S. oralis, a Gram-positive, nonmotile, alpha-hemolytic bacterium belonging to the Streptococcus mitis group, is a member of the viridans group streptococci (VGS). S. oralis comprises three subspecies including S. oralis subsp. oralis, S. oralis subsp. tigurinus, and S. oralis subsp. dentisani (Jensen et al., 2016). It has been considered a commensal colonizing the oral cavity, oropharyngeal, nasal, gastrointestinal, and genitourinary tracts with relatively low pathogenicity and virulence. Especially in the human oral cavity, S. oralis, an early colonizer of dental plaque, is one of the most abundant commensal microbiota (Li et al., 2004). In addition to humans, it has also been recognized in the commensal flora of higher primates such as great apes (Denapaite et al., 2016). However, recent studies have characterized its potential to instigate severe infections such as infective endocarditis (IE) and meningitis under specific circumstances (Chang et al., 2002). For example, in immunocompromised patients who have undergone oral interventions or with poor oral hygiene, there is a high risk for the organisms to invade sterile body sites and lead to infectious diseases (Cruz Cardoso et al., 2021).
Nonetheless, the poor assignment of this species results in an underestimation of the opportunistic infections caused by S. oralis. The taxonomy within VGS species, especially the differentiation between Streptococcus mitis and S. oralis, has been problematic for decades. Given the fact that S. mitis and S. oralis share highly identical 16S rRNA sequences (over 99%), common clinical diagnosis techniques including matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS), VI-TEK® 2 system, and API® rapid ID 32 Strep system only provide general assignments of limited VGS species but fail to accurately and reliably discriminate S. mitis and S. oralis (Suzuki et al., 2005; Teles et al., 2011; Isaksson et al., 2015). In recent years, the genotypic sequence analysis of specific genes such as the rgg gene (Park et al., 2010), the sodA gene (Teles et al., 2011), and the rnpB gene (Isaksson et al., 2015) has been proposed to discriminate S. mitis and S. oralis. Especially multilocus sequence analysis (MLSA) of seven housekeeping genes (map, pfl, ppaC, pyk, rpoB, sodA, and tuf) has been applied in prior research to provide relatively satisfactory identification within S. mitis and S. oralis (Shelburne et al., 2014; Rasmussen et al., 2016; Imai et al., 2020; Jensen et al., 2021). Nevertheless, this method does not seem feasible for clinical utilization in most laboratories since it is time-consuming (Imai et al., 2020; Menon T, 2023).
With the advancement of species-level assignment, the great variations in the distribution in infectious diseases, clinical features, antimicrobial susceptibility patterns, and prognosis among VGS species have been revealed (Chun et al., 2015; Kim et al., 2018; Chamat-Hedemand et al., 2023b). For example, a predominance of S. oralis has been recognized in several streptococcal infections such as IE and ocular infections. Identification of S. oralis within VGS is of utmost importance for the expedited diagnosis and optimization of antimicrobial therapy. In this review, we sought to summarize the infectious diseases caused by S. oralis to offer important insights into its role as an opportunistic pathogen (Figure 1).
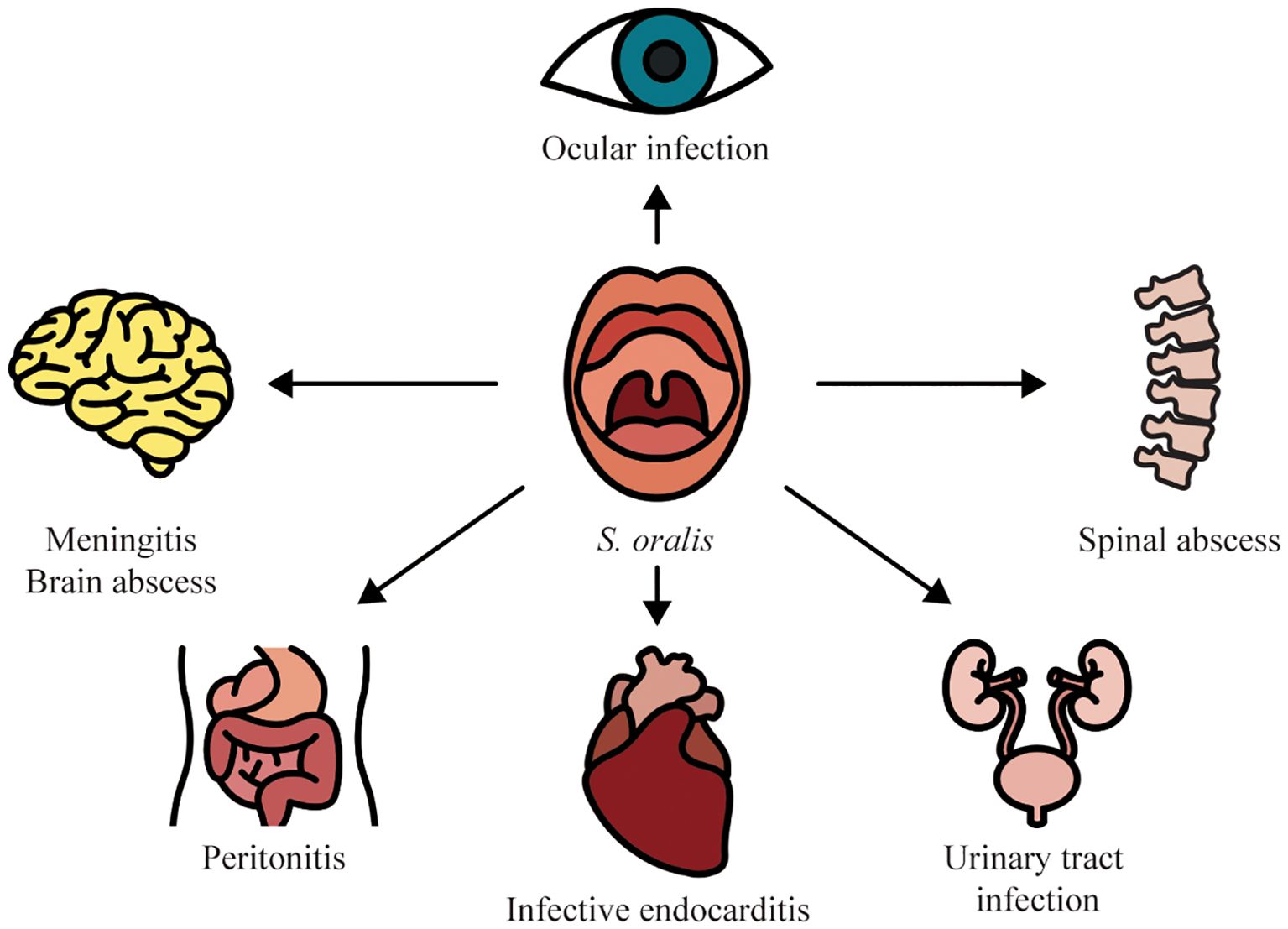
Figure 1. Infectious diseases associated with S. oralis.
2 Opportunistic infections caused by S. oralis2.1 Bloodstream infectionInvasive dental interventions such as tooth extraction and scaling or even daily oral hygiene practices such as tooth brushing and flossing can induce the spread of S. oralis from the oral cavity to the bloodstream leading to transient bacteremia (Yumoto et al., 2019). On the one hand, previous research has demonstrated that S. oralis is one of the most prevalent causes of streptococcal BSIs, particularly in neutropenic patients (Kennedy et al., 2000). A cohort study including 118 consecutive VGS BSI cases during the period from July 1, 2011, to December 1, 2012, found that S. oralis was the second leading cause (22 out of 118) following S. mitis (68 out of 118) (Shelburne et al., 2014). Furthermore, this study observed that approximately 80% of the patients had neutropenia and hematologic malignancies. It suggests that neutropenia and hematologic malignancies are important risk factors in VGS BSIs. Additionally, BSIs caused by S. oralis have also been reported in pediatric patients. A retrospective case-control study found that 53 BSI cases were caused by S. mitis/oralis in pediatric patients from January 2015 to March 2017 (Basaranoglu et al., 2019). In agreement with prior findings in adult cases (average age 50) (Shelburne et al., 2014), 34% of pediatric patients with S. mitis/oralis BSIs also presented febrile neutropenia. Likewise, another retrospective study involving 40 episodes of VGS bacteremia in 38 pediatric patients on chemotherapy for cancer also revealed S. oralis was the third leading causative pathogen accounting for 12.5% of isolates following S. mitis (55%) and Streptococcus sanguinis (25%) (Ahmed et al., 2003). In addition, Kennedy et al. (2003) reported one S. oralis BSI case in a pediatric neutropenic patient presenting severe gingivitis indicating the oral entry of S. oralis into the bloodstream. However, our understanding of why S. oralis BSI is related to neutropenia and hematologic malignancies is notably underdeveloped. Although S. oralis BSIs in both adult and pediatric patients generally present favorable clinical outcomes (Shelburne et al., 2014; Basaranoglu et al., 2019), multidrug resistance in S. oralis has gradually emerged imposing challenges to the therapy for bacteremia in neutropenic patients (Watanabe et al., 2020). Unfortunately, previous work largely failed to differentiate S. mitis and S. oralis (Basaranoglu et al., 2019). The epidemiology of S. oralis needs to be further determined.
Moreover, there is a significant relationship between S. oralis BSIs and IE. It has been established that non-beta-hemolytic streptococcal BSIs are the dominant cause of IE accounting for 13–44% of all cases (Sunnerhagen et al., 2018). In streptococcal BSIs, S. oralis presents a higher IE risk in comparison with other streptococcal species. Chamat-Hedemand et al. (2020) evaluated the risk of IE in 6,506 streptococcal BSI cases in the capital region of Denmark from 2008 to 2017. The author observed the highest IE prevalence in S. mitis/oralis BSIs (19.4%) compared with a variety of streptococcal species including Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus anginosus, and Streptococcus salivariu. Moreover, the same author revealed that one-year mortality was higher in the patients with S. mitis/oralis BSIs (Chamat-Hedemand et al., 2023b). It highlights the importance and necessity of species-level identification within streptococcal species for the prediction of prognosis in patients with streptococcal BSIs. Consistently, a retrospective cohort study in South Korea, involving 2,737 patients with streptococcal BSIs from January 2010 to June 2020, analyzed the prevalence of IE in BSIs caused by different streptococcal species (Seo et al., 2023). The results showed that 12% of BSI cases caused by S. oralis (14/115) developed IE further confirming the significant association between IE and S. oralis BSIs (Seo et al., 2023). Taken together, these findings address the different distribution of streptococcal species in the development of IE and indicate that S. oralis is one of the high-risk species in streptococcal BSIs to cause IE. In this context, routine echocardiography has been recommended in patients with S. oralis BSIs.
2.2 Ocular infectionInfectious keratitis and endophthalmitis are rare but severe sight-threatening ocular infections (Simunovic et al., 2012; Gunalda et al., 2023). Without prompt and appropriate antimicrobial therapy, these diseases may result in irreversible visual loss (Gunalda et al., 2023). The common risk factors for infectious keratitis and endophthalmitis mainly include ocular trauma, ocular surgery, ocular surface disease, the use of contact lenses, and systemic immunodeficiency (Wong et al., 2012; Gunalda et al., 2023).
Following Staphylococcus spp., Streptococcus spp. is the second prevalent etiological agent in bacterial keratitis (Teweldemedhin et al., 2017). Compared with ocular infections caused by Staphylococcus spp., endophthalmitis and keratitis caused by streptococci usually progress more acutely and aggressively (Kuriyan et al., 2014). The prognoses of ocular infections caused by streptococci are thereby greatly compromised (Teweldemedhin et al., 2017; Santin et al., 2020). A recent study has demonstrated that S. oralis was the most prevalently identified causative agent in streptococcal endophthalmitis and keratitis, while S. mitis was only isolated in a small group of keratitis patients (Santin et al., 2020). This study analyzed the distribution of VGS species in endophthalmitis and keratitis by recovering 62 VGS isolates from patients with endophthalmitis (n=27; 2002-2013) and keratitis (n=35; 2009-2013) in Brazil. The results showed that the most predominately identified species was S. oralis accounting for 32.2% of cases (n=20), while S. mitis was only detected in 8.1% of cases (n=5). Even though early diagnosis and clinical therapy had been performed, the patients showed highly unfavorable visual acuity outcomes. One of the possible explanations is the high propensity of S. oralis to invade the posterior chamber (Santin et al., 2020).
Moreover, streptococci are also one of the leading causes of endophthalmitis following ocular interventions such as intravitreal injections and phakic intraocular lens (pIOL) implantations (Chen et al., 2011; Marquart et al., 2018; Busch et al., 2019; Cioana et al., 2024). An outbreak of streptococcal endophthalmitis caused by intravitreal injections of Bevacizumab has been reported in the USA (Matthews et al., 2014). In this outbreak, 10 out of 12 cases were caused by S. mitis/oralis. The pathogens have been demonstrated to originate from the contaminations during syringe preparation in pharmacy (Matthews et al., 2014). The majority of patients with S. mitis/oralis endophthalmitis showed poor visual outcomes where even an enucleation was sometimes required to remove infections (Matthews et al., 2014; Colás-Tomás and Pérez-Trigo, 2018). Additionally, it has been reported that S. mitis/oralis can also cause infectious endophthalmitis following pIOL implantation (Chung and Lee, 2014). Endophthalmitis in pIOL implantation is a relatively rare but potentially devastating complication. Thus, an early diagnosis is critically important. Compared with endophthalmitis caused by other pathogens, S. mitis/oralis endophthalmitis presents less virulent where the antibiotic treatment is effective without the removal of pIOL (Chung and Lee, 2014). However, the above-mentioned studies failed to differentiate between S. mitis and S. oralis. The distribution of S. mitis and S. oralis is required to be further addressed.
2.3 MeningitisBacterial meningitis is a global health menace with mortality rates ranging from 6% to 54% (Hasbun, 2022). It has been reported that the incidence of acute bacterial meningitis is 5–10/100,000 per year in developed countries, while the incidence in less developed countries is estimated even higher (Heckenberg et al., 2014). Given the high mortality of acute bacterial meningitis, a prompt diagnosis is paramount for tailoring appropriate antibiotic therapy and averting severe sequela such as permanent brain damage (Cruz Cardoso et al., 2021).
VGS species can cause meningitis in patients of all age groups. About 0.3–2.4% of meningitis cases are attributed to VGS (including S. oralis) (Montejo and Aguirrebengoe, 1998). In meningitis caused by VGS species, S. oralis is rarely implicated. To date, to our knowledge, about 10 cases of S. oralis meningitis have been reported in English-language literature (Fan et al., 2023). Although sporadic, S. oralis meningitis presents an association with oral diseases or oral manipulations. In several cases of S. oralis meningitis, it has been postulated that causative organisms originated from the oral cavity (Cruz Cardoso et al., 2021; Nakamura et al., 2021). Therefore, S. oralis meningitis should be taken into account when patients with oral diseases or dental procedure history present a fever, disturbance of consciousness, and headache (Nakamura et al., 2021). In other cases, S. oralis meningitis has been observed following spinal anesthesia for elective total knee replacement (Willder et al., 2013) and cerebrospinal fluid leaks (Patel et al., 2019). Additionally, S. oralis is also a rare causative agent of neonatal meningitis and maternal sepsis (Poi et al., 2018). In another case, meningoencephalitis and ventriculitis caused by S. oralis were reported in a 71-year-old female patient (Adly et al., 2023). However, risk factor from the oral cavity was ruled out in this case. S. oralis meningitis is also implicated in uncommon but severe complications such as cerebral vasospasm (Nonaka et al., 2018). Furthermore, a close scrutiny of the potential occurrence of IE in patients with meningitis associated with S. oralis is recommended (Patel et al., 2019; Cruz Cardoso et al., 2021).
2.4 Brain abscessIn addition to meningitis, S. oralis is also associated with brain abscesses (Solanki et al., 2014; Thiagarajan et al., 2016). Brain abscess is an intraparenchymal pyogenic infection with the reported incidence ranging from 0.98 to 1.28 per 100,000 population (Iro et al., 2023; Korkmaz and Korkmaz, 2023). It is a severe disease with a high potential of fatality (Muzumdar et al., 2011). The common predisposing factors mainly include an associated contiguous focus of infection, neurosurgery, head trauma, and hematogenous dissemination from a distant focus (Brook, 2017).
Brain abscesses caused by S. oralis are extremely uncommon. So far, to the best of our knowledge, two cases have been reported in the relevant English-language literature. In one case, S. oralis brain abscess was found in an infant bitten by a monkey wherein the pathogen might spread from the oral cavity of the monkey (Thiagarajan et al., 2016). In the other case, S. oralis brain abscess was observed in a 12-year-old patient with congenital heart disease (Solanki et al., 2014). In this case, congenital heart disease is assumed to be a putative predisposing factor in S. oralis infection while independent of oral hygiene (Solanki et al., 2014).
2.5 IEIE is an infection in endocardium involving large intrathoracic vessels, native or prosthetic heart valves, or even cardiac chambers with substantial morbidity and mortality rates. As mentioned above, streptococcal BSI is one of the most dominant causes of IE (Chamat-Hedemand et al., 2020). A Spanish multicenter study has reported that VGS represented 27.5% of IE cases (Vicent et al., 2018). Among streptococcal species, S. oralis is the most common causative agent responsible for about 37.8% of streptococcal IE cases (Doyuk et al., 2002; Ercibengoa et al., 2019; Chamat-Hedemand et al., 2023a). Oral diseases such as caries and periodontitis, poor oral hygiene, and oral interventions are important risk factors in S. oralis endocarditis (Udayaraj et al., 2003; Nahhal et al., 2023). On the other hand, it has been surprisingly reported that S. oralis IE was developed in an edentulous patient without any predisposing conditions such as underlying valvular heart disease, systemic infections at other sites, or dental procedure history (Renton et al., 2009). The mortality and therapy could differ remarkably from streptococcal species. For example, IE caused by S. oralis requires heart valve surgery more frequently than that caused by Streptococcus gallolyticus (Chamat-Hedemand et al., 2023a). It is postulated that S. oralis can grow more in plasma or thrombotic vegetation compared with other oral streptococcal species (Doyuk et al., 2002; Nagata et al., 2005). Therefore, an accurate and expedited diagnosis is vitally important in the treatment of IE. However, in clinical practice, the etiologic agent of IE has been predominantly identified at the group level, whilst the species-level classification has been seldom performed (Chamat-Hedemand et al., 2023a).
In high-income countries, the epidemiology of IE presents a shift from occurring in native valves to occurring in prosthetic valves or implantable cardiovascular devices of elderly patients (Hill et al., 2007). In addition to native valve endocarditis, S.oralis has also been recognized in prosthetic valve endocarditis (Turnier et al., 2009). The prevalence of S. oralis among patients with native valve endocarditis is 7%, while the prevalence among patients with prosthetic valve endocarditis is 5% (Chamat-Hedemand et al., 2020). The beta-lactam-resistant S. oralis has emerged in prosthetic valve IE imposing formidable clinical challenges (Tanaka et al., 2022). In this case, a patient who had native valve IE caused by beta-lactam-susceptible S. oralis has undergone prosthetic valve replacement (Tanaka et al., 2022). Subsequently, prosthetic valve IE caused by highly beta-lactam-resistant S. oralis was developed. This case confirms the evolution of antibiotic resistance in S. oralis and reflects the importance of antimicrobial therapy based on the susceptibility of specific species.
Notably, S. oralis has also been identified in uncommon IE cases such as pulmonic valve endocarditis and IE in pregnancy. Right-sided endocarditis occurs less frequently than left-sided endocarditis accounting for approximately 10% of IE cases (Shmueli et al., 2020). Furthermore, right-sided endocarditis largely involves the tricuspid valve but rarely involves the pulmonic valve (Fishbein and Fishbein, 2019). S. oralis has been recognized as a causative agent in pulmonic valve endocarditis (Goud et al., 2015; Nahhal et al., 2023). In one case, pulmonic valve endocarditis was caused by S. oralis BSI secondary to a dental abscess (Nahhal et al., 2023). IE in pregnancy is also uncommon with an estimated incidence of 1 per 100,000 per year (Montoya et al., 2003). Nonetheless, IE in pregnancy is devasting with maternal and fetal mortalities of 22.1% and 14.7%, respectively (Yuan, 2015). Wydall et al. (2021) identified S. oralis as the causative microorganism for IE and bacterial meningitis in a pregnant patient.
In the pathogenesis of IE, binding to platelets is a crucial step (Werdan et al., 2014). Previous work has identified several virulence factors such as serine-rich repeat protein (SRRP), associated with sialic acid adhesion A (AsaA), and neuraminidases A (NanA) in S. oralis IE isolates (Singh et al., 2017; Ronis et al., 2019; Gaytán et al., 2021). Additionally, it has been demonstrated that these virulence factors play a crucial role in regulating S. oralis adherence to platelets by binding to sialic acid (Singh et al., 2017; Ronis et al., 2019; Gaytán et al., 2021). Furthermore, Gaytán et al. (2021) have observed a significant reduction in colony-forming units (CFUs) in the rabbits with aortic valve damage inoculated with the asaA mutant compared with the parent strain. The evidence collectively indicates the pathogenic potential of these virulence factors in the development of IE caused by S.oralis.
2.6 PeritonitisPeritoneal dialysis (PD)-related peritonitis is the most common and severe complication of PD resulting in PD catheter removal transition to hemodialysis, encapsulation peritoneal sclerosis, and mortality. About 5-10% of all cases of PD-related peritonitis are attributed to VGS species (Chao et al., 2015; Liu et al., 2018). Nonetheless, the information concerning the identification and distribution of different VGS species is extremely scarce. To date, few cases of PD-related peritonitis have been reported to be caused by S. oralis. Two of them assumed that the pathogen originated from the oral cavity via hematogenous spread (Koruk et al., 2005; Mihara et al., 2023), while the other two cases excluded the oral entry route (Kotani et al., 2021). Favorable clinical outcomes have been observed in patients with PD-related peritonitis caused by S. oralis, and the outcomes do not differ from VGS species (Liu et al., 2018; Kotani et al., 2021).
2.7 Urinary tract infectionUTI is one of the most common infectious diseases in clinical practice owing to the anatomic features of the human urinary tract (Foxman, 2010). It is estimated that approximately 150 million people are affected by UTIs every year worldwide (Flores-Mireles et al., 2015). Albeit the low morbidity, the high incidence of UTIs causes a substantial society economic burden by costing over US $3.5 billion each year in the USA alone (Neugent et al., 2020). Predominant UTI pathogens include Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Enterococcus faecalis, and Staphylococcus saprophyticus. The identification of S. mitis/oralis in urine has been taken as commensals or contamination leading to an underestimation of UTIs associated with this species (Nelson et al., 2010; Mores et al., 2021). Nevertheless, growing case reports have revealed that S. mitis/oralis could cause UTIs in both adults and children with compromised immune systems due to alcoholic liver disease, renal transplant, and diabetes (Ong et al., 1998; Swain and Otta, 2013; Zhang et al., 2023). Furthermore, S. mitis/oralis has emerged as multidrug-resistant rendering antibiotic therapy difficult (Zhang et al., 2023). However, previous research failed to differentiate between S. mitis and S. oralis. The species-level identification is recommended for the determination of appropriate antibiotic regimens and understanding of the epidemiology of S. oralis UTIs.
2.8 Spinal abscessPyogenic spinal infection is a rare but highly fatal disease with a mortality rate of 2-20% in developed countries (Lener et al., 2018). Common predisposing factors for pyogenic spinal infection encompass immunodeficiency, intravenous drug use, and spine procedures (Schwab and Shah, 2020). S. oralis can be transmitted hematogenously to the brain, spinal cord, and spine due to spinal instrumentation, anesthesia, or oral infectious diseases (Willder et al., 2013; Prod’homme et al., 2021). It has been reported that S. oralis led to spinal abscesses in immunocompromised patients (diabetes) (Chu et al., 2022). In this case, the patient presented gingivitis. It has been speculated that S. oralis was spread hematogenously from gingivitis to the spine.
3 ConclusionsMolecular techniques have illuminated the role of S. oralis as one of the major causative agents among VGS infections and suggested variations in distribution, clinical features, and prognosis within VGS (Kitten et al., 2012; Sahasrabhojane et al., 2014; Chun et al., 2015; Kim et al., 2018). The present study has offered a concise summary of S. oralis opportunistic infections (Table 1). The insights gained here may be of assistance to the correct diagnosis and optimizing antimicrobial therapy of relative infectious diseases in clinical practice. Although the role of S. oralis as an opportunistic pathogen has been uncovered, the epidemiology of S. oralis infections is poorly understood owing to the lack of species-level identification of VGS, especially the correct differentiation between S. mitis and S. oralis. Therefore, on the one hand, future investigations are required to provide a simple, precise, and reliable assignment of S. oralis. Additionally, clinicians should pay more attention to S. oralis opportunistic infections to further clarify the epidemiology of S. oralis infectious diseases.
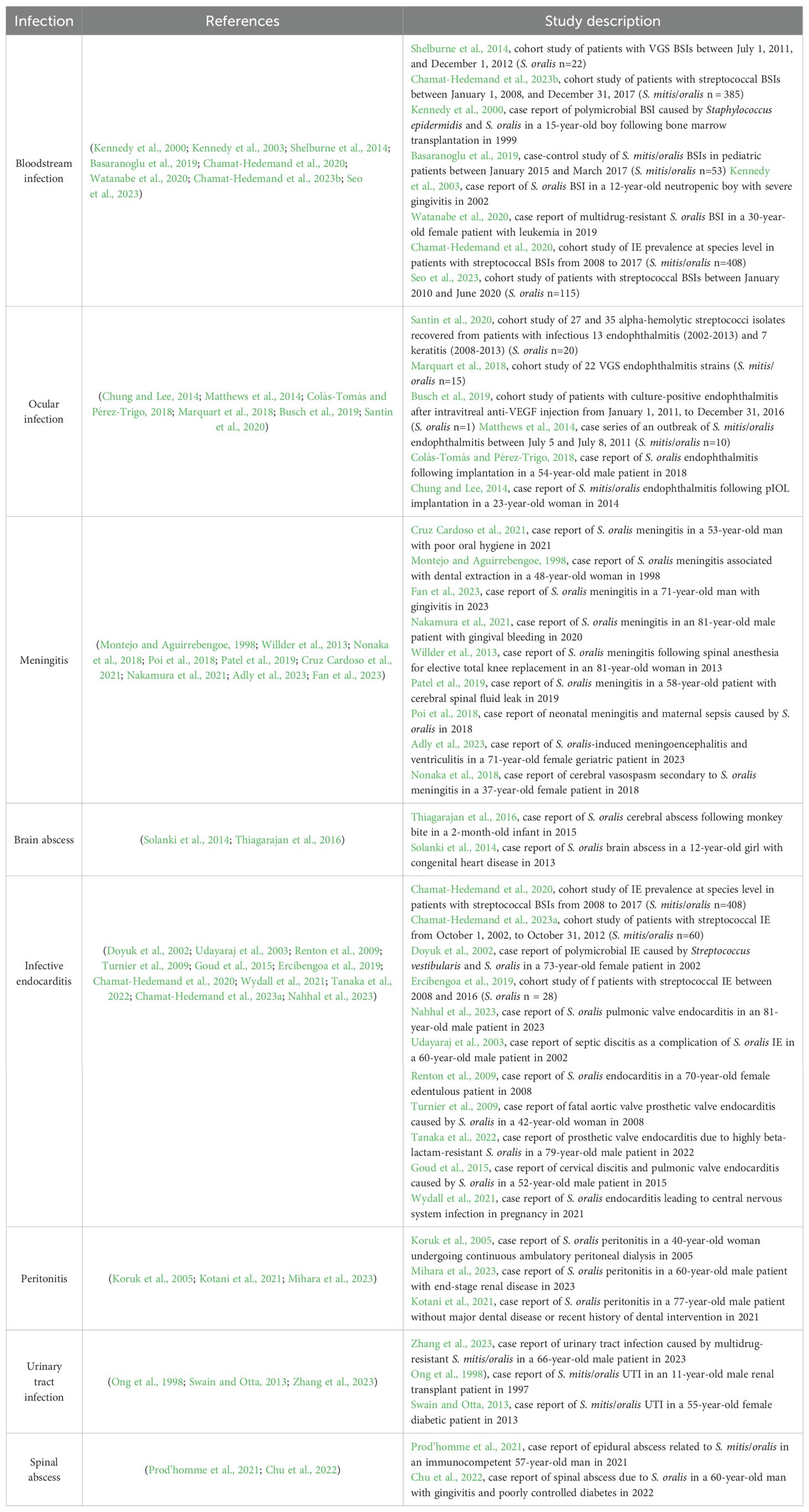
Table 1. S. oralis opportunistic infections.
In addition to the epidemiology, the pathogenesis of S. oralis also remains elusive. On the one hand, many previous studies failed to illustrate the entry route of S. oralis in infectious diseases. The association between the oral entry route and S. oralis infections needs to be explored. Another potentially fruitful avenue for future research is the pathogenic potential of S. oralis. It has been established that as one of the closest relatives of S. pneumoniae, S. oralis shares a variety of common virulence factors with S. pneumoniae such as choline-binding proteins, neuraminidases A, immunoglobulin A1 proteases, and zinc metalloproteases (Kilian and Tettelin, 2019). Moreover, the interspecies gene transfer between S. pneumoniae and S. oralis allows the emergence of more common virulence factors (Joyce et al., 2022). Owing to the role of S. pneumoniae as a major human pathogen, the functions of these virulence factors involving the adhesion and invasiveness of S. pneumonia have been extensively studied (Sadowy and Hryniewicz, 2020). By contrast, the exact functions of these virulence factors in the pathogenesis of S. oralis infections are still unexplored. Particularly very little is currently known about the underlying pathogenic mechanisms that trigger the alteration from commensals to opportunistic pathogens of S. oralis. Further research on this topic is needed to develop a full picture of S. oralis opportunistic infections.
Author contributionsJR: Writing – original draft, Writing – review & editing. PS: Investigation, Writing – review & editing, Writing – original draft. MW: Software, Writing – review & editing, Investigation. WZ: Conceptualization, Writing – review & editing. ZL: Conceptualization, Funding acquisition, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Yantai School-City; Integration Development Project (2023XDRHXMPT11).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesAdly, M., Elmoheen, A., Helmi Ahmed, M. M., Hanbouly, A., Mishreky, L. M. (2023). Streptococcus oralis-induced meningoencephalitis and ventriculitis in a geriatric female patient. Cureus 15, e46101. doi: 10.7759/cureus.46101
PubMed Abstract | Crossref Full Text | Google Scholar
Ahmed, R., Hassall, T., Morland, B., Gray, J. (2003). Viridans streptococcus bacteremia in children on chemotherapy for cancer: an underestimated problem. Pediatr. Hematol. Oncol. 20, 439–444. doi: 10.1080/08880010390220144
PubMed Abstract | Crossref Full Text | Google Scholar
Basaranoglu, S. T., Ozsurekci, Y., Aykac, K., Aycan, A. E., Bıcakcigil, A., Altun, B., et al. (2019). Streptococcus mitis/oralis causing blood stream infections in pediatric patients. Jpn J. Infect. Dis. 72, 1–6. doi: 10.7883/yoken.JJID.2018.074
PubMed Abstract | Crossref Full Text | Google Scholar
Busch, C., Iglicki, M., Okada, M., Gabrielle, P. H., Cohen, S., Mariussi, M., et al. (2019). Causative pathogens of endophthalmitis after intravitreal anti-VEGF injection: an international multicenter study. Ophthalmologica 241, 211–219. doi: 10.1159/000496942
PubMed Abstract | Crossref Full Text | Google Scholar
Chamat-Hedemand, S., Dahl, A., Hassager, C., Arpi, M., Østergaard, L., Bundgaard, H., et al. (2023a). Streptococcal infective endocarditis: clinical features and outcomes according to species. Infection 51, 869–879. doi: 10.1007/s15010-022-01929-1
PubMed Abstract | Crossref Full Text | Google Scholar
Chamat-Hedemand, S., Dahl, A., Østergaard, L., Arpi, M., Fosbøl, E., Boel, J., et al. (2020). Prevalence of infective endocarditis in streptococcal bloodstream infections is dependent on streptococcal species. Circulation 142, 720–730. doi: 10.1161/CIRCULATIONAHA.120.046723
PubMed Abstract | Crossref Full Text | Google Scholar
Chamat-Hedemand, S., Dahl, A., Østergaard, L., Arpi, M., Fosbøl, E., Boel, J., et al. (2023b). Streptococcal species as a prognostic factor for mortality in patients with streptococcal bloodstream infections. Infection 51, 1513–1522. doi: 10.1007/s15010-023-02025-8
PubMed Abstract | Crossref Full Text | Google Scholar
Chang, W. N., Wu, J. J., Huang, C. R., Tsai, Y. C., Chien, C. C., Lu, C. H. (2002). Identification of viridans streptococcal species causing bacterial meningitis in adults in Taiwan. Eur. J. Clin. Microbiol. Infect. Dis. 21, 393–396. doi: 10.1007/s10096-002-0727-z
PubMed Abstract | Crossref Full Text | Google Scholar
Chao, C. T., Lee, S. Y., Yang, W. S., Chen, H. W., Fang, C. C., Yen, C. J., et al. (2015). Viridans streptococci in peritoneal dialysis peritonitis: clinical courses and long-term outcomes. Perit Dial Int. 35, 333–341. doi: 10.3747/pdi.2013.00108
PubMed Abstract | Crossref Full Text | Google Scholar
Chen, E., Lin, M. Y., Cox, J., Brown, D. M. (2011). Endophthalmitis after intravitreal injection: the importance of viridans streptococci. Retina 31, 1525–1533. doi: 10.1097/IAE.0b013e318221594a
PubMed Abstract | Crossref Full Text | Google Scholar
Chu, E. C., Trager, R. J., Chen, A. T. C., Shum, J. S. F. (2022). A 60-year-old man with gingivitis and poorly controlled diabetes developing low back pain 1 week following recovery from COVID-19 diagnosed with spinal abscess due to streptococcus oralis. Am. J. Case Rep. 23, e937517. doi: 10.12659/AJCR.937517
PubMed Abstract | Crossref Full Text | Google Scholar
Chun, S., Huh, H. J., Lee, N. Y. (2015). Species-specific difference in antimicrobial susceptibility among viridans group streptococci. Ann. Lab. Med. 35, 205–211. doi: 10.3343/alm.2015.35.2.205
PubMed Abstract | Crossref Full Text | Google Scholar
Chung, J. K., Lee, S. J. (2014). Streptococcus mitis/oralis endophthalmitis management without phakic intraocular lens removal in patient with iris-fixated phakic intraocular lens implantation. BMC Ophthalmol. 14, 92. doi: 10.1186/1471-2415-14-92
PubMed Abstract | Crossref Full Text | Google Scholar
Cioana, M., Naidu, S., Far, P. M., Yeung, S. C., You, Y., Yan, P. (2024). Postintravitreal injection and postcataract extraction endophthalmitis visual outcomes by organism: A systematic review and meta-analysis. Retina 44, 1608–1618. doi: 10.1097/IAE.0000000000004143
PubMed Abstract | Crossref Full Text | Google Scholar
Colás-Tomás, T., Pérez-Trigo, S. (2018). Delayed-onset endophthalmitis following implantation of a XEN45 glaucoma device: A case report. J. Glaucoma. 27, 936–938. doi: 10.1097/IJG.0000000000001064
PubMed Abstract | Crossref Full Text | Google Scholar
Cruz Cardoso, J., Ferreira, D., Assis, R., Monteiro, J., Coelho, I., Real, A., et al. (2021). Streptococcus oralis meningitis. Eur. J. Case Rep. Intern. Med. 8, 002349. doi: 10.12890/2021_002349
PubMed Abstract | Crossref Full Text | Google Scholar
Denapaite, D., Rieger, M., Köndgen, S., Brückner, R., Ochigava, I., Kappeler, P., et al. (2016). Highly Variable Streptococcus oralis Strains Are Common among Viridans Streptococci Isolated from Primates. mSphere 1, e00041-15. doi: 10.1128/mSphere.00041-15
PubMed Abstract | Crossref Full Text | Google Scholar
Doyuk, E., Ormerod, O. J., Bowler, I. C. (2002). Native valve endocarditis due to Streptococcus vestibularis and Streptococcus oralis. J. Infect. 45, 39–41. doi: 10.1053/jinf.2002.1004
PubMed Abstract | Crossref Full Text | Google Scholar
Ercibengoa, M., Goenaga, M. A., Ardanuy, C., Grau, I., García-de-la-Maria, C., Almela, M., et al. (2019). Epidemiological and clinical characteristics of Streptococcus tigurinus endocarditis. BMC Infect. Dis. 19, 291. doi: 10.1186/s12879-019-3914-6
PubMed Abstract | Crossref Full Text | Google Scholar
Fan, D., Fisher, C., Douglas, N. M., Hurrell, M. A., Freeman, J. T., Jardine, D. L. (2023). A rare case of Streptococcus oralis meningitis in New Zealand. Intern. Med. J. 53, 1931–1933. doi: 10.1111/imj.v53.10
PubMed Abstract | Crossref Full Text | Google Scholar
Flores-Mireles, A. L., Walker, J. N., Caparon, M., Hultgren, S. J. (2015). Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 13, 269–284. doi: 10.1038/nrmicro3432
PubMed Abstract | Crossref Full Text | Google Scholar
Gaytán, M. O., Singh, A. K., Woodiga, S. A., Patel, S. A., An, S.-S., Vera-Ponce de León, A., et al. (2021). A novel sialic acid-binding adhesin present in multiple species contributes to the pathogenesis of Infective endocarditis. PloS pathog. 17, e1009222. doi: 10.1371/journal.ppat.1009222
PubMed Abstract | Crossref Full Text | Google Scholar
Goud, A., Abdelqader, A., Dahagam, C., Padmanabhan, S. (2015). Isolated pulmonic valve endocarditis presenting as neck pain. J. Community Hosp Intern. Med. Perspect. 5, 29647. doi: 10.3402/jchimp.v5.29647
PubMed Abstract | Crossref Full Text | Google Scholar
Hill, E. E., Herijgers, P., Claus, P., Vanderschueren, S., Herregods, M. C., Peetermans, W. E. (2007). Infective endocarditis: changing epidemiology and predictors of 6-month mortality: a prospective cohort study. Eur. Heart J. 28, 196–203. doi: 10.1093/eurheartj/ehl427
PubMed Abstract | Crossref Full Text | Google Scholar
Imai, K., Nemoto, R., Kodana, M., Tarumoto, N., Sakai, J., Kawamura, T., et al. (2020). Rapid and accurate species identification of mitis group streptococci using the minION nanopore sequencer. Front. Cell Infect. Microbiol. 10, 11. doi: 10.3389/fcimb.2020.00011
PubMed Abstract | Crossref Full Text | Google Scholar
Iro, M. A., Goldacre, M. J., Goldacre, R. (2023). Central nervous system abscesses and empyemas in England: epidemiological trends over five decades. J. Infect. 86, 309–315. doi: 10.1016/j.jinf.2023.01.040
PubMed Abstract | Crossref Full Text | Google Scholar
Isaksson, J., Rasmussen, M., Nilson, B., Stadler, L. S., Kurland, S., Olaison, L., et al. (2015). Comparison of species identification of endocarditis associated viridans streptococci using rnpB genotyping and 2 MALDI-TOF systems. Diagn. Microbiol. Infect. Dis. 81, 240–245. doi: 10.1016/j.diagmicrobio.2014.12.007
PubMed Abstract | Crossref Full Text | Google Scholar
Jensen, C. S., Iversen, K. H., Dargis, R., Shewmaker, P., Rasmussen, S., Christensen, J. J., et al. (2021). Streptococcus pseudopneumoniae: use of whole-genome sequences to validate species identification methods. J. Clin. Microbiol. 59, e02503-20. doi: 10.1128/JCM.02503-20
PubMed Abstract | Crossref Full Text | Google Scholar
Jensen, A., Scholz, C. F. P., Kilian, M. (2016). Re-evaluation of the taxonomy of the Mitis group of the genus Streptococcus based on whole genome phylogenetic analyses, and proposed reclassification of Streptococcus dentisani as Streptococcus oralis subsp. dentisani comb. nov., Streptococcus tigurinus as Streptococcus oralis subsp. tigurinus comb. nov., and Streptococcus oligofermentans as a later synonym of Streptococcus cristatus. Int. J. Syst. Evol. Microbiol. 66, 4803–4820. doi: 10.1099/ijsem.0.001433
PubMed Abstract | Crossref Full Text | Google Scholar
Joyce, L. R., Youngblom, M. A., Cormaty, H., Gartstein, E., Barber, K. E., Akins, R. L., et al. (2022). Comparative genomics of streptococcus oralis identifies large scale homologous recombination and a genetic variant associated with infection. mSphere 7, e0050922. doi: 10.1128/msphere.00509-22
PubMed Abstract | Crossref Full Text | Google Scholar
Kennedy, H. F., Morrison, D., Kaufmann, M. E., Jackson, M. S., Bagg, J., Gibson, B. E. S., et al. (2000). Origins of Staphylococcus epidermidis and Streptococcus oralis causing bacteraemia in a bone marrow transplant patient. J. Med. Microbiol. 49,
留言 (0)