Globally, lung cancer holds the highest mortality rate among all cancers, with smoking identified as the leading risk factor, followed by air quality concerns (1, 2). Recent data reveal a stabilization and gradual decline in the number of male patients, while the number of female patients has increased significantly. Many of these women are non-smokers, which is largely associated with EGFR mutations (3). Lung cancer is categorized into small cell lung cancer and non-small cell lung cancer (NSCLC), with NSCLC comprising 85% of lung cancer cases. The predominant types of NSCLC are squamous cell carcinoma, large cell carcinoma, and adenocarcinoma. For early-stage NSCLC, the primary treatment method is surgical resection, with about 30% considered resectable (4). Traditional chemotherapy is often the first line of treatment for advanced NSCLC, but its efficacy is limited (5), and it is only suitable for patients without genetic mutations (6). The 5-year overall survival rate is 2 to 13% for NSCLC recurrence and 4% for advanced NSCLC patients (6–8). Among clinical checkpoint inhibitors, anti-programmed death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors are the most effective, as they inhibit downstream pathways and restore T cell antitumor functions (9). Numerous studies indicate that combination therapy can enhance immunotherapy effects by exposing tumor antigens and increasing tumor mutational burden, and preoperative use of immune checkpoint inhibitors (ICIs) can improve survival rates (10). The highest mutation rate in lung adenocarcinoma involves EGFR, reaching up to 20%, with an incidence rate of NSCLC in Asians between 40-60%, making EGFR a critical factor for NSCLC. Resistance is inevitable following the use of TKIs, necessitating combination therapy (11). Current research shows that chemotherapy can induce PD-L1 expression, creating a synergistic effect leading to better survival outcomes (12). Nivolumab combined with platinum-based doublet chemotherapy has received FDA approval for preoperative treatment in resectable NSCLC patients, and atezolizumab is approved for postoperative treatment in patients with PD-L1 > 1%, due to its significant effects and improved clinical outcomes (13–15). Toripalimab, China’s first domestically produced PD-1 antibody (9), is used in melanoma, nasopharyngeal carcinoma, and urothelial carcinoma, with relatively fewer studies in NSCLC. In the treatment of unresectable or metastatic melanoma, Toripalimab was first approved and can provide long-term survival benefits. Additionally, it is approved as a first-line treatment for unresectable locally advanced or recurrent/metastatic esophageal squamous cell carcinoma, ALK non-squamous NSCLC, and metastatic nasopharyngeal carcinoma (12). Therefore, adding PD-1 drugs as a first-line treatment could represent a new breakthrough for most late-stage cancer patients. This meta-analysis focuses on evaluating the effectiveness and safety of Toripalimab in NSCLC patients.
2 MethodsThis study is registered in the International Prospective Register of Systematic Reviews database (PROSPERO: CRD42024519806).
2.1 Search strategyWe searched for articles published up to March 2024 in PubMed, Embase, Cochrane, and Web of Science databases related to the use of Toripalimab in non-small cell lung cancer (NSCLC) patients. General search terms included “non-small cell lung cancer” and “Toripalimab.” The detailed search strategies are provided in Supplementary Tables S1-S4.
2.2 Selection criteriaWe included single-arm studies or randomized controlled trials (RCTs) with at least 10 participants. Patients included must be diagnosed with non-small cell lung cancer (NSCLC), primarily consisting of subtypes such as pulmonary adenocarcinoma, squamous cell carcinoma, adenosquamous carcinoma, large cell carcinoma, and sarcomatoid carcinoma, among others. Additionally, patients must receive treatment with Toripalimab either as monotherapy or in combination with standard chemotherapy agents such as pemetrexed, carboplatin, cisplatin, and paclitaxel. The primary endpoints were objective response rate (ORR), progression-free survival (PFS), overall survival (OS), and adverse events (AE). We have excluded literature and cases involving small cell lung cancer or tumors that have metastasized to the lungs from other parts of the body. In terms of interventions, we have also excluded studies where Toripalimab is used in combination with other immunotherapies or targeted drugs. Additionally, We excluded case reports, reviews, conference proceedings, animal studies, meta-analyses, and abstracts. Studies with fewer than 10 participants and those without available full text and data were also excluded. After excluding these articles and removing duplicates, we conducted a meta-analysis.
2.3 Data extractionTwo researchers independently performed data extraction. In cases of discrepancies or differing opinions, a third researcher participated in the discussion and decision-making. Extracted data included the author’s name, publication year, basic patient characteristics (gender, average age), tumor staging, ECOG performance status, PD-1 expression levels, intervention measures, and outcome indicators.
2.4 Quality assessmentFor non-randomized single-arm studies, we used the Methodological Index for Non-Randomized Studies (MINORS), applying the first eight items of this scale, each scored from 0 to 2, for a total maximum score of 16 (16), as shown in Supplementary Table S5. For RCTs, we used Version 2 of the Cochrane tool for assessing risk of bias in randomized trials (RoB2) to evaluate the risk of bias, detailed in Supplementary Figure S1. The included studies were categorized as having high, moderate, or low risk of bias. Additionally, we define I²<25% as low heterogeneity, 25-75% as moderate heterogeneity, and >75% as high heterogeneity (17).
2.5 Statistical analysisTwo researchers independently conducted data extraction and analysis, with any disagreements resolved through discussion with a third researcher. For single-arm studies, effect size (ES) and 95% confidence intervals were used to determine the objective response rate (ORR) and adverse events (AE), while median progression-free survival (PFS) was represented by median PFS. For RCT studies, overall survival (OS) was assessed using hazard ratios (HR) and 95% confidence intervals. Heterogeneity among studies was evaluated using Cochran’s Q test and the I² statistic, with a P-value of less than 0.05 considered statistically significant, indicating substantial differences. Due to variability in participant populations across studies, heterogeneity was expected. Consequently, a random-effects model was employed for the meta-analysis when I² exceeded 50%; otherwise, a fixed-effects model was used. Sensitivity analysis was performed for analyses with significant heterogeneity. Egger’s test was used for linear regression testing, and funnel plots were employed to visually assess publication bias. Analyses were conducted using Stata 17.0 (Stata Corporation, College Station, TX, USA) and R version 3.6.0.
3 Results3.1 Literature searchInitially, we retrieved a total of 743 articles from these databases. We excluded 129 duplicates. After reviewing titles and abstracts, 597 articles were further excluded, including reviews, conference proceedings, case reports, and animal studies. Of the remaining 17 studies, 9 were excluded due to incomplete data. Finally, 8 studies were deemed eligible for inclusion in this meta-analysis. The process of retrieval and screening is illustrated in Figure 1.
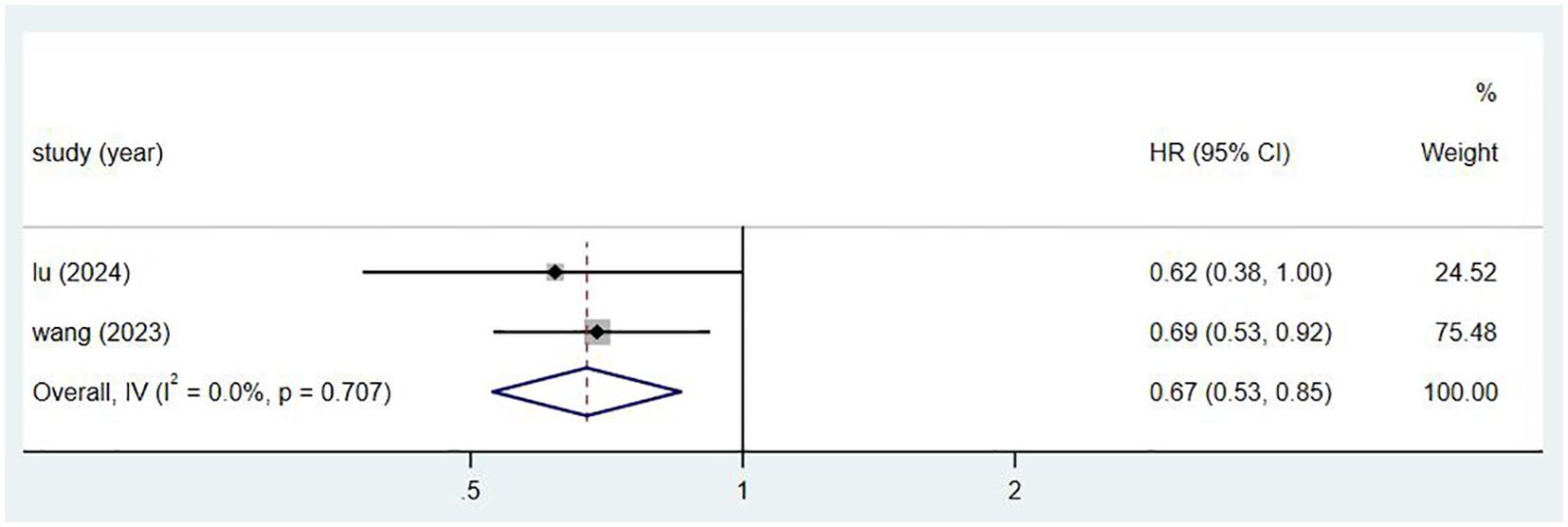
Figure 1. Research search process and results chart.
3.2 Study characteristicsAmong the 8 included studies, 2 were randomized controlled trials (RCTs) with complete data (8, 18). Both the control and experimental groups in these studies included chemotherapy, using pemetrexed plus platinum for adenocarcinoma and paclitaxel plus platinum for squamous cell carcinoma. The remaining 6 studies were single-arm studies, each with more than 10 participants (19–24). It is hereby noted that the chemotherapy drugs used in all studies may not be identical; however, all of these drugs are standard chemotherapy agents for the treatment of lung cancer. Age, gender, tumor staging, ECOG performance status, PD-1 expression, intervention measures, and outcome measures were recorded in Table 1. The study by Wang (21) discussed the efficacy of Toripalimab produced by different processes, which divided the study into two groups. However, due to the small number of subjects included in this study, we combined the two groups to process the data. Since there are only two RCT studies, we extracted part of the data and treated it as single-arm study data. This approach enhances the persuasiveness of the data in this paper.
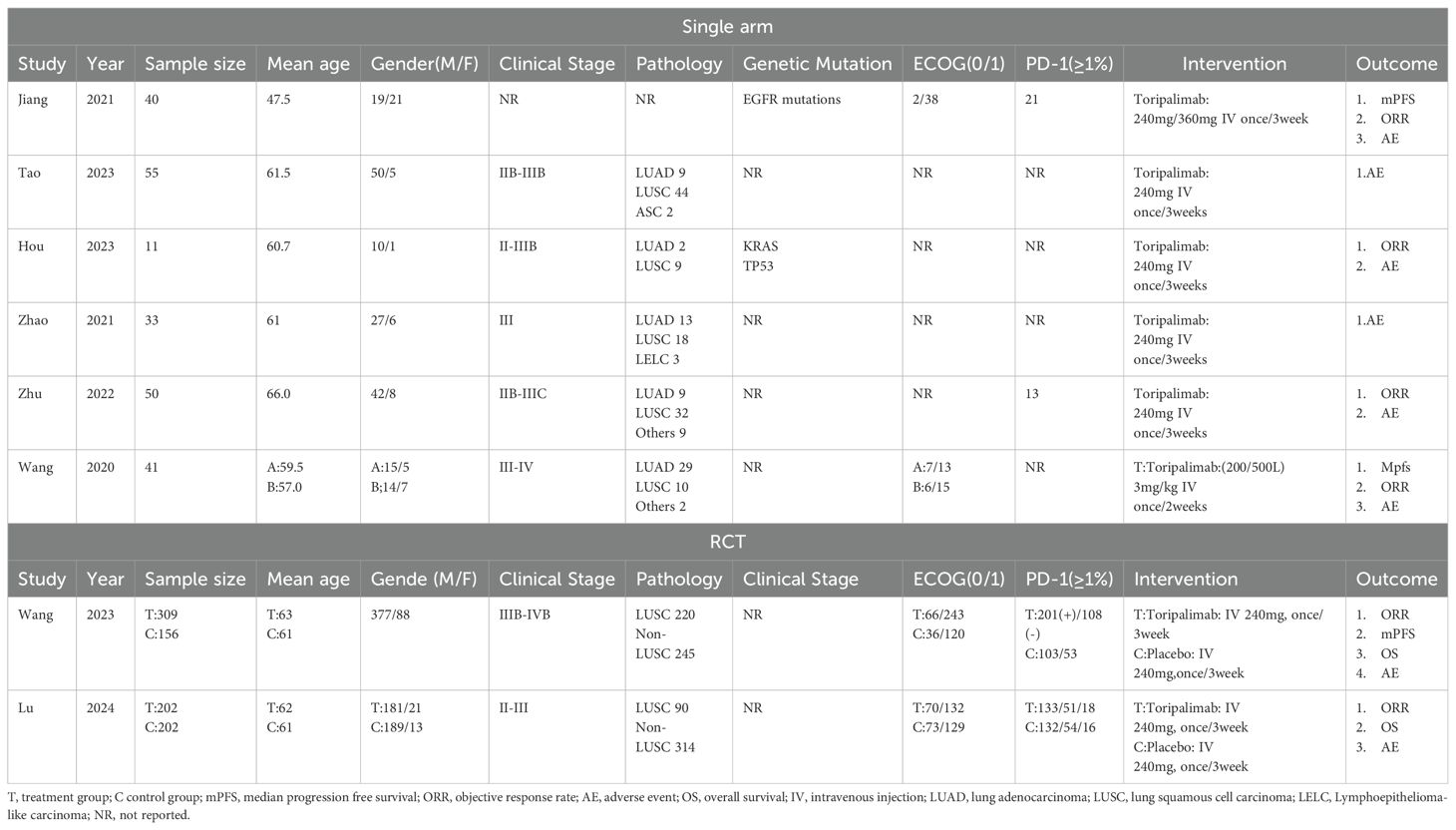
Table 1. Research abstracts included in the meta-analysis.
3.3 Study data for the single-arm studies3.3.1 ORRObjective response rate (ORR) refers to the proportion of tumor patients who achieve complete response (CR) or partial response (PR) after treatment, primarily assessed by imaging to evaluate changes in tumor size. Pathological complete response (pCR) refers to the absence of residual viable cancer cells in the resected surgical specimen after neoadjuvant therapy, as determined by pathological examination (25). Of the 8 studies, 6 reported the Objective Response Rate (ORR) (8, 18, 19, 21, 23, 24). These six studies included a total of 639 patients, with an I² value of 91% and P<0.01, indicating significant heterogeneity. Therefore, a random-effects model was applied, as shown in Figure 2. The figure demonstrates that the ORR for NSCLC patients treated with Toripalimab in combination with chemotherapy was [ES=0.58, CI(0.6-0.81), P=0.00]. A funnel plot, shown in Supplementary Figure S2, visually suggests the presence of publication bias (results of Egger and Begg tests). A sensitivity analysis conducted in Supplementary Figure S3 indicated that removing the study by Wang (21) significantly decreased the heterogeneity (I²=57%, P<0.01), suggesting that this study could be a major source of the observed heterogeneity. We believe that the main cause of heterogeneity is the low ORR in this study, coupled with the small sample size. However, this result is also within our expectations.
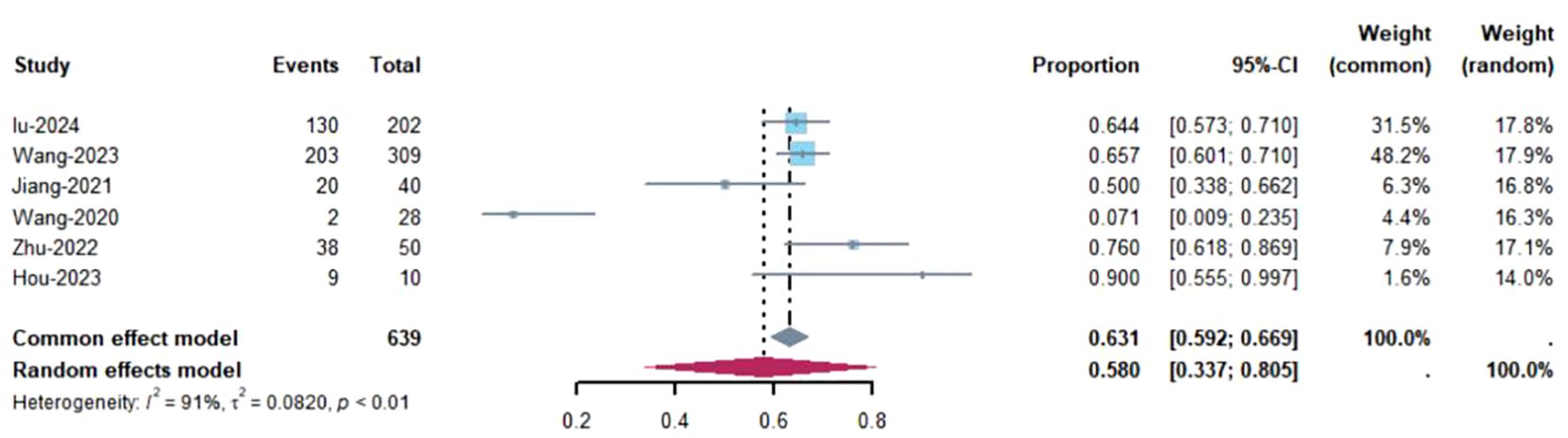
Figure 2. Estimated proportion of ORR in Toripalimab-treated patients for single-arm studies.
3.3.2 PFSThree studies reported the median Progression-Free Survival (mPFS) (18, 21, 23), involving a total of 546 patients, with an I² value of 96.3% and P<0.00. Figure 3 shows that the mPFS for NSCLC patients treated with Toripalimab in combination with chemotherapy was [median PFS = 5.49 months, 95% CI (2.88, 10.49); P=0.00]. A sensitivity analysis, shown in Supplementary Figure S4, proved to be relatively stable. The possibility of publication bias appears to be low, as indicated by the Egger test (0.469) and Begg test (0.469).
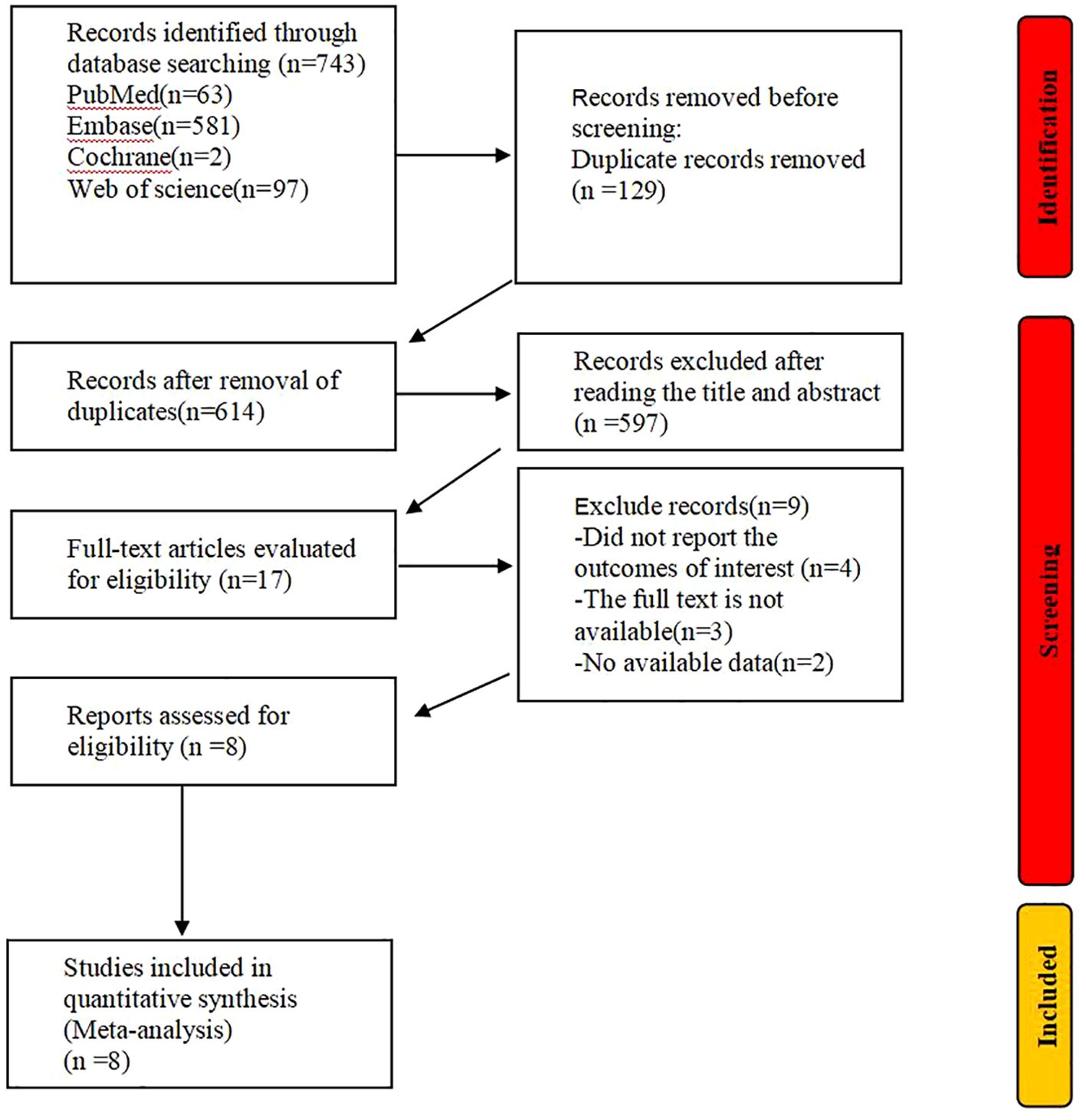
Figure 3. Estimated proportion of mPFS in Toripalimab-treated patients for single-arm studies.
3.3.3 Meta-analysis for adverse eventsIn this study, in addition to efficacy, we also investigated the safety by examining the types of adverse events or the occurrence of more severe conditions. Below are the results of the adverse events. The 8 studies reported adverse event (AE) information (8, 18–24), involving a total of 740 patients. The main adverse events included anemia, neutropenia, leukopenia, thrombocytopenia, nausea, rash, thyroid dysfunction, ALT increase, and AST increase. The effect sizes (ES) for any grade of AEs were: anemia=64%, neutropenia=50%, leukopenia=45%, thrombocytopenia=30%, nausea=27%, rash=19%, thyroid dysfunction=20%, hemoptysis=10%, ALT increase=31%, and AST increase=31%. For severe adverse events (Grade ≥3), the ES were: anemia=11%, neutropenia=28%, leukopenia=19%, thrombocytopenia=7%, decreased appetite=1%, pneumonia=4%, and rash=1%. These data are visually presented in Table 2.
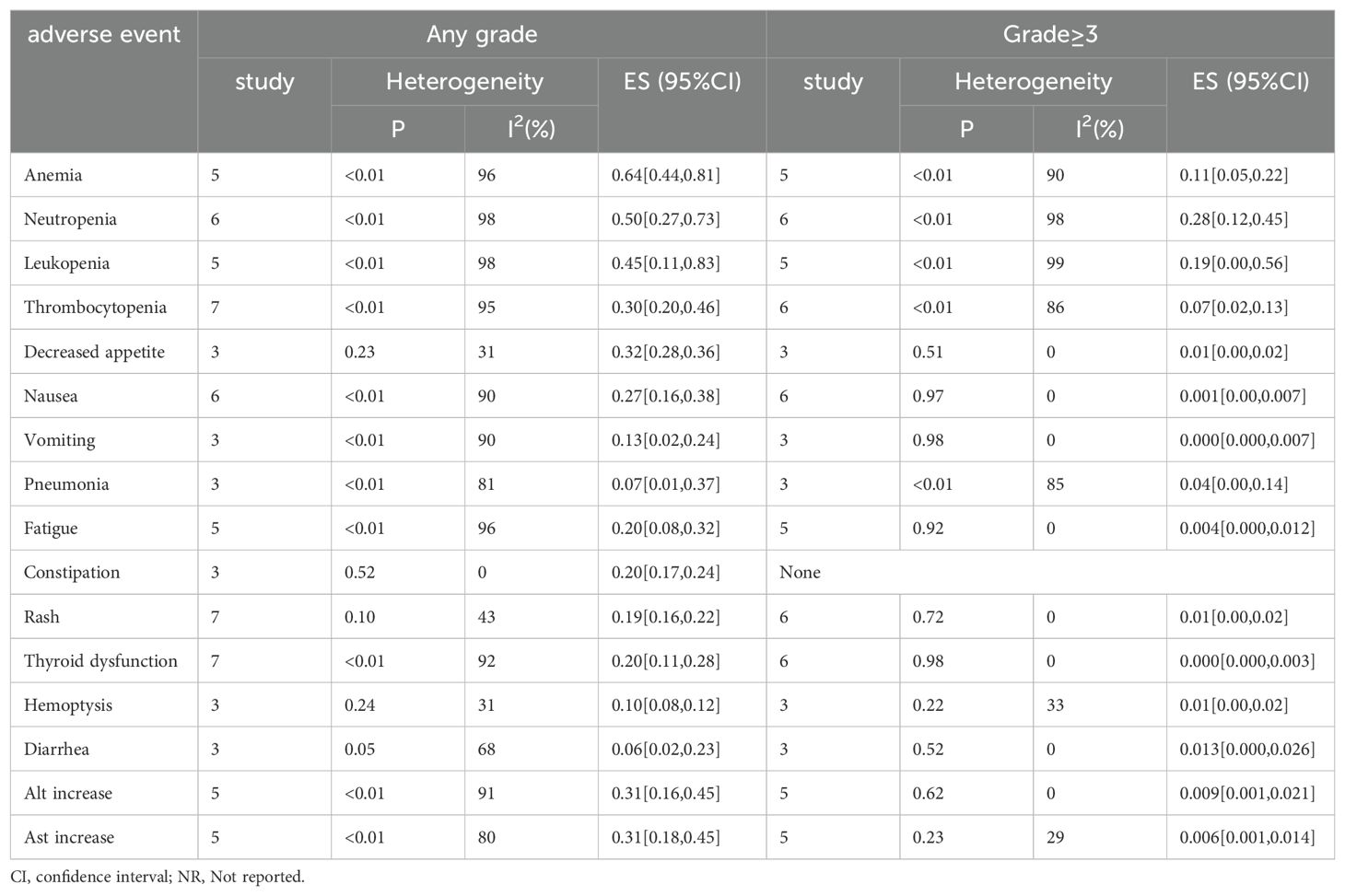
Table 2. Adverse events of toliparibumab for NSCLC therapy.
3.4 Meta-analysis for RCT3.4.1 OSBoth randomized controlled trials (RCTs) reported on overall survival (OS) (8, 18), involving a total of 869 patients. Figure 4 shows that the effect size (hazard ratio, HR) for OS is 0.69, with a confidence interval (CI) of (0.53, 0.85).
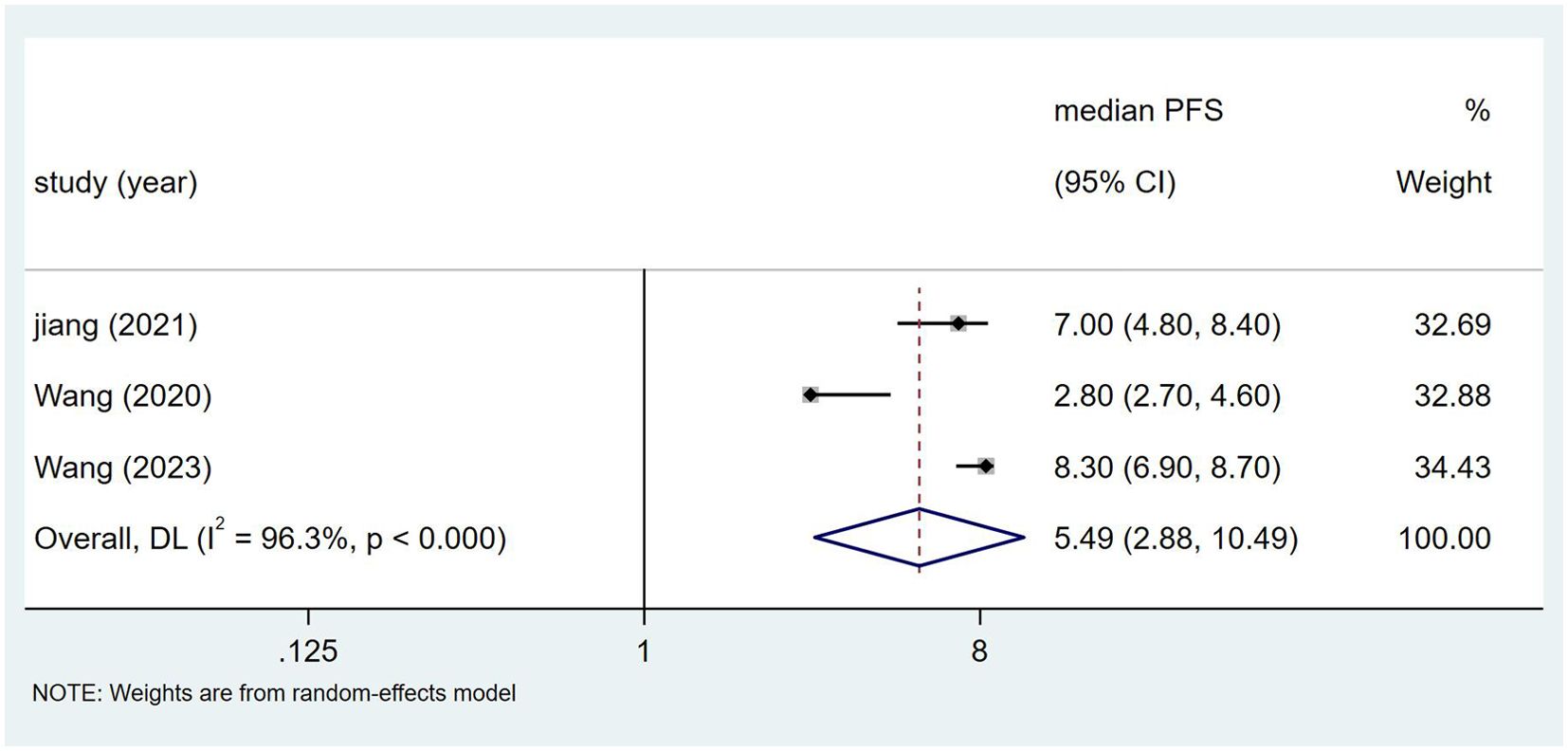
Figure 4. Estimated proportion of OS in Toripalimab-treated patients for RCT studies.
4 DiscussionAccording to our investigation, this is the inaugural study on Toripalimab in the context of non-small cell lung cancer (NSCLC), with the latest literature emerging in 2024 by Lu et al. (8). The novelty of this research lies in examining domestically developed immunotherapy agents for NSCLC, demonstrating their efficacy while revealing that the adverse effects of this medication remain within an acceptable limit. In first-line treatment for NSCLC lacking driver gene mutations, conventional therapies primarily include chemotherapy. Nevertheless, some research indicates that the anti-tumor immune response triggered by chemotherapy following tumor cell death could augment the effectiveness of tumor immunotherapy (26). The FDA has granted approval for Toripalimab to treat advanced nasopharyngeal carcinoma. According to Luo’s meta-analysis (27), the median progression-free survival (PFS) for patients treated with Toripalimab alongside gemcitabine and cisplatin is 10.8 months, with a duration of response (DOR) averaging 9.9 months and an overall response rate (ORR) of 88.1%. In contrast, patients with non-squamous NSCLC undergoing treatment with alternative immunotherapy drugs combined with chemotherapy achieved a median PFS of only 8.5 months and a median overall survival (OS) of 17.2 months (26, 28, 29). This underscores the significant promise of this drug or treatment regimen in oncological therapy, suggesting that combining chemotherapy with immunotherapy may yield improved patient outcomes.
For single-arm studies, the ORR was reported as [ES=0.58, CI (0.34, 0.81)], while the mPFS was [ES=5.49, CI (2.9, 10.5)]. The evaluation of randomized controlled trials (RCTs) indicated an OS of [HR=0.67, 95% CI (0.53, 0.85)]. When compared to standard chemotherapy (30), the combination of Toripalimab and chemotherapy showed greater survival advantages. It is important to acknowledge the effectiveness of Toripalimab, but we should treat this research data with caution given the limited number of studies, most of which are single-arm trials. Regarding the ORR, our findings suggest that the variability is largely due to Wang’s study (21), potentially linked to its smaller sample size. In two RCTs, Wang’s research indicated a median PFS of 8.3 months, which is significantly higher than the 5.6 months survival rate observed in the placebo cohort. Furthermore, although Lu’s study did not specify mPFS, it noted that 48.5% of patients receiving Toripalimab exhibited a major pathological response following surgical resection, highlighting Toripalimab’s promising efficacy in treating non-small cell lung cancer. In China, Toripalimab is the first approved PD-1 inhibitor, primarily utilized for nasopharyngeal carcinoma, melanoma, and urothelial carcinoma (9). It is sanctioned as a first-line treatment for adults with metastatic or recurrent locally advanced nasopharyngeal carcinoma, administered in combination with cisplatin and gemcitabine chemotherapy (31). In the study NCT03513666 (23), forty patients were treated with Toripalimab alongside either carboplatin or pemetrexed. The treatment regimen, given every three weeks, resulted in a median OS of 23.5 months, a median PFS of 7.0 months, and an ORR of 50%, substantially enhancing patient survival.
Previous research has highlighted the pathological response as a critical endpoint, which can be categorized into major pathological response (MPR) and complete pathological response (pCR) (29). MPR has been identified as a significant predictor of long-term overall survival (OS) in patients with NSCLC, functioning as a predictive marker. The application of neoadjuvant immunotherapy is on the rise, with both MPR and pCR gaining importance in randomized controlled trial (RCT) studies (29). Neoadjuvant therapies, including immunotherapy or chemotherapy, have demonstrated safety and efficacy for stage I-III NSCLC, particularly in the context of combination neoadjuvant chemo-immunotherapy (32). In the majority of NSCLC cases that proceeded to surgical resection following neoadjuvant chemotherapy, the pathological response acted as an endpoint for treatment, effectively managing tumor micro-metastases and enhancing mediastinal lymph node clearance rates (33). According to Zhao’s study (20), 50% of patients achieved pCR, while two-thirds (20 out of 30) attained MPR. Although the sample size is not sufficiently large to validate Toripalimab as a primary or secondary therapy, it lays the groundwork for future neoadjuvant strategies. The recent research by Lu et al. (8) is particularly noteworthy, encompassing 501 patients, with 404 receiving Toripalimab treatment. Participants underwent three cycles preoperatively and one cycle postoperatively, facilitating an extended treatment duration. The hazard ratio (HR) was recorded at 0.66. In comparison to the placebo group (MPR=0.01), the treatment group yielded a value of 0.24, reflecting an intergroup difference of 23.7% [95% CI, 17.6%-29.8%], P <.001. The article also indicated that the pathological response might serve as a potential surrogate marker for event-free survival. Nonetheless, in our country, there is a tendency toward non-invasive treatments, making its application in preoperative neoadjuvant therapy promising, while post-chemotherapy biopsies continue to present challenges. Chemotherapy enhances immunotherapy by inducing tumor cell apoptosis and releasing antigens that stimulate the immune response. Common side effects associated with standard chemotherapy include nausea, vomiting, hair loss, and suppression of bone marrow function. The risk of experiencing these adverse effects tends to rise, particularly when multiple chemotherapy drugs are used concurrently, resulting in an increased number of complications (34). In contrast, the rate of adverse reactions linked to immunotherapy is notably lower than that associated with chemotherapy, with the majority being gastrointestinal, dermatological, and systemic issues such as fatigue and fever. These reactions can be effectively managed with symptomatic treatments (35). Additionally, chemotherapy enhances the infiltration of cytotoxic T cells into the tumor microenvironment and stimulates inflammatory signaling, which supports the recruitment and activation of immune cells (24). It also reduces the cell burden that immune cells must handle and lowers the levels of immunosuppressive substances produced by tumor cells. Moreover, since immunotherapy alone is often insufficient for tumor eradication, its combination with chemotherapy becomes essential (36). Adverse events related to Toripalimab mainly involve anemia, neutropenia, leukopenia, and reduced appetite, with grade 3 neutropenia occurring in 28% of cases, while other events are less frequent. Overall, the incidence of adverse effects is considered acceptable, and pre-medication can mitigate or eliminate these reactions. Research indicates that the rates of treatment-related adverse events (TRAE) vary from 54% to 76%, with fatal adverse events occurring at a rate between 0.36% and 0.63% (9). Adverse events can be classified into five categories, with the most prevalent reactions—such as nausea, vomiting, decreased appetite, hypothyroidism, rash, fever, diarrhea, constipation, peripheral neuropathy, musculoskeletal pain, cough, upper respiratory infections, insomnia, fatigue, dizziness, and discomfort—occurring at rates of 20% or higher, all within acceptable severity limits. Firstly, the existing literature is limited, particularly regarding studies on Toripalimab for non-small cell lung cancer, especially randomized controlled trials (RCTs). Secondly, non-small cell lung cancer encompasses both squamous cell carcinoma and adenocarcinoma, yet the literature fails to clearly differentiate between them, hindering subgroup analysis. Furthermore, the baseline chemotherapy regimens for squamous cell and adenocarcinomas differ in combined chemotherapy, potentially resulting in significant heterogeneity. Some studies also overlook factors such as smoking history, genetic mutations, family medical history, and prior treatment history, further contributing to variability. Lastly, comparing the incidence of adverse events between immunotherapy and traditional chemotherapy lacks persuasiveness due to differing baselines and a scarcity of studies.
5 ConclusionIn summary, this meta-analysis compiled the latest study along with previous literature, and we found that Toripalimab is beneficial for patient survival. It provides some support for the research on Toripalimab for non-small cell lung cancer and also offers new insights into treatment plans for advanced patients with PD-L1 > 1%.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributionsYL: Data curation, Formal analysis, Investigation, Software, Writing – original draft, Writing – review & editing, Methodology, Supervision, Validation. LY: Data curation, Formal analyis, Data analysis, Software, Writing – review & editing. ZD: Data curation, Writing – review & editing. QC: Data curation, Writing – review & editing. ML: Data curation, Writing – review & editing. HBZ: Data curation, Writing – review & editing. HLZ: Data curation, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. Support was provided by the Tianjin Municipal Health Commission and the Tianjin Key Medical Discipline Sub-project (TJLCMS2021-06), as well as the Tianjin Municipal Education Commission through the General Project of the Natural Science Foundation (2020KJ162).
AcknowledgmentsWe thank all the staff involved in this research, especially LY, ZD, QC, ML, HBZ, and HLZ for their hard work. All authors meet the criteria for authorship, and everyone contributed to data collection, writing, revisions, and management of the article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1444312/full#supplementary-material
References1. Rodak O. Current landscape of non-small cell lung cancer: epidemiology, histological classification, targeted therapies, and immunotherapy. Cancers (Basel). (2021) 13:4705. doi: 10.3390/cancers13184705
PubMed Abstract | Crossref Full Text | Google Scholar
2. Alberg AJ, Ford JG, Samet JM, et al. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. (2007) 132:29S–55S. doi: 10.1378/chest.07-1347
PubMed Abstract | Crossref Full Text | Google Scholar
3. Dogan S, Shen R, Ang DC, Johnson ML, D'Angelo SP, Paik PK, et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res. (2012) 18:6169–77. doi: 10.1158/1078-0432.CCR-11-3265
PubMed Abstract | Crossref Full Text | Google Scholar
4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
PubMed Abstract | Crossref Full Text | Google Scholar
5. Mok T, Yang JJ, Lam KC. Treating patients with EGFR-sensitizing mutations: first line or second line–is there a difference? J Clin Oncol. (2013) 31:1081–8. doi: 10.1200/JCO.2012.43.0652
PubMed Abstract | Crossref Full Text | Google Scholar
6. Chen W, et al. Network meta-analysis of first-line immune checkpoint inhibitor therapy in advanced non-squamous non-small cell lung cancer patients with PD-L1 expression >/= 50. BMC Cancer. (2023) 23:791. doi: 10.1186/s12885-023-11285-4
PubMed Abstract | Crossref Full Text | Google Scholar
7. Wong ML, McMurry TL, Stukenborg GJ, Francescatti AB, Amato-Martz C, Schumacher JR, et al. Impact of age and comorbidity on treatment of non-small cell lung cancer recurrence following complete resection: A nationally representative cohort study. Lung Cancer. (2016) 102:108–17. doi: 10.1016/j.lungcan.2016.11.002
PubMed Abstract | Crossref Full Text | Google Scholar
8. Lu S, Zhang W, Wu L, Wang W, Zhang P, Fang W, et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the neotorch randomized clinical trial. JAMA. (2024) 331:201–11. doi: 10.1001/jama.2023.24735
PubMed Abstract | Crossref Full Text | Google Scholar
11. Hellmann MD, Nathanson T, Rizvi H, Creelan BC, Sanchez-Vega F, Ahuja A, et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell. (2018) 33:843–852.e4. doi: 10.1016/j.ccell.2018.03.018
PubMed Abstract | Crossref Full Text | Google Scholar
12. Huo G, et al. Toripalimab plus chemotherapy vs. chemotherapy in patients with advanced non-small-cell lung cancer: A cost-effectiveness analysis. Front Pharmacol. (2023) 14:1131219. doi: 10.3389/fphar.2023.1131219
PubMed Abstract | Crossref Full Text | Google Scholar
13. Lazzari C, Spagnolo CC, Ciappina G, Pietro Di M, Squeri A, Passalacqua MI, et al. Immunotherapy in early-stage non-small cell lung cancer (NSCLC): current evidence and perspectives. Curr Oncol. (2023) 30:3684–96. doi: 10.3390/curroncol30040280
PubMed Abstract | Crossref Full Text | Google Scholar
14. Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet. (2021) 398:1344–57. doi: 10.1016/S0140-6736(21)02098-5
Crossref Full Text | Google Scholar
15. Pisters K, et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I-IIIA completely resected non-small-cell lung cancer: ASCO guideline rapid recommendation update. J Clin Oncol. (2022) 40:1127–9. doi: 10.1200/JCO.22.00051
PubMed Abstract | Crossref Full Text | Google Scholar
16. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for nonrandomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
PubMed Abstract | Crossref Full Text | Google Scholar
17. Sterne J, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
PubMed Abstract | Crossref Full Text | Google Scholar
18. Wang Z, Wu L, Li B, Cheng Y, Li X, Wang X, et al. Toripalimab plus chemotherapy for patients with treatment-naive advanced non-small-cell lung cancer: A multicenter randomized phase III trial (CHOICE-01). J Clin Oncol. (2023) 41:651–63. doi: 10.1200/JCO.22.00727
PubMed Abstract | Crossref Full Text | Google Scholar
19. Zhu X, Sun L, Song N, He W, Xie B, Hu J, et al. Safety and effectiveness of neoadjuvant PD-1 inhibitor (toripalimab) plus chemotherapy in stage II-III NSCLC (LungMate 002): an open-label, single-arm, phase 2 trial. BMC Med. (2022) 20:493. doi: 10.1186/s12916-022-02696-4
PubMed Abstract | Crossref Full Text | Google Scholar
20. Zhao ZR, Yang C-P, Chen S, Yu H, Lin Y-B, Lin Y-B, et al. Phase 2 trial of neoadjuvant toripalimab with chemotherapy for resectable stage III non-small-cell lung cancer. Oncoimmunology. (2021) 10:1996000. doi: 10.1080/2162402X.2021.1996000
PubMed Abstract | Crossref Full Text | Google Scholar
21. Wang Z, Ying J, Xu J, Yuan P, Duan J, Bai H, et al. Safety, antitumor activity, and pharmacokinetics of toripalimab, a programmed cell death 1 inhibitor, in patients with advanced non-small cell lung cancer: A phase 1 trial. JAMA Netw Open. (2020) 3:e2013770. doi: 10.1001/jamanetworkopen.2020.13770
PubMed Abstract | Crossref Full Text | Google Scholar
22. Tao Y, Li X, Liu B, Wang J, Lv C, Li S, et al. Association of early immune-related adverse events with treatment efficacy of neoadjuvant Toripalimab in resectable advanced non-small cell lung cancer. Front Oncol. (2023) 13:1135140. doi: 10.3389/fonc.2023.1135140
PubMed Abstract | Crossref Full Text | Google Scholar
23. Jiang T, Wang P, Zhang J, Zhao Y, Zhou J, Fan Y, et al. Toripalimab plus chemotherapy as second-line treatment in previously EGFR-TKI treated patients with EGFR-mutant-advanced NSCLC: a multicenter phase-II trial. Signal Transduct Target Ther. (2021) 6:355. doi: 10.1038/s41392-021-00751-9
PubMed Abstract | Crossref Full Text | Google Scholar
24. Hou H, Wang Y, Sun D, Zhu J, Jiang M, Zhang X, et al. Neoadjuvant toripalimab plus platinum-paclitaxel chemotherapy in stage II-III non-small cell lung cancer: a single-center, single-arm, phase I study in China. Invest New Drugs. (2023) 41:86–92. doi: 10.1007/s10637-022-01324-5
PubMed Abstract | Crossref Full Text | Google Scholar
25. Shohdy KS, Almeldin DS, Fekry MA, Ismail MA, AboElmaaref NA, ElSadany EG, et al. Pathological responses and survival outcomes in patients with locally advanced breast cancer after neoadjuvant chemotherapy: a single-institute experience. J Egypt Natl Canc Inst. (2021) 33:39. doi: 10.1186/s43046-021-00096-y
PubMed Abstract | Crossref Full Text | Google Scholar
26. Saxena P, et al. Immunotherapy alone or in combination with chemotherapy as first-line treatment of non-small cell lung cancer. Curr Treat Options Oncol. (2020) 21:69. doi: 10.1007/s11864-020-00768-2
PubMed Abstract | Crossref Full Text | Google Scholar
27. Luo J, Xiao W, Hua F, Cao Y, Wang D, Wang X, et al. Efficacy and safety of PD-1 inhibitors in recurrent or metastatic nasopharyngeal carcinoma patients after failure of platinum-containing regimens: a systematic review and meta-analysis. BMC Cancer. (2023) 23:1172. doi: 10.1186/s12885-023-11318-y
PubMed Abstract | Crossref Full Text | Google Scholar
28. Li R, Liang H, Li J, Shao Z, Yang D, Bao J, et al. Paclitaxel liposome (Lipusu) based chemotherapy combined with immunotherapy for advanced non-small cell lung cancer: a multicenter, retrospective real-world study. BMC Cancer. (2024) 24:107. doi: 10.1186/s12885-024-11860-3
PubMed Abstract | Crossref Full Text | Google Scholar
29. Liu W, et al. A systematic review and meta-analysis of neoadjuvant chemoimmunotherapy in stage III non-small cell lung cancer. BMC Pulmonary Med. (2022) 22:1–9. doi: 10.1186/s12890-022-02292-5
PubMed Abstract | Crossref Full Text | Google Scholar
30. Ma HC, Liu Y-H, Ding K-L, Liu Y-F, Zhao W-J, Zhu Y-J, et al. Comparative efficacy and safety of first-line treatments for advanced non-small cell lung cancer with ALK-rearranged: a meta-analysis of clinical trials. BMC Cancer. (2021) 21:1–11. doi: 10.1186/s12885-021-08977-0
PubMed Abstract | Crossref Full Text | Google Scholar
32. Jiang J, Wang Y, Gao Y, Sugimura H, Minervini F, Uchino J, et al. Neoadjuvant immunotherapy or chemoimmunotherapy in non-small cell lung cancer: a systematic review and meta-analysis. Transl Lung Cancer Res. (2022) 11:277–94. doi: 10.21037/tlcr-22-75
PubMed Abstract | Crossref Full Text | Google Scholar
33. Wu Y, Verma V, Gay CM, Chen Y, Liang F, Lin Q, et al. Neoadjuvant immunotherapy for advanced, resectable non-small cell lung cancer: A systematic review and meta-analysis. Cancer. (2023) 129:1969–85. doi: 10.1002/cncr.v129.13
留言 (0)