the adult brain, while VMAT1 is mainly expressed during embryonic development and in peripheral tissues. Mice lacking VMAT2 die soon after birth, while heterozygotes develop normally, but have increased sensitivity to amphetamine (51).
Dopamine metabolismThe enzymes monoamine oxidase (MAO) A and B and catechol-O-methyltransferase (COMT) metabolize DA and similar monoamine substrates. MAOs are found in the outer membrane of mitochondria in most cell types (Figure 1). In contrast, COMT acts both intracellularly and extracellularly, as the membrane bound form of this enzyme is oriented with its C-terminal catalytic domain facing the extracellular space (52).
Presynaptic regulationTH is the key regulatory enzyme in DA biosynthesis and its activity is under regulation by several mechanisms (28). Thus, availability of its substrates (tyrosine (Tyr), tetrahydrobiopterin (BH4) and oxygen), feedback inhibition by catecholamines, active-site iron redox-state, serine (Ser)-phosphorylation, protein-protein interactions, protein localization, turnover and expression all regulate TH activity in the different neuronal compartments (53, 54). Tyr availability is governed by transport across the blood brain barrier and is influenced by the level of other amino acids that compete for the same transport mechanism (55). TH is also characterized by extensive substrate inhibition of Tyr, which could compromise its activity in conditions with significantly elevated Tyr levels (56). The activity of TH is shut down via feedback inhibition from high affinity binding of catecholamines to the active site iron, but the enzyme can be reactivated by phosphorylation at Ser40 (57). The cAMP dependent protein kinase (PKA) is a prominent TH-Ser40 kinase in the brain that is further regulated by D2 autoreceptors (57). There are several signaling pathways that regulate TH phosphorylation on Ser19, 31 and 40, locally in the striatum and likely in other brain regions (57–59). Ser19 phosphorylation enables binding of TH to the 14-3-3 proteins, which regulate activity, secondary protein interactions and multi-site phosphorylation of the enzyme (57, 60). Much less is known about the regulation of other proteins in the DA synthesis pathway. However, there are reports of phosphorylation-mediated regulation of DDC, VMAT2 and DAT (61, 62).
Regulation of dopamine signalingThe cyclic nucleotide signaling pathways are central to DA signaling. The activation or inhibition of cAMP signaling is affected by receptor configurations and the expression and protein regulation of downstream components. The adenylate cyclases and guanylate cyclases that synthesize cyclic nucleotides and the phosphodiesterases (PDEs) that degrade the cyclic nucleotide second messengers, are important in shaping downstream DA signaling. PDEs in particular are well explored drug targets (63, 64). The mammalian PDEs comprise a superfamily of 11 members, originating from 21 different genes that give rise to > 100 different isoforms (65). These are differentially expressed in different tissues and have diverse substrate selectivity (towards cAMP and cGMP), regulation, and cellular localization. Brain regional differences in PDE isoform expression have been reported for several of the PDE genes. For example, the dual specificity PDEs PDE1B1 and PDE10A are highly expressed in striatal medium spiny neurons (MSN) (63, 64). Knock out mice of the Ca2+/calmodulin stimulated PDE1B show increased locomotor activity and amphetamine sensitivity. The opposite has been reported for inhibition of PDE10A (66). The PDE10A has high affinity for cAMP and has been shown to be important for maintaining baseline cAMP levels and PKA activity in striatal MSN (67). The cGMP activated PDE2A has been found to be mainly located in the axonal and terminal compartments of pyramidal neurons in the cortex and hippocampus, striatal MSN, and in the medial habenula. The terminal location was confirmed by high immunoreactivity in the globus pallidus, substantia nigra pars reticulata, and interpeduncular nucleus (68). The presence of this PDE in the terminal compartment would contribute to nitric oxide mediated inhibition of presynaptic activity as cGMP will stimulate PDE2A and decrease the cAMP levels. For the dopaminergic terminals, less is known about the key PDEs that control PKA activation and thus TH Ser40 phosphorylation. So far, only members of the PDE4 family have been put forward, based on inhibitor studies (69). More research is needed to understand the key players in DA signaling at the cellular detail of different brain regions.
Together these examples illustrate some of the extensive signaling diversity that can be generated even in a simple signaling pathway such as cAMP signaling. There are many additional layers of complexity as illustrated in Figure 1. The involvement of these signaling modules across multiple pathways and biological functions also implies that it is virtually impossible to define a single “DA-specific” biological system.
Genetic perspectiveAnimal modelsWidely studied animal models of ADHD include spontaneously hypertensive rats (SHR), DAT knockout (KO) mice and Coloboma mice. The latter model has a mutation in the SNAP-25 gene, affecting synaptic vesicle release and resulting in hyperactivity and attention deficits (70). As SNAP-25 has multiple functions and affects many transmitters and ion channels, the superficial phenotypic resemblance of Coloboma mice to ADHD can hardly be attributed only to the DA system. SHRs exhibit many metabolic alterations, hypertension, hyperactivity, impulsivity, attention deficits, and altered monoamine levels, but the exact origin of this complex phenotype is still being debated. Several of the known proteins and genes involved in DA signaling have been studied in rodents, zebrafish and fruit fly models, which have partially confirmed their role in ADHD-like behaviors. A recent review listed 111 different genetically modified mouse strains implicating 103 different causal genes that show “exclusively ADHD-related behaviors” (70). Many of these genes have also been implicated in trans-synaptic signaling and forebrain development, including but not limited to dopaminergic synapses. Genetic variants in transcription factors (e.g. FOXP1, FOXP2 and MEFC2) are among the most robustly associated genetic findings in ADHD, autism and other neurodevelopmental disorders (see below) (71, 72). The forkhead box (fox) transcription factors FOXP1/FOXP2 regulate expression of many downstream proteins involved in neurogenesis, synaptic development and functions and are highly expressed in neurons involved in striatal dopaminergic signaling (73). Loss of Foxp1 and Foxp2 results in impaired motor and social behavior in mice, possibly by altering potassium channel functions (Figure 1) (73). This example illustrates the complexity of the dopamine system, how different animal models can be used to explore various aspects of these functions and how they relate to human genetic findings. However, it has also been shown that different animal models with different genetic backgrounds can give conflicting results (70), which demonstrate the complexity of animal (and human) behavior and the inherent limitations of animal models. For a summary of animal studies exploring monoamine functions in ADHD models, see Viggiano et al. (74). Although many genetic ADHD models with altered DA signaling have been examined, there is probably not a single animal model that can adequately recapitulate all clinically relevant aspects of this complex human phenotype (70).
Animal models, particularly DAT, NET and SERT KO mice, have also been used to elucidate the molecular targets of ADHD medications. Mice lacking DAT were reported to show hyperlocomotion and indifference to cocaine and amphetamine (75), but self-administration of cocaine persisted in these DAT-KO mice, possibly indicating involvement of multiple targets (e.g. NET and SERT) and compensatory mechanism in such animal models (76). A recent study compared the behavioral effects of monoamine transporter drugs on DAT-KO and NET-KO mice (77). The NET blocker desipramine increased PFC DA and NE levels in DAT-KO but not in NET-KO mice. Furthermore, desipramine or the dopamine beta-hydroxylase (DBH) inhibitor nepicastat reduced hyperactivity in DAT-KO mice. DBH catalyzes the conversion of DA to NE so inhibition of DBH depletes the brain of NE, while increasing levels of DA. Based on these observations, the researchers concluded that increased PFC levels of DA rather than NE was the common mechanism explaining the ‘paradoxical’ calming effects of psychostimulants. However, these animal models also show developmental compensatory effects, such as alterations in striatal D2 receptors in DAT KO mice, that could contribute to the phenotype (78).
Human studiesAlthough twin studies have shown that ADHD in children and adults has a high heritability (79), it is considered a polygenic disorder, and it has been difficult to find consistent genetic markers for this trait (80). Since the 1980s linkage, candidate gene, genome wide association (GWA), whole exome sequencing and whole genome sequencing studies have been used to study the molecular genetics of ADHD (80–82). As stimulants target DATs and other monoamine transporters, like SERT, NET and VMAT2, the genes encoding these transporters, as well as the five human DRs (DRD1-5), have been intensively explored in candidate gene studies (83–87). Genetic variants of DRD4 have been investigated particularly intensively in connection with ADHD (88, 89). In 1993 it was reported that the DRD4 gene has a hypervariable segment (VNTR) encoding an intracellular domain of the DA receptor (90). The frequency of the different 4R, 7R, and 2R alleles varies widely across ethnic groups. For instance, the prevalence of the 7R allele was reported to be <2% in Asian populations and ∼48% in native Americans. It was suggested that these differences could be due to positive selection (91), but later studies have not confirmed a positive selection favoring the 7-repeat allele of VNTR in the DRD4 gene. Instead, the increased population frequencies of the DRD4 7R allele in African and European populations can be explained by random genetic drift (92, 93). Although recent meta-analyses and original studies in various populations show possible associations with DRD4 variants in clinical subgroups of ADHD, no unifying or generally accepted hypothesis for the role of DRD4 in ADHD has been presented (94, 95).
Early candidate gene studies also suggested associations between various genetic markers in the other DRDs, DAT1 (SLC6A3), COMT, and DBH, but later meta-analyses have given mixed results (88, 89, 96–98). In a comprehensive meta-analysis, Gizer et al. reported significant associations for DAT1, DRD4, DRD5, 5HTT, HTR1B, and SNAP25, but also significant heterogeneity for the associations between ADHD and DAT1, DRD4, DRD5, DBH, ADRA2A, 5HTT, TPH2, MAOA, and SNAP25 (88). Many subsequent studies have since investigated these candidate genes in different populations, reporting nominal associations in clinical subgroups with different genetic markers in some of the genes (94, 99). Compared to recent GWA studies (see below), these studies were all based on small clinical samples (hundreds of individuals) with variable reporting practices and borderline significant results, compared to tens of thousands of individuals, harmonized genotyping platforms and protocols and stringent significance levels used in recent GWA studies. In the absence of sufficiently powered and replicated studies, e.g., on the DAT1 or DRD4 VNTRs, there is still some uncertainty regarding the robustness of these findings and the role of individual genetic variants in core DA related genes in ADHD.
Unlike candidate gene studies that have a limited focus, GWA studies aim to investigate common genetic markers across the entire genome. Due to the polygenic nature of ADHD, early GWA studies did not provide any genome wide significant findings. However, using a sample of >20 000 ADHD cases and >35 000 controls, the first 12 genome wide significant hits were published in 2019 (71). When this sample was expanded to >38 691 cases and 187 843 controls, 27 significantly associated signals were obtained, many of which could be linked to credible functional variants and risk genes (72). None of the previously nominated candidate genes, including DA related genes or known drug targets for ADHD, appeared among the top ranked risk genes (100). Among the core DA related genes, DRD2 appeared promising, but not at the genome wide significance level. Human DRD2 is found in a gene rich region on chromosome 11. Some of the most studied DRD2 variants are found in neighboring genes, such as the common variant rs1800497, which is located downstream of DRD2 within the ankyrin repeat of ANKK1 that encodes a Ser/Thr protein kinase. The rs1800497 variant correlates with expression levels of DRD2 and has been associated with multiple psychiatric and somatic disorders and common traits. The rs1800497 and many other common variants in proximity to the DRD2 locus are significantly associated (p< 10-8 in GWA studies) with smoking (101), alcohol consumption (102), risk tolerance (103), schizophrenia (104), depression (105), suicide attempts (106), neuroticism (107), educational attainment (108), as well as cross-disorder (103) psychiatric disorders (8 diagnoses) (109). However, it is not clear whether these phenotypes are mediated by the DRD2 protein or by other neighboring genes or regulatory DNA sequences. In comparison to these well-established associations, the connection of psychiatric disorders and behavioral traits with other DR genes are much less consistent, although there are some GWA studies showing that DRD1 is associated with educational attainment (108) and DRD3 with neuroticism (110). As the DRD2 receptor protein is expressed at higher levels than other DRs in the human brain (Human Protein Atlas) and has important autoregulatory functions (Figure 1), it is not surprising that DRD2 variants also show the strongest associations with human disease and behavioral traits. However, in comparison to the cited associations with other traits, the largest GWA study of ADHD did not show genome significant associations with DRD2 variants or any other core DA related genes (72).
The failure to replicate candidate gene studies, e.g. the DAT1 or DRD4 VNTRs, in large GWA studies of ADHD could be due to the poor tagging of such variants (i.e. not being in linkage disequilibrium) with SNPs typically included in GWAS. Other explanations could be that the patient and control samples included in the early reports were different from more recent and larger GWA studies or due to publication bias where some early positive studies have dominated the literature. Furthermore, the lack of genome wide significant associations (p <10-8) with core DA-related genes could still be a statistical power issue, as the ADHD sample sizes are still relatively small compared to those used for some other complex traits. Although modest associations were found for the core DA genes, pathway analyses showed enrichment in dopaminergic gene transcripts (72). Overall, it seems clear that genetic alterations in DA-related genes are found across several behavioral traits but that ADHD does not currently stand out as one disease uniquely or predominantly associated with such genetic variants.
Early candidate studies in ADHD and other traits often prioritized coding variants with functional effects. In contrast, GWA studies typically explore common, noncoding variants with unclear functional effects of their own. A study comparing rare and common variants in early and late diagnosed ADHD found that childhood ADHD had stronger genetic overlap with hyperactivity and autism compared with late-diagnosed ADHD and the highest burden of rare protein-truncating variants in evolutionarily constrained genes (111). As more whole exome sequencing and whole genome sequencing data become available, we expect that a clearer picture of phenotypic correlates of different human genes and variants will emerge across the full spectrum of common and rare genetic variants.
In addition to analyzing single genetic variants, it is possible to collapse multiple variants into a single gene or analyze many related genetic variants and genes together, e.g. multiple genes in the dopaminergic pathway, using MAGMA or similar software tools (112, 113). In a review from 2003 it was concluded that “SLC6A3 and DRD4 genes in ADHD appears to be one of the most replicated in psychiatric genetics and strongly suggests the involvement of the brain DA system in the pathogenesis of ADHD” (114). However, as detailed above, not all subsequent data have supported this conclusion. Such early gene set analyses were limited by small sample sizes and the low number of genetic variants available for testing. With access to GWA data it has been possible to perform gene set and pathway analyses more systematically and at a larger scale.
In 2022 Cabana-Domínguez et al. published a gene set analysis using publicly available GWAS data and manual curation to explore combined genetic signals from DA and serotonin (DA core with 12 genes, and SERT core with 23 genes) (115). The analysis contained the well-known dopaminergic or serotonergic genes (neurotransmitter receptors: DRD1-5, 5-hydroxytryptamine receptor (HTR)1A-B, HTR1D- F, HTR2A-C, HTR3A-E, HTR4, HTR5A, HTR6, HTR7; transporters: DAT1/SLC6A3, 5HTT/SLC6A4 (serotonin transporter gene); and related enzymes DDC, TH, tryptophan hydroxylase (TPH)1, TPH2, DBH, COMT, MAOA, MAOB). Using the MAGMA software for gene-based analyses, no significant multiple testing corrected association was observed with either the DA or SERT gene sets and ADHD, but the SERT genes were weakly nominally associated with ADHD. However, when an expanded list of 265 “wider dopaminergic genes” using Gene Ontology and KEGG pathway databases (as updated in 2016) was tested, they found significant associations for ADHD and autism spectrum disorder (115). Notably, a major caveat of such studies is that few of the included genes or genetic variants are uniquely associated with dopaminergic neurotransmission. For example, the genes and proteins involved in DA synthesis, storage, release, and degradation are also involved in synthesis of other neurotransmitters such as NE, serotonin, and nitric oxide. As detailed above, even the DA receptors are known to interact with multiple transmitters. Cabana-Domínguez et al. noted that 20% of the genes in the DA set were also present in the SERT set and 32% the other way round, but this estimate may be too low (115).
Gene-environment interactionsLow birth weight, preterm birth and perinatal asphyxia are among the best documented environmental risk factors of ADHD (116, 117). Fetal hypoxia can result in neuronal death, white matter damage and reduce the growth of neural processes (118). Perinatal hypoxia and exposure to neurotoxins such as 6-hydroxydopamine and MPTP (1-methyl-4-phenyl-1,2,3,5-tetrahydropyridine) have also been shown to reduce brain DA syntheses in animal models (119, 120). As human TH is also very sensitive to reduced oxygen tension, it is likely that DA synthesis is also reduced in the human brain under hypoxic conditions (54).
The generation and maintenance of dopaminergic neurons also requires a precise transcriptional cascade at specific time points during brain development. For instance, Fox1a, Foxa2 and Nurr promote a dopaminergic phenotype in the mouse brain (121). However, most of these transcription factors are also expressed in other cell types and transmitter systems. The DA neuron specifying transcription factors could be promising risk genes for psychiatric disorders that should be more systematically explored. As many risk genes for neuropsychiatric disorders are mainly expressed during embryonic development, it is possible that any putative altered dopaminergic functions in ADHD patients could be present before birth, but not necessarily in children or adults. Furthermore, it might be possible that the causative genes are not among core dopaminergic genes, but rather in other developmental or regulatory factors.
As mentioned above, alterations in the DA system can be secondary to other genetic or environmental changes in transcription factors or other proteins involved in neurodevelopment. Linkage studies in large multigenerational families in Colombia led to the discovery of the latrophilin 3 gene LPHN3 (ADGRL3) as a risk gene in ADHD (122, 123). Latrophilins 1-3 are postsynaptic adhesion G protein-coupled receptors that have been implicated in brain development and synaptic maturation (124) and linked to developmental behavior abnormalities in rodents and humans (125). Animal studies have shown that Latrophilin 1 or 3 haploinsuffiency (ADGRL1 or ADGRL3 knockouts) lead to developmental abnormalities and altered dopamine signaling (126). Similarly, the Wnt/mTOR (wingless-related integration site/mammalian target of rapamycin) pathway has been implicated in ADHD based on genetic and pharmacological evidence (127). The Wnt-signaling pathway is involved in cellular proliferation and differentiation and mTOR has been implicated in neurodevelopment, dopamine signaling and synaptic plasticity (128). Interestingly, exposure to manganese in neonatal rats results in attention deficits, dysregulation of mTOR signaling and downregulation of DA synthesis in brain prefrontal cortex (129). These examples show that alterations in brain DA functions may be due to a primary genetic predisposition, environmental exposures such as pharmacological agents, or specific interactions between genes and the environment (130).
Concluding remarks: Many different animal models, including some models with altered DA function, show phenotypic resemblance to ADHD, including hyperactivity and inattention. However, it is unclear whether these behaviors are specific and how they can be used to trace the biological origins of human ADHD. Although the DA-system is indirectly implicated in several of the most robustly associated ADHD risk genes, associations with the core set of genes involved in DA synthesis, DA receptors or transporters are less consistent. There is also a need for larger and more diverse human samples, as well as for integrative approaches that combine genetic and environmental data in order to provide a more comprehensive perspective on ADHD neurobiology.
Neurometabolic perspectiveRecent genetic advances have revealed risk genes for hundreds of different diseases and traits, including genetic causes of recessive lethality (131). Nearly 5000 human genes are known to exist as homozygous complete knockouts in the human population (131). Phenotypic characterization of such genetic variation constitutes a rich source of information that is highly relevant for studying normal gene function, rare genetic syndromes, as well as common, polygenic and complex human disorders, such as ADHD. Thus, rare monogenic syndromes may reveal novel biological functions of the affected gene(s) and also shed light on the role of these genes in common complex disorders (132). Disrupted brain metabolism as a potential causative element to the ADHD phenotype is worth exploring from this perspective.
Inborn errors of metabolism constitute a subgroup of “genetic” diseases that typically affect enzymes, transmembrane signaling proteins or co-factor synthesis relevant to the function of key enzymes (133). The International Classification of Inherited Metabolic Disorders (134) currently recognizes over 1,450 conditions, hundreds of which belong to the heterogeneous and expanding group of inherited neurometabolic disorders (NMDs;//www.omim.org/) that affect metabolic processes relevant to brain function. The immediate molecular consequence of such enzyme deficiencies – whether they are present within the CNS or occur peripherally – may present as a decrease/absence of critical metabolites and/or the accumulation of toxic intermediates. Such metabolic defects typically interfere with cellular processes involved in neuronal signaling or produce tissue damage, potentially affecting multiple organs. Human Mendelian “model” diseases have been characterized for virtually every known gene and protein involved in monoamine functions. Some of these genes and conditions are briefly reviewed here. As for animal models, interpretation of the phenotypic consequences of genetic variants, including complete loss of function (LOF) variants, must consider early (prenatal) developmental effects, as well as redundant, overlapping and compensatory mechanisms of different genes.
Human variants of DAT1 SLC6A3 are of particular interest in the context of ADHD. Infants with early onset SLC6A3-related DA transporter deficiency syndrome typically manifest nonspecific findings with irritability, feeding difficulties and hypotonia and delayed motor development, but not typical ADHD symptoms. In contrast, these children typically show a “neurological” type of hyperkinetic movement disorder with chorea, dystonia, ballismus and orolingual dyskinesia and later also show typical neurological symptoms (135).
Similar observations have been made for children and adults with severe defects in DA synthesis. LOF mutations in DDC - the gene encoding the enzyme immediately responsible for DA synthesis - have been associated with severe neurodevelopmental delay, hypotonia, oculogyric crises, and a complex movement disorder with autonomic features, but again not typical ADHD symptoms (132, 136). Since DDC has a broad range of substrates and is involved in the synthesis of other monoamines (histamine, serotonin, NE), such symptoms are not necessarily related to altered dopaminergic functions and could also be due to early prenatal neurodevelopmental alterations. As the enzyme TH is a specific marker of catecholaminergic neurons and the rate limiting enzyme in DA synthesis, it has also been explored in the context of ADHD. Central TH deficiency, resulting in mild-to-severe DA deficiency, has been associated primarily with movement symptoms - or in severe cases, progressive encephalopathy with mental retardation – but apparently no reports of ADHD symptoms (5). For comparison, as Parkinson’s disease is characterized by very low levels and dysregulated TH, it has been termed a TH deficiency syndrome (28). LOF mutations of DBH typically present with very low levels of NE and severe orthostatic hypotension and eyelid ptosis, but normal intellectual development and no record of ADHD symptoms (137). Animal studies show that total lack of DA or NE is incompatible with life, but less severe missense variants in DBH may be beneficial as they are protective against age related arterial hypertension (138).
Phenylalanine, tyrosine, and tryptophan are aromatic amino acid (AAA) precursors to the production of monoamine neurotransmitters such as DA, NE, adrenaline, serotonin, and melatonin. Deficiencies in the enzymes governing the conversion and breakdown of AAA precursors result in various metabolic disorders categorized as aminoacidopathies. Associations have been observed between aminoacidopathies, which purportedly result in low prefrontal DA levels, and neuropsychiatric disorders, including ADHD (139). Specifically, tyrosinemia type 1 (TYRSN1) and phenylketonuria (PKU) have been linked to core symptoms of ADHD, mainly presenting as impaired executive functioning in affected patients (140, 141). Shared pathophysiological mechanisms have been suggested (139, 140), partly supported by the improvement of cognitive symptoms in response to amphetamine treatment (142). However, in treated TYRSN1 there is debate over whether elevated tyrosine levels lead to increased or attenuated DA synthesis (140, 143). Furthermore, not all patients respond positively to stimulants, and the general response in healthy adults to these drugs is rather similar (see above).
The synthesis of DA and other transmitter molecules also depends on an adequate supply of enzyme cofactors, i.e. tetrahydrobiopterin (BH4) for TH, pyridoxal phosphate (vitamin B6) for DDC and ascorbate (vitamin C) for DBH (132). A lack of tetrahydrobiopterin is the most common and best-established genetic cause of DA deficiency in humans. LOF mutations in genes encoding the BH4 synthetic enzymes are found in the autosomal recessive neurological disorder DOPA responsive dystonia (Segawa syndrome) (144). This condition is clinically similar to TH deficiency and characterized by low levels of homovanillic acid and 5-hydroxyindoleacetic acid in CSF, microcephaly, early onset neurological symptoms, but not typical ADHD or other psychiatric symptoms or disorders (145). Although BH4 is also an essential cofactor for synthesis of serotonin, nitric oxide and other metabolites, the clinical picture is dominated by hyperphenylalaninemia and DA deficiency (145). As the cofactor for DDC (pyridoxal phosphate, a derivative of pyridoxamine, vitamin B6) is needed for hundreds of different enzymes and metabolic pathways (146), vitamin B6 deficiency has multiple manifestations from different organ systems and has been associated with increased risk of diabetes, heart disease, and cancer (147). Although again, ADHD is not among the defining symptoms in these conditions of DA deficiency. Similarly, severe ascorbate deficiency interferes with NE synthesis via DBH and causes scurvy, which is a syndrome primarily affecting connective tissue but apparently with less consequences for brain function (148).
Concluding remarks: Hundreds of patients with different monogenic diseases that lead to abnormally low DA levels in the brain have been characterized. The clinical features reported for these patients are dominated by motor neurological symptoms, not by ADHD or other psychiatric disorders. Although these observations do not directly implicate low DA levels as the defining feature in ADHD, partially overlapping cognitive deficiencies with aminoacidopathies and related conditions suggest that aspects of disrupted monoaminergic brain metabolism could contribute to the broader clinical picture of ADHD.
Neurophysiological perspectivePositron emission tomography (PET) and single photon emission computerized tomography (SPECT) can measure neuronal activity indirectly through measurements of metabolism, blood flow, and ligand–receptor interactions. Studies using these methods are therefore worthwhile examining in more detail as they can visualize neurotransmission in vivo and provide relatively strong support for/against the DA hypothesis.
One of the earliest ADHD PET studies suggesting decreased extracellular DA levels was a 1999 preliminary study with children by Ernst and colleagues (149). Extracellular DA was decreased in the midbrain but not the striatum or frontal regions. Despite the high citation count, the authors acknowledged that the main finding of decreased extracellular DA in the right substantia nigra/ventral tegmental area compared to healthy controls did not survive Bonferroni correction. In theory, a higher [18F]DOPA uptake reflects elevated presynaptic AADC activity (i.e. increased DA synthesis). The authors reasoned that increased synthesis occurs in response to a disruption of DA neurotransmission leading to abnormally low extracellular levels, as has been shown for the effect of L-DOPA on the dorsal striatum in Parkinson’s disease (150, 151). Presynaptic DA autoreceptor stimulation is thought to play a role in this inverse effect on synthesis/tracer influx (152). The finding of decreased extracellular DA in ADHD has since been replicated through increased prefrontal [18F]DOPA influx (153) and increased striatal DAT binding (154–157).
There have, however, been several PET/SPECT studies over the years which have reported findings which either fail to support or are contradictory to the hypo-dopaminergic hypothesis. Studies have found no differences in endogenous DA between ADHD and healthy controls within striatal (158–161) and midbrain regions (160). Of particular interest, a 1998 study be Ernst et al. (162) reported lower [18F]DOPA uptake in ADHD as opposed to the nonsignificant higher uptake in their 1999 study, by extension implying increased extracellular DA in prefrontal regions. Similarly, increased extracellular DA (i.e. lower [18F]DOPA influx/DAT binding/receptor binding) has been reported in the striatum (153, 163, 164) and midbrain (153, 158, 165) of ADHD patients relative to controls. This collection of studies would therefore suggest a generalized hyperdopaminergic state in ADHD. For a recent comprehensive meta-analysis of the inconsistent PET and SPECT findings for adult and adolescent ADHD see (166). It may be possible to reconcile the hyper- vs. hypo-dopaminergic findings by considering dynamic versus generalized DA activity. For example, in a task-based study, Badgaiyan and colleagues reported decreased tonic but enhanced phasic release of DA in the right caudate of people with ADHD (167).
In addition to contradictory experimental findings, result interpretations also differ between studies. For example, some authors interpret lower DA synthesis and lower DAT density as evidence of reduced DA signaling and therefore a hypo-functioning dopaminergic system (165), as opposed to the interpretation by Ernst and colleagues in 1999. This discrepancy between interpretations is somewhat unsurprising as translating DA metabolism findings into functional changes in dopaminergic networks can be difficult, given that DA can have both facilitatory and inhibitory effects depending on the dominant local DA receptor population. Nevertheless, the opposing directions for changes in PET/SPECT measures demonstrate incongruent links between ADHD and DA. Possible reasons for these discrepancies include small sample sizes, level of psychostimulant exposure, focus on several kinds of existing ligands, and a questionable validity of the diagnosis through a lack of biological diagnostic criteria (168). The review by Yamamoto and Inada (168) suggests that whether alterations of monoamine function may be involved in the pathophysiological mechanism of adult ADHD remains to be clarified. Indeed, other recent reviews (169) have concluded that there seems to be a greater consensus on other potential disease mechanisms e.g. prefrontal hypo-connectivity and prefrontal glucose hypo-metabolism.
A role for neurovascular coupling?As mentioned, all clinically approved stimulant drugs for ADHD either directly or indirectly affect multiple transmitter systems in addition to DA, most notably NE and serotonin neurotransmission. Ultrastructural investigation of DA and NE innervation of cortical structures has revealed a similar morphology of their terminals. Of note, some clinically effective non-stimulant drugs specifically target NE neurotransmission, apparently without directly affecting DA transmission (170). NE is an important modulator of local blood flow in the brain. The clinical efficacy of these medications may imply that the effect of some ADHD medications is not mediated directly on neurons, but rather indirectly via effects on blood flow. However, as clinical studies indicate that stimulant drugs that also block the dopamine transporter are more effective than non-stimulant drugs in ADHD, an involvement of DA in their mode of action still seems plausible (13). Nevertheless, such a symptomatic effect of dopaminergic drugs doesn’t necessarily imply that there is an a priori defect in the dopamine system.
Neurovascular perfusion is tightly regulated to accommodate changes in neuronal activity and metabolic demand. This neurovascular coupling occurs at the level of the microvasculature – the neurovascular unit – and at larger arteries of the vascular tree. The larger blood vessels are regulated by peripheral innervation, with NE post-ganglionic orthosympathetic fibers and cholinergic parasympathetic fibers. Within the brain parenchyma, precapillary arterioles and capillaries are innervated by NE fibers originating from the locus coeruleus (LC). The brain microvascular compartment is almost completely covered by end-feet projections from nearby astrocytes, leaving a space between the pericyte or smooth muscle layer and the astrocyte end-feet. This space contains the basal matrix of the vasculature and circulating cerebrospinal fluid and is part of the glymphatic system. Most NE varicose release sites target the microvasculature via the astrocyte end-feet. Astrocytes have a rich expression of adrener
留言 (0)