In holometabolous insects, precise hormone regulation facilitates development from eggs to sexually mature adults through several molts, thereby controlling population dynamics (Liu et al., 2018; Smykal et al., 2014). Juvenile hormone (JH), a structurally unique sesquiterpenoid hormone, influences metamorphic development in larvae/nymphs by antagonizing the ecdysone-signaling pathways (Mukai et al., 2022). In adults, JH predominantly functions as a gonadotropin, promoting reproduction by regulating various physiological and behavioral processes in both males and females. Bactrocera dorsalis larvae exhibit premature pupation when there is insufficient JH, significantly compromising pupal survival (Liu et al., 2017). In Aedes aegypti, the JH signal is crucial for regulating ribosomal protein synthesis, i.e., RPL32 and RRS1, and facilitates vitellogenin (Vg) synthesis in the fat body (Wang et al., 2017). JH also induces the expression of genes associated with Vg uptake, such as vitellogenin receptor (VgR) and lipophorin receptor (LpR), thereby promoting ovarian development (Marchal et al., 2014; Parra-Peralbo and Culi, 2015; Ahmed et al., 2022). Thus, the JH signaling pathway has emerged as a prominent area of investigation in entomological research.
Regardless of its chemical structure, form, or function, most regulatory effects of JH in insects are mediated by its receptors. The receptor Methoprene-tolerant (Met) and its partner Taiman (Tai), also known as βFtz-F1 steroid hormone receptor coactivator (FISC)/steroid receptor coactivator (SRC), are central to JH signaling, together influencing insect growth and development (Zhang et al., 2011). However, vitellogenesis and oocyte maturation in insects are regulated by JH receptor complexes through different pathways. JH-Met-Tai promotes Vg expression via insulin-like peptides (ILPs) in Tribolium castaneum (Li et al., 2011; Zhang et al., 2023). The JH receptor complex directly activates the expression of Krüppel-homolog 1 (Kr-h1) and Hairy in mosquitoes to facilitate post-eclosion ovarian maturation (Saha et al., 2019). Additionally, JH and 20-hydroxyecdysone (20E) are key hormones regulating insect molting and metamorphosis. In the Bombyx mori Met1 knockout strain, 20E signaling is significantly activated in the larval epidermis, leading to early epidermal development and advanced pupation (Daimon et al., 2015). The Broad gene, an early responder in the 20E signaling pathway, is regulated by JH signaling genes, such as Kr-h1, which affect metamorphosis (Minakuchi et al., 2009).
Met was initially identified as a transcription factor having a basic helix-loop-helix Per/Arnt/Sim (bHLH-PAS) structure and directly binding to JH via the PAS-B domain, forming JH-Met, in Drosophila melanogaster (Ashok et al., 1998). This complex enters the nucleus using heat shock proteins. JH-Met forms a transcription complex in the nucleus with Tai/FISC, targeting downstream gene promoters at the Ebox element, thereby inducing gene expression and activating the JH signaling cascade. Insect Met is essential in various physiological processes, including reproductive development, metamorphosis, and toxin response (Julide et al., 2013; Lozano and Belles, 2014). RNA interference (RNAi)-mediated silencing of Met significantly reduced Vg and VgR expression, causing abnormal ovarian morphology and decreased egg production and hatchability in Neoseiulus barkeri females (Wu et al., 2021). The JH receptor, Met, and its target genes, Kr-h1 and Broad-Complex (BR-C), are crucial for metamorphosis in holometabolous insects. In A. aegypti, knockout the Met gene decreased the expression of Kh-h1 and caused severe molt block in larval–pupal transition (Zhu et al., 2019). Met1 phosphorylation via prokaryotic expression enhances the binding of the Met1-Tai complex to the JHRE E-box, thereby regulating Kr-h1 transcription in Helicoverpa armigera (Li et al., 2021). Met deficiency significantly boosts BR-C expression in B. mori larvae, leading to early metamorphosis and deformed adult wings (Hu et al., 2019). Met binds to the E-box in the diptericin gene’s regulatory region in A. aegypti mosquitoes, indicating that JH suppresses diptericin (Dpt) gene expression via the Met receptor (Chang et al., 2021).
The tomato leafminer Tuta absoluta (Meyrick) is an important invasive Lepidopteran pest that arrived from North America to Xinjiang in 2007 and rapidly spread across China, including Sichuan, Shandong, and Tianjin (Biondi et al., 2017). Larvae damage plants by eating leaves and penetrating leaf veins, leading to yellowing, wilting, and up to 80% crop loss (Uulu et al., 2017). Chemical pesticides, such as pyrethroids and avermectin, and some fungicides are effective for rapidly controlling T. absoluta. The large and widespread use of these pesticides hass inevitably resulted in varying resistance in T. absoluta (Cherif et al., 2017; Guedes et al., 2019; Siqueira et al., 2000). In Brazil and Chile, T. absoluta has developed resistance to many insecticides, making it necessary to identify new green approaches for tomato leaf miner control. Met is known to play critical roles in insect growth and development, but specific information about its functions in T. absoluta remains limited despite some studies having cloned the TaKr-h1 and TaMet genes (Wang et al., 2023). Focusing on insect reproduction and molting, the RNAi-based functional study of Met in T. absoluta may enhance pest control strategies and potentially replace traditional chemical pesticides.
2 Materials and methods2.1 InsectsThe tested T. absoluta population was originally collected from Kunming City, Yunnan Province, China, in 2023. The insects were reared in the sunlight insectary room of Guiyang University under the following conditions: temperature 27°C ± 1°C, relative humidity 55% ± 5%, and light cycle 16 L: 8 D. The larvae were used to inoculate fresh tomato plant leaves. Each cage is equipped with 15% honey water for adults.
2.2 Developmental and tissue-specific expression analysisThe same batch of hatched larvae were used to inoculate the leaves of tomato seedlings, and T. absoluta samples were collected at different developmental stages (1–7 days for male and female pupae, and 1–2 days for adults). Insects were collected as one sample at each developmental stage, and three biological replicates were prepared. Nine tissues, including the head, epidermis, intestinal tract, Malpighian tubule, fat body, ovary, seminal vesicle, male wing, and female wing, were dissected from day-2 adults, with 50 insects in each sample and three biological replicates. Total RNA was extracted from the samples using TransZol reagent (TransGen Biotech, Beijing, China). Subsequently, the first-strand cDNA was synthesized using the PrimeScriptTM RT Reagent Kit (TaKaRa, Tokyo, Japan), according to the manufacturer’s instructions. qPCR was used to investigate the expression profiles of TaMet. The qPCR primers were designed using primer3 (https://primer3.ut.ee). The qPCR mix included 10 μL of TransStart® Top Green qPCR SuperMix (TransGen Biotech), 7 μL of ddH2O, 1 μL of the cDNA template, and 1 μL each of the forward and reverse primers (10 μmol/L). The qPCR reaction procedure was performed as follows: predenaturation at 95°C for 5 min and 40 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. The melting curve was analyzed at 60–95°C. The EF1α gene (GenBank: MZ054826) of T. absoluta was used as the reference gene (Yan et al., 2021). The relative expression levels of TaMet were calculated using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
2.3 Effects of TaMet RNAi on ovarian development and female reproductionSpecific primers containing the T7 RNA polymerase promoter sequence were designed using Primer 5.0 (Supplementary Table S1), and double-stranded RNA (dsRNA) was synthesized using the TranscriptAid T7 High Yield Transcription Kit (Thermo Fisher Scientific, America). After purification, the synthesized dsRNA was diluted to 1 μg/μL with RNAi injection buffer. The same-aged female insects were collected for microinjection at the pupal stage (3-day-old), and samples were collected at different times (24, 48, and 72 h) for total RNA isolation. The RNAi silencing efficiency was evaluated using qPCR, as described above. Two-day-old female adults were dissected and photographed with a stereomicroscope VHX-2000C (Keyence Corporation, Osaka, Japan), and their ovaries were graded according to the previously published ovarian classification (Yang et al., 2024). After their emergence, female adults were reared with same-aged male insects in a 1:3 ratio in a new cage. Egg production and hatching were recorded within 10 days, and the changes in the ovarian morphology of the females were dissected and observed by measuring the ovarian tube length and egg size. Similarly, qPCR was used to detect the mRNA levels of key genes (TaVg and TaVgR) that may affect reproduction. Another batch of 3-day-old female pupae was also injected with dsTaMet, and the samples were frozen with liquid nitrogen after 24 h. The vitellogenin content was measured using the Insect Vitellogenin Enzyme-linked immunosorbent assay (ELISA) Kit (Mlbio, Shanghai, China).
2.4 Phenol fuchsin stainThe ovarioles from T. absoluta females were dissected in phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde fixative. The ovarioles were randomly selected from the dsTaMet- and dsGFP-injected groups, rinsed 3 times with PBS, stained with improved phenol fuchsin stain (Yuanye, Shanghai, China) for 15 min at room temperature, and squashed under a coverslip. All images were captured using an LSM 900 confocal laser-scanning microscope (Zeiss, Oberkochen, Germany).
2.5 Effect of TaMet RNAi on male molting and wing developmentThree-day-old male pupae were collected for dsRNA injection, and the mortality rate and the death phenotype were recorded for 20 days. The expression of four wing development genes, including TaWG (wing less), TaAP (apterous), TaSRF (serum response), and TaVG (vestigial), four chitin synthesis genes including TaChs (chitin synthase), TaTre1 (trehalase 1), TaTre2 (trehalase 2), and TaUAP (UDP-N-acetylglucosamine pyrophosphorylase), and five chitin degradation genes including TaCDA1 (chitin deacetylase 1), TaCDA2 (chitin deacetylase 2), TaCht5 (chitinase 5), TaCht7 (chitinase 7), and TaCht10 (chitinase 10) were detected using qPCR.
2.6 Statistical analysisStatistical analyses were performed using SPSS 20.0 software (IBM, Chicago, IL, USA), and the data were visualized using GraphPad Prism 6.01 (GraphPad, La Jolla, CA, USA). The survival rates were analyzed using the Kaplan-Meier method, and the Log-Rank test was used to determine the significant difference between the RNAi-treatment and control groups. Differences between the two groups were compared using a Student’s t-test at a significance level of p < 0.05. A one-way analysis of variance (ANOVA) was applied to compare the differences among more than two samples.
3 Results3.1 Developmental and tissue-specific expression of TaMetQuantitative real-time PCR (qPCR) results showed that TaMet was highly expressed during the early pupal and early adult stages in males. Specifically, TaMet expression in day-1 male pupae was 12.58-fold higher than that in day-7 male pupae. A similar expression pattern was observed in females, with TaMet expression peaking on the first day of pupation and being 16.45-fold higher than on the seventh day (Figure 1A) (F = 6.781; df = 6; p < 0.001). Tissue expression profiling revealed that TaMet was highly expressed in the epidermis and ovaries of T. absoluta, with 9.55- and 10.65-fold higher expression than in the Malpighian tube, respectively. Besides, TaMet was also highly expressed in the female wing and male wing (Figure 1B).
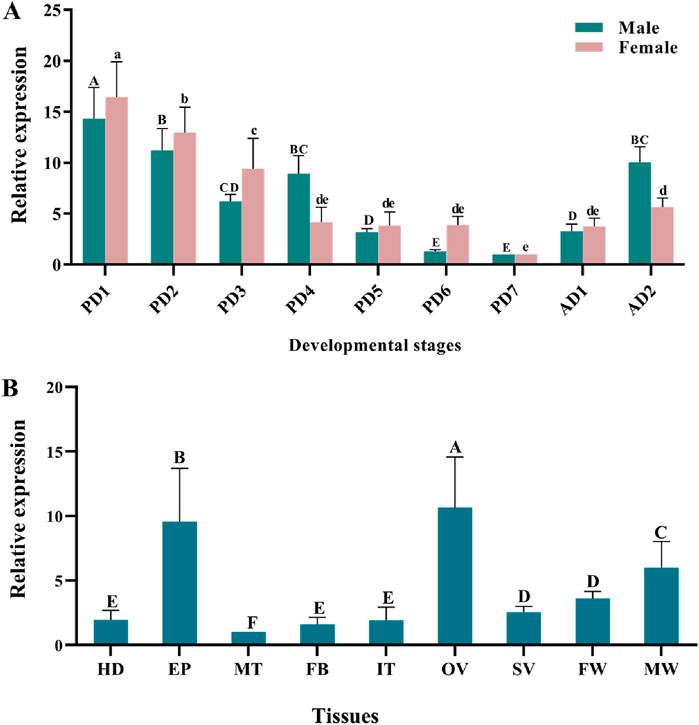
Figure 1. Relative expression of TaMet in Tuta absoluta at different developmental stages (A) and adult tissues (B). Total RNA was isolated from pools of 1–7-day pupae (PD1-PD7), 1–2-day adults (AD1-AD2), and the tissues of the head (HD), epidermis (EP), intestinal tract (IT), Malpighian tubule (MT), fat body (FB), ovary (OV), seminal vesicle (SV), female wing (FW), and male wing (MW). Different letters above bars indicate significant differences for females or males or the tissues based on one-way ANOVA, followed by a least significant difference test (p < 0.05).
3.2 TaMet knockdown resulted in abnormal ovarian developmentTaMet expression was significantly suppressed up to 70.8% at 72 h post-dsRNA injection in females (Figure 2A) (t = 32.977, p < 0.001). The ovaries were mainly at level III and IV (high production period) in the control group (dsGFP), while those of the treatment group were significantly delayed. Specifically, the ovarian development levels were multipolar (II, III, and IV were present), with level II ovaries (development period) being dominant (Figure 2B). The average length of the ovarian tube in the dsTaMet-injected group was 2601.4 μm, which was 27.5% lower than that of the control group at 3,588.6 μm (Figure 2C) (t = 6.017, p < 0.01). In addition, the dsGFP group had more mature oocytes with a mean length of 343.2 µm, while the dsTaMet group had fewer mature oocytes with a mean length of 194.7 µm (Figure 2D) (t = 10.172, p < 0.001). The ovaries of 2-day-old female adults in the dsGFP group were mature and contained many mature egg particles. In contrast, the ovaries of the treatment group were atrophied, and the ovarian tubes contained fewer egg particles. Phenol margin staining showed that the follicular epithelial cells on the surface of the secondary oocytes in the dsGFP group were closely packed, with sufficient yolk precipitation. However, follicular epithelial cells in the dsTaMet experimental group were unobstructed, with some of the epithelial cells showing an irregular distribution (Figure 2E).

Figure 2. Effect of TaMet silencing on ovarian development in Tuta absoluta females. (A) Relative expression levels of TaMet at 24, 48, and 72 h after TaMet or GFP dsRNA injection. (B) Changes in the ovarian grade ratio after silencing TaMet. (C) Length of ovarian tubules in females after dsRNA injection. (D) Average length of egg grains after dsRNA injection. (E) Effects of TaMet RNAi on the follicular epithelium of primary oocytes. Morphology of the follicular epithelium of primary oocytes in 2-day-old adults. PO, primary oocyte; SPO, sub-primary oocyte; FEB, follicular epithelium between. Significant differences between the treatment and control groups were determined using a Student’s t-test (*p < 0.05, **p < 0.01, ***p < 0.001). II: ovarian grade II, yolk deposition stage; III: ovarian grade III, mature and waiting period; IV: ovarian grade IV, peak spawning stage.
3.3 TaMet knockdown resulted in decreased reproductive ability in femalesSuppressing TaMet expression significantly decreased the female moths’ oviposition calendar period, which was reduced to 5.1 days compared to 7 days in the control group (Figure 3A). TaVg and TaVgR in T. absoluta significantly decreased after injection with dsTaMet (Figure 3B) (tvg = 196.892, tVgR = 60.432, p < 0.001). Females in the dsTaMet-injected group average laid 54.70 eggs per individual, which was significantly less than the 167.05 eggs per individual laid in the control group (Figure 3C) (t = 18.508, p < 0.001). The hatching rate of progeny in the dsTaMet-treated group was only 27%, which was significantly less than the 82.2% observed in the control group (Figure 3D) (t = 26.887, p < 0.001). The vitellogenin content was significantly lower (0.89 μg/mL) in 1-day-old T. absoluta females than in the control group (2.33 μg/mL; t = 60.432; p < 0.001) (Figure 3E).
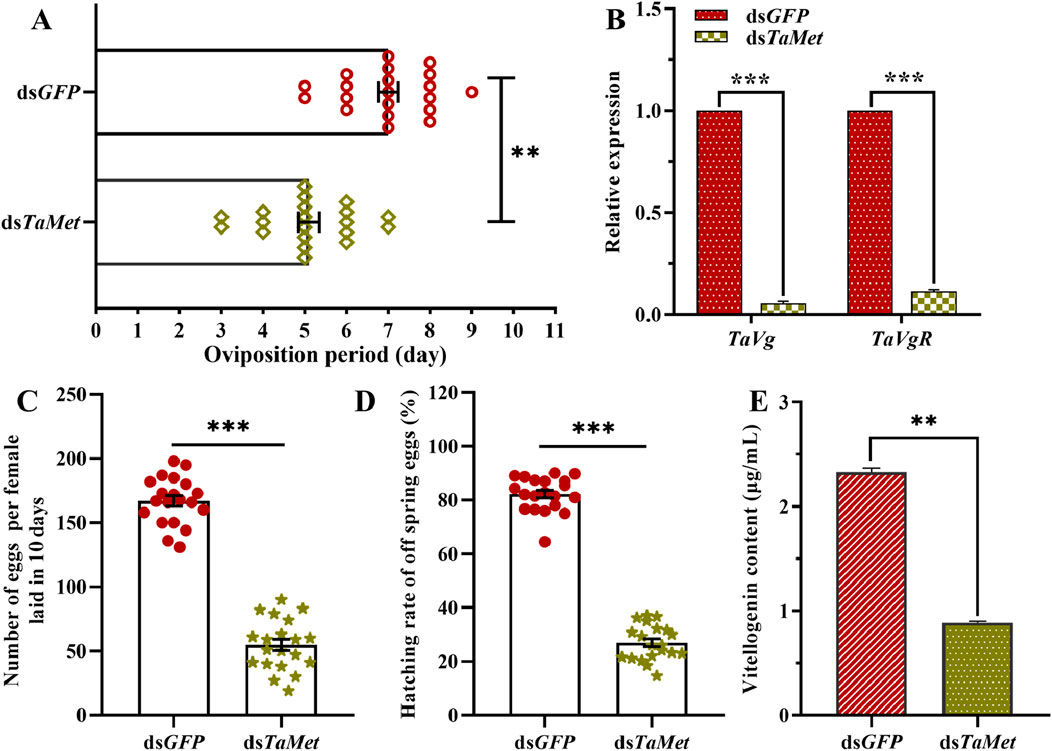
Figure 3. Effect of TaMet silencing on female reproduction in Tuta absoluta. (A) Oviposition period of females after dsRNA injection. (B) Expression levels of Vg and VgR after dsRNA injection. (C) Total number of eggs laid per female in 10 days (D) The hatch rate of offspring eggs. (E) Vitellogenin content in 2-day-old females after dsRNA injection. Significant differences between the treatment and control groups were determined using a Student’s t-test (*p < 0.05, **p < 0.01, ***p < 0.001).
3.4 TaMet knockdown resulted in molt failure and wing deformityThe expression level of TaMet was significantly reduced after injection with dsRNA at 24, 48, and 72 h (Figure 4A) (t24h = 8.353; t48h = 14.349; t72h = 16.218; p < 0.001). The dsTaMet-injected group had only a 20% male survival rate after 20 days compared to 85% in the dsGFP group (Figure 4B). Statistical observations revealed that silencing the TaMet gene resulted in 47.5% of deaths during the pupal stage due to abnormal pigmentation or the inability to molt pupal sheaths, and 32.5% of deaths during the adult stage. Males injected with dsTaMet had a 60% chance of developing wing deformities in adulthood. Both the forewing and hindwing veins displayed varying degrees of atrophy and notching (Figure 4C). Silencing TaMet in males led to a significant reduction in wing development genes TaWG and TaVG by 76.8% and 95.6%, respectively. The expression of wing primordial formation genes TaAP and TaSRF was reduced by 90.6% and 87.1%, respectively. The expression of eight genes associated with chitin degradation and synthesis were variably suppressed (Figure 4D), suggesting that TaMet regulates chitin degradation and synthesis genes during the pupal–adult transition.
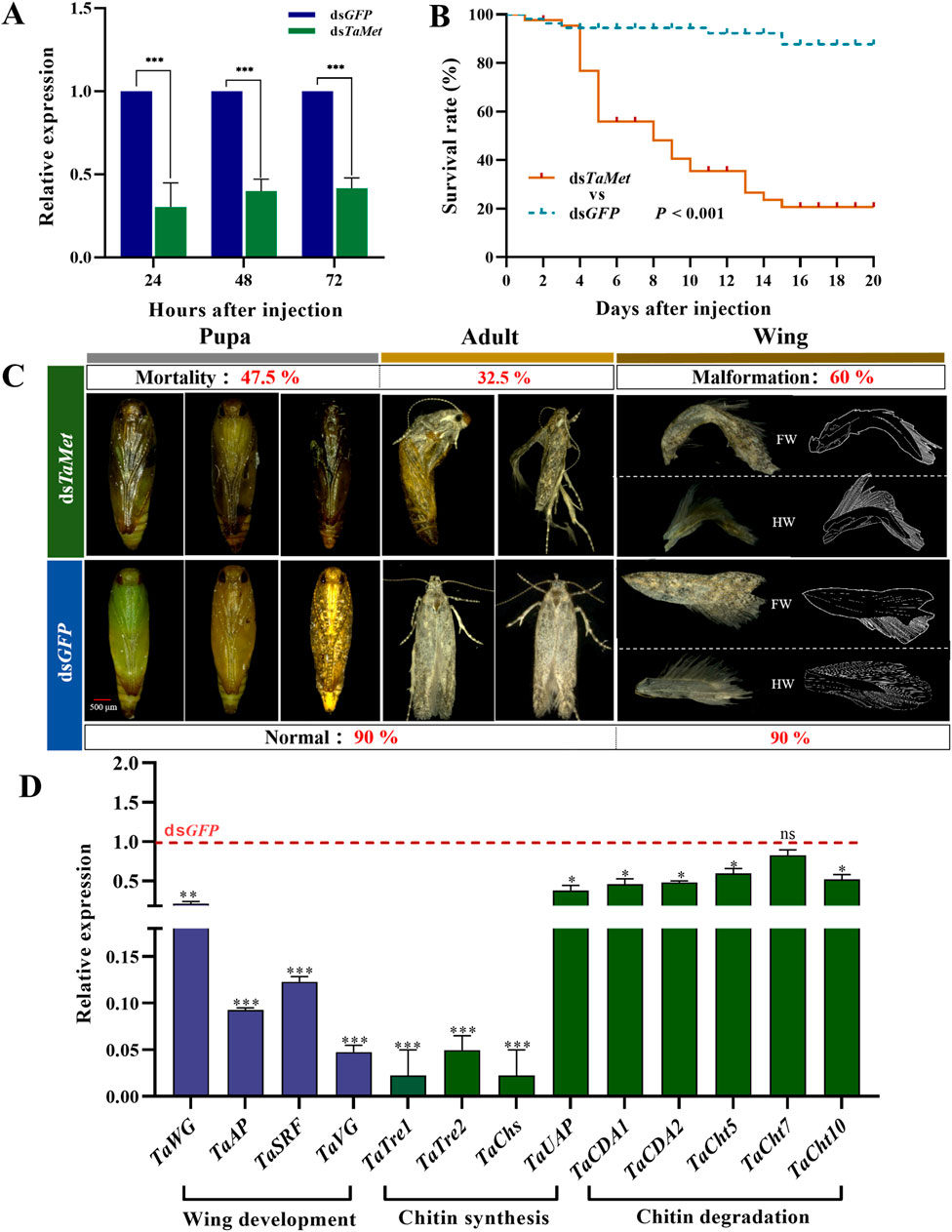
Figure 4. Effect of TaMet silencing in male Tuta absoluta. (A) Relative expression levels of TaMet at 24, 48, and 72 h after TaMet or GFP dsRNA injection. (B) Survival rate of males within 20 days of dsRNA injection. (C) Analysis of death phenotypes and wing deformity in males after dsRNA injection. FW: Forewings; HW: Hind wings. (D) The expression levels of genes involved in wing development, chitin synthesis, and degradation after dsRNA injection. Significant differences between the treatment and control groups were determined using a Student’s t-test (*p < 0.05, **p < 0.01, ***p < 0.001).
4 DiscussionThe expression of hormone receptor genes at different insect development stages is regulated by JH (Yan et al., 2021). Specifically, during the transition from the nymphal to adult stages in the wheat red sucker, SmMet expression was significantly lower in females than in males, indicating that SmMet may play a crucial role in sustaining the effects in insects (Cheng et al., 2020). Elevated expression of the Met gene during the early pupation stages facilitates tissue reconstruction (Tettamanti and Casartelli, 2019). HaMet was predominantly expressed in the pupal and egg stages in Harmonia axyridis (Han et al., 2022). In this study, the TaMet expression pattern exhibited cyclical variations across developmental stages. In T. absoluta, TaMet expression was significantly higher in the early pupal and adult stages, which are critical periods for pupal growth and adult reproduction.
Insects exhibit a range of notable characteristics, particularly their pronounced reproductive and migratory capabilities. Consequently, our research concentrated on examining TaMet expression within the reproductive system and during the wing formation process in T. absoluta. TaMet expression in the adult ovary was significantly higher than in the spermatophore. HaMet expression in H. armigera was significantly higher in the ovary than in other tissues, including seminal vesicles (Ma et al., 2018). TaMet expression in T. absoluta was higher in the wings of males than in females during the same developmental period. These results are consistent with previous studies. BmMet2 was highly expressed in the wing primordia of B. mori larvae and pupae (Kayukawa et al., 2012). Thus, it can be concluded that TaMet plays dual roles in reproduction and wing development.
In most female insects, Vg synthesis in fat bodies and oocyte maturation are regulated by JH (Luo et al., 2021; Liu et al., 2019). To investigate the function of TaMet in T. absoluta reproduction, we suppressed its expression using RNAi, and egg production, egg hatchability, ovary tube length, and egg granule length decreased as a result. We also noted a delay in ovary development. SgMet deficiency in Schistocerca gregaria results in delayed ovarian development and reduced female reproductive capacity (Gijbels et al., 2019). SfMet knockdown in Sogatella furcifera significantly reduces SfVg expression and yolk protein deposition and impairs oocyte maturation and ovarian development (Hu et al., 2019). Thus, TaMet is integral to female reproduction in T. absoluta. Furthermore, females injected with dsTaMet showed a decreased vitellogenin content and reduced TaVg and TaVgR gene expression, similar to the results for TcMet in T. castaneum (Naruse et al., 2020). Although silencing RpMet does not affect female egg production in Rhodnius prolixus, it reduces the hatching ratio (Leyria et al., 2022). Therefore, it is hypothesized that TaMet plays a crucial role in oocyte development and yolk formation. After gene silencing, the test insects were further stained using phenol magenta, showing that TaMet injection caused a loss of normal morphology in follicular epithelial cells and increased the cell gap. JH regulates vitellogenesis and oogenesis in insects via Met (Gujar and Palli, 2016). Met acts on Mcm4 and Mcm7 to regulate DNA replication and polyploidy for vitellogenesis and oocyte maturation (Guo et al., 2014). Similar results have been observed in S. gregaria (Holtof et al., 2021). Therefore, the decrease in TaMet expression may affect follicular epithelial cell development and yolk deposition, ultimately leading to ovarian morphological abnormalities and decreased fertility.
A decrease in the JH titer and an increase in the 20E titer at the end of the larval and pupal stages promote the completion of metamorphosis (Huang et al., 2013). JH suppresses the expression of the laccase2 gene during embryonic development in Blattella germanica, preventing premature hardening and cuticle darkening (Fernandez and Belles, 2017). Similarly, reducing AaMet expression in A. aegypti results in mortality during the pupal stage of the offspring (Zhu et al., 2019). In this study, TaMet knockdown in male pupae led to significant mortality. During the transformation of pupae into adults, most dsTaMet-treated pupae exhibited blackening, abnormal molting, and death. We propose that TaMet suppression influences the inhibitory effect of JH on the laccase2 gene, consequently resulting in the melanization and mortality of male T. absoluta pupae. During insect molting, abnormal regulation of stratum corneum formation can be fatal. Thus, the expression levels of genes involved in chitin metabolism are crucial for the degradation of the old epidermis and the synthesis of the new stratum corneum (Liu et al., 2022; Zhao et al., 2019). We found that silencing TaMet in male pupae resulted in significant reductions in the expression of genes critical for wing development and those involved in chitin metabolism. Depletion of hormone receptor three gene, another typical transcription factor in Lasioderma serricorne, disrupted the larval–pupal molting and downregulated the expression of chitin synthesis and degradation genes (Ma et al., 2022). These results suggest that different transcription factors are involved in the regulation of chitin metabolism during the insect molting and metamorphosis. In the larval stage of Leptinotarsa decemlineata, silencing of LdMet led to an increase in the 20E titer, resulting in shorter larval stages and early pupal weight loss (Meng et al., 2018). The effect of JH on insect metamorphosis through the “MEKRE93” pathway has been demonstrated in B. germanica (Belles and Santos, 2014). Additionally, Met knockdown also resulted in a reduction of insulin-like peptide (ILP) expression (Sheng et al., 2011). Therefore, we propose that TaMet knockdown in male T. absoluta pupae leads to plumage failure, possibly due to an increase in the 20E titer and insufficient energy reserves.
In the present study, TaMet showed functional differentiation in tomato leafminer moths of different sexes. Similarly, in B. mori, BmMet1 is required for JH antagonism of larval metamorphosis, while BmMet2 is involved in JH regulation of adult reproduction (Kayukawa and Shinoda, 2015). Only one TaMet gene has been identified in T. absoluta. It has become common for the same gene to exhibit functional differentiation in insects. Pleiotropy, which plays an important role in many organisms, refers to the phenomenon in which one gene determines the formation of multiple characteristics (Horabin and Schedl, 1993). In D. melanogaster, the α1,4-galactosyltransferase one gene has dual roles in spermatogenesis, including maintaining the survival of sperm bundles and regulating the sperm individualization process (Xiao et al., 2024). However, the gender-based functional differentiation of genes has rarely been reported in insects. In this study, TaMet mediated the male molting and female reproduction processes. Similar results have been found for the triacylglycerol lipase gene in Sitotroga cerealella, which regulates the sperm number in male moths and egg production in females (Yan et al., 2022). At present, there are few reports on the functional differentiation of the same gene in male and female insects, necessitating further research. Therefore, this study provides important materials for subsequent research on gene functional differentiation. We believe that TaMet is essential for the reproductive ability of T. absoluta females. TaMet mediates the male molting and wing development processes, making it a new potential target for RNAi-based control of T. absoluta.
Data availability statementThe datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statementThe manuscript presents research on animals that do not require ethical approval for their study.
Author contributionsTZ: Conceptualization, Investigation, Methodology, Writing–original draft. KX: Validation, Writing–original draft. DL: Investigation, Writing–original draft. HM: Data curation, Writing–review and editing. WL: Investigation, Writing–original draft. WY: Conceptualization, Data curation, Funding acquisition, Writing–review and editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by Project of Research and Development (YTH-4320-WB-FW-2022-033507-00), Natural Science Foundation of Guizhou Province (QKHJC-ZK-[2022]003), Program of Excellent Innovation Talents in Guizhou Province (GCC[2023]071), Program for Natural Science Research in Guizhou Education Department (QJJ-[2023]-024), and Program of Postgraduate Research Fund in Guiyang University (GYU-YJS-SH[2022]-01).
AcknowledgmentsThe authors gratefully acknowledge Yi Yan, Min Deng, Xin Jiang, and Yian Chen for laboratory assistant work.
Conflict of interestAuthors HM and WL are employed by the R&D Center of Yunnan Yuantianhua Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2024.1500391/full#supplementary-material
ReferencesAhmed T. H., Saunders T. R., Mullins D., Rahman M. Z., Zhu J. (2022). Molecular action of pyriproxyfen: role of the Methoprene-tolerant protein in the pyriproxyfen-induced sterilization of adult female mosquitoes. PLoS Negl. Trop. D. 148, e0008669. doi:10.1371/journal.pntd.0008669
PubMed Abstract | CrossRef Full Text | Google Scholar
Ashok M., Turner C., Wilson T. G. (1998). Insect juvenile hormone resistance gene homology with the bHLH-PAS family of transcriptional regulators. Proc. Natl. Acad. Sci. U. S. A. 95, 2761–2766. doi:10.1073/pnas.95.6.2761
PubMed Abstract | CrossRef Full Text | Google Scholar
Belles X., Santos C. G. (2014). The MEKRE93 (Methoprene tolerant-Krüppel homolog 1-E93) pathway in the regulation of insect metamorphosis, and the homology of the pupal stage. Insect biochem. Mol. Biol. 52, 60–68. doi:10.1016/j.ibmb.2014.06.009
PubMed Abstract | CrossRef Full Text | Google Scholar
Biondi A., Guedes R. N. C., Wan F. H., Desneux N. (2017). Ecology, Worldwide spread, and management of the invasive south American tomato pinworm, Tuta absoluta: past, Present, and Future. Annu. Rev. Entomol. 63, 239–258. doi:10.1146/annurev-ento-031616-034933
PubMed Abstract | CrossRef Full Text | Google Scholar
Chang M. M., Wang Y. H., Yang Q. T., Wang X. L., Wang M., Raikhel A. S., et al. (2021). Regulation of antimicrobial peptides by juvenile hormone and its receptor, methoprene-tolerant, in the mosquito Aedes aegypti. Insect biochem. Mol. Biol. 128, 103509. doi:10.1016/j.ibmb.2020.103509
PubMed Abstract | CrossRef Full Text | Google Scholar
Cheng W. N., Li X. J., Zhao J. J., Zhu S. K. (2020). Cloning and characterization of methoprene-tolerant (Met) and krüppel homolog 1 (Kr-h1) genes in the wheat blossom midge, Sitodiplosis mosellana. Insect Sci. 27, 292–303. doi:10.1111/1744-7917.12638
PubMed Abstract | CrossRef Full Text | Google Scholar
Cherif A., Harbaoui K., Zappalà L., Grissa L. K. (2017). Efficacy of mass trapping and insecticides to control Tuta absoluta in Tunisia. J. Plant Dise. Prot. 125, 51–61. doi:10.1007/s41348-017-0140-6
CrossRef Full Text | Google Scholar
Daimon T., Uchibori M., Nakao H., Sezutsu H., Shinoda T. (2015). Knockout silkworms reveal a dispensable role for juvenile hormones in holometabolous life cycle. Proc. Natl. Acad. Sci. U. S. A. 112, E4226–E4235. doi:10.1073/pnas.1506645112
PubMed Abstract | CrossRef Full Text | Google Scholar
Gijbels M., Lenaerts C., Vanden B. J., Marchal E. (2019). Juvenile hormone receptor met is essential for ovarian maturation in the desert locust, Schistocerca gregaria. Sci. Rep. 9, 10797. doi:10.1038/s41598-019-47253-x
PubMed Abstract | CrossRef Full Text | Google Scholar
Guedes R. N. C., Roditakis E., Campos M. R., Haddi K., Bielza P., Siqueira H. A., et al. (2019). Insecticide resistance in the tomato pinworm Tuta absoluta: patterns, spread, mechanisms, management and outlook. J. Pest Sci. 92, 1329–1342. doi:10.1007/s10340-019-01086-9
CrossRef Full Text | Google Scholar
Guo W., Wu Z. X., Song J. S., Jiang F., Wang Z. M., Deng S., et al. (2014). Juvenile hormone-receptor complex acts on Mcm4 and Mcm7 to promote polyploidy and vitellogenesis in the migratory locust. PLoS Genet. 10, e1004702. doi:10.1371/journal.pgen.1004702
PubMed Abstract | CrossRef Full Text | Google Scholar
Han H., Feng Z. Y., Han S. P., Chen J., Wang D., He Y. Z. (2022). Molecular identification and functional characterization of methoprene-tolerant (Met) and krüppel-homolog 1 (Kr-h1) in Harmonia axyridis (Coleoptera: coccinellidae). J. Econ. Entomol. 115, 334–343. doi:10.1093/jee/toab252
PubMed Abstract | CrossRef Full Text | Google Scholar
Holtof M., Lommel V. J., Gijbels M., Dekempeneer E., Nicolai B., Broeck V. J., et al. (2021). Crucial role of juvenile hormone receptor components methoprene-tolerant and taiman in sexual maturation of adult male desert locusts. Biomolecules 11, 244. doi:10.3390/biom11020244
PubMed Abstract | CrossRef Full Text | Google Scholar
Hu K., Tian P., Yang L., Qiu L., He H., Ding W., et al. (2019). Knockdown of methoprene-tolerant arrests ovarian development in the Sogatella furcifera (Hemiptera: Delphacidae). J. Insect Sci. 19, 5. doi:10.1093/jisesa/iez113
PubMed Abstract | CrossRef Full Text | Google Scholar
Huang J. H., Lozano J., Belles X. (2013). Broad-complex functions in postembryonic development of the cockroach Blattella germanica shed new light on the evolution of insect metamorphosis. Biochi. Biophys. Acta 1830, 2178–2187. doi:10.1016/j.bbagen.2012.09.025
PubMed Abstract | CrossRef Full Text | Google Scholar
Julide B., Jade A., Reza A., Levineb J. D., Riddiford L. M. (2013). Regulation of onset of female mating and sex pheromone production by juvenile hormone in Drosophila melanogaster. Proc. Natl. Acad. Sci. U. S. A. 110, 18321–18326. doi:10.1073/pnas.1318119110
PubMed Abstract | CrossRef Full Text | Google Scholar
Kayukawa T., Minakuchi C., Namiki T., Togawa T., Yoshiyama M., Kamimura M., et al. (2012). Transcriptional regulation of juvenile hormone-mediated induction of Krüppel homolog 1, a repressor of insect metamorphosis. Proc. Natl. Acad. Sci. U. S. A. 109, 11729–11734. doi:10.1073/pnas.1204951109
PubMed Abstract | CrossRef Full Text | Google Scholar
Kayukawa T., Shinoda T. (2015). Functional characterization of two paralogous JH receptors, methoprene-tolerant 1 and 2, in the silkworm, Bombyx mori (Lepidoptera: bombycidae). Appl. Entomol. Zool. 50, 383–391. doi:10.1007/s13355-015-0345-8
CrossRef Full Text | Google Scholar
Leyria J., Orchard I., Lange A. B. (2022). Impact of JH signaling on reproductive physiology of the classical insect model, Rhodnius prolixus. Int. J. Mol. Sci. 23, 13832. doi:10.3390/ijms232213832
PubMed Abstract | CrossRef Full Text | Google Scholar
Li M., Mead E. A., Zhu J. S. (2011). Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc. Natl. Acad. Sci. U. S. A. 108, 638–643. doi:10.1073/pnas.1013914108
PubMed Abstract | CrossRef Full Text | Google Scholar
Li Y. X., Wang D., Zhao W. L., Zhang J. Y., Kang X. L., Li Y. L., et al. (2021). Juvenile hormone induces methoprene-tolerant 1 phosphorylation to increase interaction with taiman in Helicoverpa armigera. Insect biochem. Mol. Biol. 130, 103519. doi:10.1016/j.ibmb.2021.103519
PubMed Abstract | CrossRef Full Text | Google Scholar
Liu H., Li H. M., Yue Y., Song Z. H., Wang J. J., Dou W. (2017). The alternative splicing of BdTai and its involvement in the development of Bactrocera dorsalis (Hendel). J. Insect Physiol. 101, 132–141. doi:10.1016/j.jinsphys.2017.07.012
PubMed Abstract | CrossRef Full Text | Google Scholar
Liu S. N., Li K., Gao Y., Liu X., Chen W. T., Ge W., et al. (2018). Antagonistic actions of juvenile hormone and 20-hydroxyecdysone within the ring gland determine developmental transitions in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 115, 139–144. doi:10.1073/pnas.1716897115
PubMed Abstract | CrossRef Full Text | Google Scholar
Liu W., Guo S., Sun D., Zhu L., Zhu F., Lei C. L., et al. (2019). Molecular characterization and juvenile hormone-regulated transcription of the vitellogenin receptor in the cabbage beetle Colaphellus bowringi. Comp. Biochem. Phys. A 229, 69–75. doi:10.1016/j.cbpa.2018.12.004
PubMed Abstract | CrossRef Full Text | Google Scholar
Liu Z. Q., Nanda S., Yang C. X., Chen S. M., Guo M. J., Khan M. M., et al. (2022). RNAi suppression of the nuclear receptor FTZ-F1 impaired ecdysis, pupation, and reproduction in the 28-spotted potato ladybeetle, Henosepilachna vigintioctopunctata. Pestic. Biochem. Physiol. 182, 105029. doi:10.1016/j.pestbp.2021.105029
PubMed Abstract | CrossRef Full Text | Google Scholar
Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408. doi:10.1006/meth.2001.1262
PubMed Abstract | CrossRef Full Text | Google Scholar
Lozano J., Belles X. (2014). Role of Methoprene-tolerant (Met) in adult morphogenesis and in adult ecdysis of Blattella germanica. PLoS One 9, e103614. doi:10.1371/journal.pone.0103614
PubMed Abstract | CrossRef Full Text | Google Scholar
Luo W., Liu S., Zhang W. Q., Yang L., Huang J. H., Zhou S. T., et al. (2021). Juvenile hormone signaling promotes ovulation and maintains egg shape by inducing expression of extracellular matrix genes. Proc. Natl. Acad. Sci. U. S. A. 118, e2104461118. doi:10.1073/pnas.2104461118
PubMed Abstract | CrossRef Full Text | Google Scholar
Ma L., Zhang W. N., Liu C., Chen L., Xu Y., Xiao H. J., et al. (2018). Methoprene-tolerant (Met) is indispensable for larval metamorphosis and female reproduction in the cotton bollworm Helicoverpa armigera. Front. Physiol. 9, 1601. doi:10.3389/fphys.2018.01601
PubMed Abstract | CrossRef Full Text | Google Scholar
Ma L. X., He R. T., Yan S. Y., Yang W. J. (2022). RNAi suppression of hormone receptor HR3 blocks larval molting and metamorphosis in the cigarette beetle, Lasioderma serricorne. Agriculture 12, 1257. doi:10.3390/agriculture12081257
CrossRef Full Text | Google Scholar
Marchal E., Hult E. F., Huang J., Pang Z., Stay B., Tobe S. S. (2014). Methoprene-tolerant (Met) knockdown in the adult female cockroach, Diploptera punctata completely inhibits ovarian development. PLoS One 9, e106737. doi:10.1371/journal.pone.0106737
PubMed Abstract | CrossRef Full Text | Google Scholar
Meng Q. W., Xu Q. Y., Deng P., Fu K. Y., Guo W. C., Li G. Q. (2018). Involvement of methoprene-tolerant (Met) in the determination of the final body size in Leptinotarsa decemlineata (Say) larvae. Insect biochem. Mol. Biol. 97, 1–9. doi:10.1016/j.ibmb.2018.04.003
PubMed Abstract | CrossRef Full Text | Google Scholar
Minakuchi C., Namiki T., Shinoda T. (2009). Krüppel homolog 1, an early juvenile hormone-response gene downstream of methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle Tribolium castaneum. Dev. Biol. 325, 341–350. doi:10.1016/j.ydbio.2008.10.016
留言 (0)