Sarcomas are a heterogeneous group of malignant tumors that include more than 70 different types, primarily arising from bone or soft tissue. The clinical manifestations of malignant soft tissue sarcomas in particular are often nonspecific, which often leads to delayed diagnosis. Therefore, by the time of detection, the tumor has usually progressed significantly and may have metastasized to distant organs (1). Soft tissue sarcomas (STS) are malignant soft tissue tumors that include multiple histological subtypes (e.g., liposarcoma, rhabdomyosarcoma, synovial sarcoma, leiomyosarcoma, and fibrosarcoma) that can occur almost anywhere in the body. STS mainly occurs in the lower limbs, followed by the upper limbs and trunk. Other common locations include the head, neck, and retroperitoneal space (2). Late-stage STS often metastasizes through the bloodstream, lymphatic system, and intra-abdominal pathways, which is an important reason for the poor prognosis of STS patients (3). The lung is the most common organ of metastasis. STS metastasis accounts for 80% of the first metastasis sites of STS, thus emphasizing the critical role of pulmonary therapy in patients with the disease (3, 4). The risk of metastasis depends largely on tumor grade; approximately 60% of patients with highly malignant tumors will develop lung metastasis (LM), the vast majority of which occur within 2 years after resection of the primary tumor (5). The highest-risk tumors are those >5 cm in size and of intermediate or high grade (6). The median life expectancy of STS patients with LM has been reported to be 10–12 months (7, 8). Given the rapid progression of high-grade STS, timely detection of LM may improve prognosis based on currently available therapeutic interventions. Despite their diversity, sarcomas are relatively rare, accounting for approximately 1% of all cancer diagnoses (9).
Treatment strategies for sarcoma lung metastases are mainly based on limited evidence due to the lack of large randomized trials. However, surgical resection of resectable metastatic tumors remains the mainstream treatment approach (10). The gold standard for patients with localized STS is surgical-wide resection of the tumor margins (2, 11). 2,9 In contrast, STS patients with metastatic disease receive systemic chemotherapy, radiation therapy, and possible resection of the primary tumor or metastatic disease (12, 13). Doxorubicin is a first-line palliative chemotherapy and is recommended as a treatment option for most STS patients with LM (14). Pulmonary metastasectomy is also a well-established standard method for treating STS patients with LM, and some published studies have shown that the 5-year overall survival (OS) rate of patients undergoing this surgery ranges from 43% to 43%. 50.9% (15–17). Furthermore, Matsuoka et al. reported that surgical resection of primary tumors in the extremities improves survival in metastatic STS (18). Despite efforts to improve clinical outcomes in these patients, prognosis remains poor (19). The effectiveness of metastasectomy in improving patient prognosis and survival is an area of ongoing research and the focus of our current study.
In addition to primary sarcomas, the metastatic behavior of these tumors is an important area of concern. Metastatic sarcomas, particularly those that metastasize to the lungs, present significant challenges in management and treatment. The complexity of this disease, coupled with the lack of specific clinical manifestations, often leads to delays or misdiagnosis, further complicating the treatment process. Several published studies have evaluated LM-related risk factors and prognostic factors in STS patients with LM, including number of LMs, histology, stage, location of primary disease, and pulmonary metastasis resection (8, 19, 20). However, to date, there is a lack of standard predictive models predicting risk and prognosis of STS vs. LM for clinicians. Therefore, this study aimed to elucidate current treatment strategies for sarcoma lung metastases, the role of metastasectomy in patient prognosis, and the challenges of diagnosing and treating this complex disease. Through this comprehensive review of the latest research and clinical practice, we hope to contribute to the ongoing discussion on improving outcomes for patients with sarcoma lung metastases.
In addition, this study is a survey of cases from the National Taiwan University Hospital. To compare with the results of past studies, this study summarizes the median postoperative outcomes of pulmonary metastasectomy and lymph node dissection in the past ten years. The survival period is shown in Table 1.
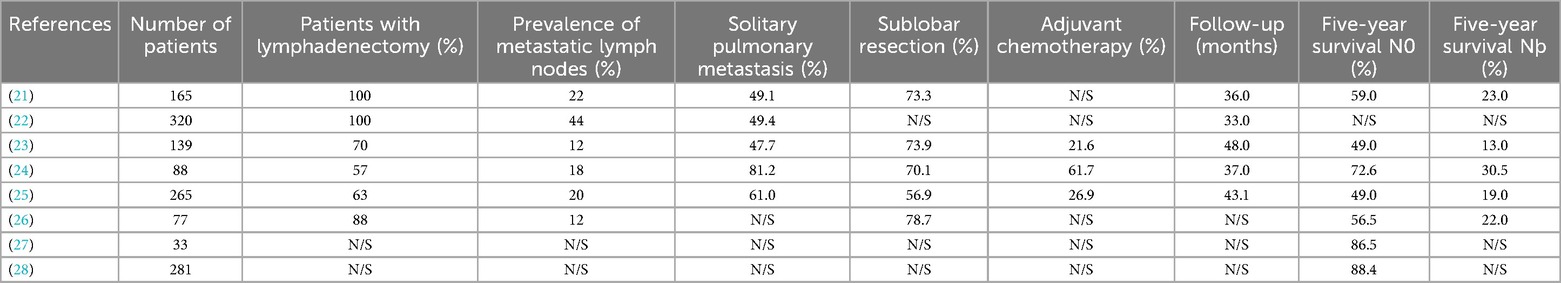
Table 1. Characteristics and results of relevant studies.
2 Materials and methods 2.1 Study designThis study is a single-center, retrospective observational study evaluating survival outcomes in patients who have undergone metastasectomy. We reviewed the data of patients who underwent LM metastasectomy at the National Taiwan University Hospital (including branches) from February 2007 to November 2020. The items discussed and analyzed included data of patients with sarcoma lung metastases who underwent metastasis resection such as demographic, clinical, and postoperative pathological characteristics of patients undergoing pulmonary metastasectomy, perioperative outcomes of patients undergoing metastasectomy, and histological subtypes of patients with metastatic sarcoma. These characteristics included the patient's physiological background information, whether he or she has a smoking habit, the duration of postoperative symptom-free disease, whether all the patient's pulmonary metastatic nodules had been completely resected, the location of the primary tumor, the maximum size of metastatic nodules, the laterality of lung metastases, and the surgical method.
In the National Taiwan University Hospital (including branches), the resection criteria for LM metastases are in principle based on the National Comprehensive Cancer Network (NCCN) guidelines (13) and include the following: primary cancer control, whether the lung lesions are resectable, whether the patients can tolerate pneumonectomy, and whether the patient does not have unresectable extrapulmonary disease. When patients do not meet these criteria, physicians recommend systemic chemotherapy with LM. When a patient begins LM treatment, the patient's treatment plan is determined based on discussions between the cancer surgeon and medical oncologist. Since the data for this study come from the database of National Taiwan University Hospital and the names of the patients are not disclosed, that is, the data are non-identifiable, so cases within the statistical interval are included in the analysis of this study.
2.2 PatientsThis study conducted a retrospective analysis of patients treated at various branches of the National Taiwan University Hospital from February 2007 to December 2020. The patients include those who were treated at the main hospital of National Taiwan University Hospital from February 2007 to December 2020; at the Yunlin branch from February 2020 to December 2020; at the Yunlin branch from January 2014 to December 2020; and at the Hsinchu Branch From November 2014 to December 2020. The Research Ethics Committee of National Taiwan University Hospital approved this retrospective study (Project Approval Number: 202103065RIND).
Eligibility for inclusion in the study required patients to meet the following criteria: (1) be 18 years or older, (2) have a pathological diagnosis of sarcoma malignancy, and (3) have undergone lung resection as a metastasis resection suitable for lung metastases. Patients were excluded if the pathology report showed no evidence of primary sarcoma with concomitant lung metastases. All surgeries were performed at the National Taiwan University Hospital and its branches, and postoperative care continued until patients were discharged.
Prior to admission for surgery, the patient underwent a comprehensive preoperative evaluation. This included blood tests, chest x-rays, and electrocardiograms to assess the patient's overall health. For patients diagnosed with primary sarcoma presenting lung tumors, we almost invariably perform a transthoracic biopsy to differentiate between metastatic sarcoma and primary lung cancer, except in cases where the tumor's proximity to major vessels significantly increases the risk associated with the biopsy. In such instances, after thorough discussions with the patient about the risks, we proceed directly to surgical resection. Every patient selected for surgery underwent comprehensive scanning using CT or PET to rule out metastasis beyond the lungs, ensuring a focused and effective surgical approach. The surgical method is determined based on the patient's physical condition and the distribution of the tumor. For patients scheduled to undergo lobectomy, additional preoperative evaluation included pulmonary function testing and echocardiography to assess whether the patient's heart and lungs were suitable for surgery (29).
The screening principles for the patients in this study are detailed in Figure 1. The samples were obtained from two pathology databases at National Taiwan University Hospital. During the 18-year period, the initial screening results showed that patients with lung metastases had been treated in our hospital. A total of 341 patients underwent resection, and samples with incomplete pathological data were further removed. Finally, a total of 110 patients were obtained as the sample number for this study.
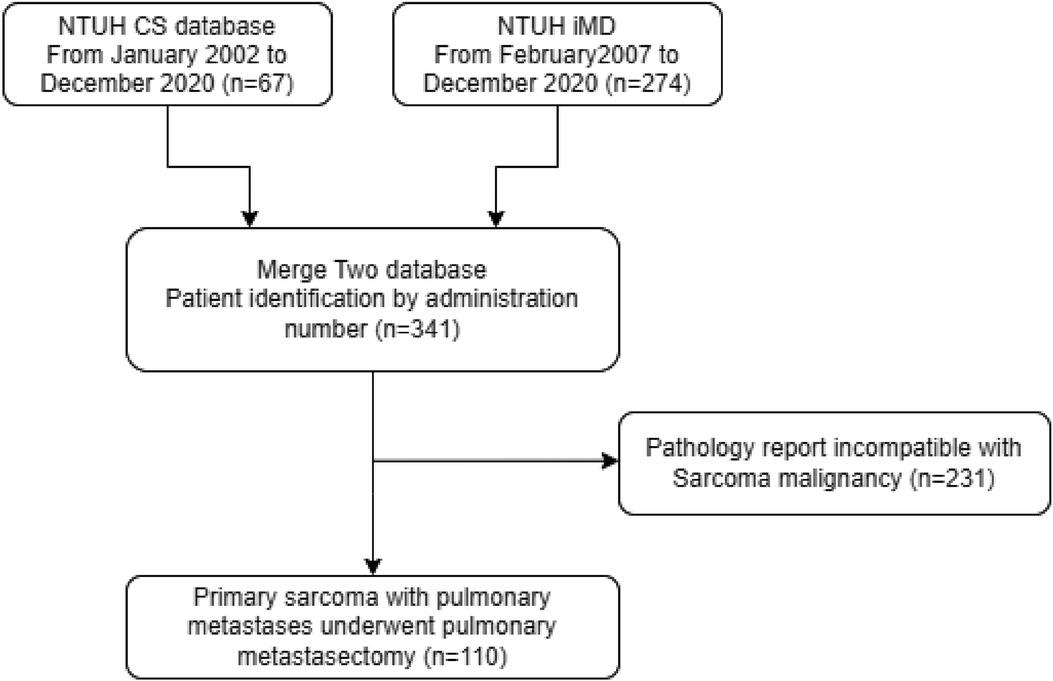
Figure 1. Algorithm for patient selection. NTUH, National Taiwan University Hospital; iMD, Integrative Medical Database.
2.3 Data collectionClinicopathological information on patients, primary sarcomas, and LM was obtained from medical records. After resection of the primary tumor or LM, follow-up was performed according to NCCN guidelines. LM was diagnosed through chest computed tomography (CT) by multiple doctors, including radiologists. The date of pulmonary recurrence was recorded as the date when LM was first detected on retrospective chest CT.
When patients developed LM at the time of primary tumor resection, we defined LM as synchronous LM and recorded the date of lung recurrence as the date of surgery. The disease-free interval (DFI) was calculated from the date of knot surgery to the date of pulmonary recurrence. If the patient had synchronous LM, the DFI was recorded as 0. If the patient had multiple LM, the diameter of the largest tumor was used as the metastatic tumor size. For patients receiving chemotherapy, tumor markers were measured monthly and CT scans were performed every 3–4 months to assess disease progression during treatment. The response of LM to chemotherapy was evaluated according to the Response Evaluation Criteria in Solid Tumors version 1.1 (30). Adverse events were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 (31).
2.4 OutcomesThe primary outcomes were 5-year overall survival (OS), cancer-specific survival (CSS), and disease-free survival (DFS) in patients treated with pulmonary metastasectomy. The secondary outcome was to identify factors that have a significant impact on OS. We discussed the impact of different surgical methods on patient prognosis. In addition, the subtype distribution of sarcoma lung metastases in Asia, Europe, and America was discussed.
2.5 Statistical analysisFirst, descriptive statistics were used to describe the ethnic characteristics of the study patients, and numerical variables are expressed as mean ± standard deviation. Categorical variables are shown as counts (percentages). Five-year OS, CSS, DFS, and progression-free survival (PFS) were then assessed using the Kaplan-Meier method, and univariate analyses were performed using the log-rank test. The distribution of characteristics between surgical and chemotherapy patients was assessed using Fisher's exact test for categorical variables and Student's t-test for numerical variables. All data were analyzed using Cox regression models for multivariate analysis. All statistical analyses were performed using IBM SPSS Statistics 26.0. All p values are two-sided, and differences were considered statistically significant at p < 0.05.
3 Results 3.1 Patient characteristicsThis study conducted an in-depth analysis of the demographic and clinical characteristics of 110 patients diagnosed with pulmonary metastatic sarcoma. The clinicopathological data of the 110 patients are shown in Tables 2, 3. These patients have been treated in a certain institution and its affiliated branches since January 2002. As of December 2020. The average age of these patients was 48.5 years, with an almost equal distribution of men and women. The majority (70.9%) of these patients did well with an Eastern Cooperative Oncology Group (ECOG) score of 0. 18.3% of patients were smokers and 81.7% were non-smokers. In addition, 33.6% of patients had underlying comorbidities, including diabetes (11.6%), hypertension (23.9%), and other types of cancer (6.6%).
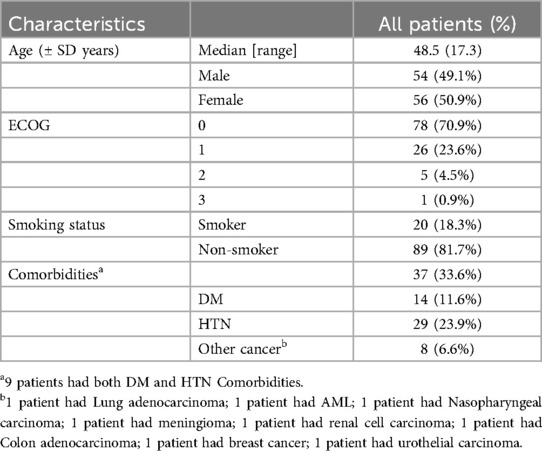
Table 2. Demographic and clinical features of sarcoma patients with lung metastasis patients who underwent metastasectomy.
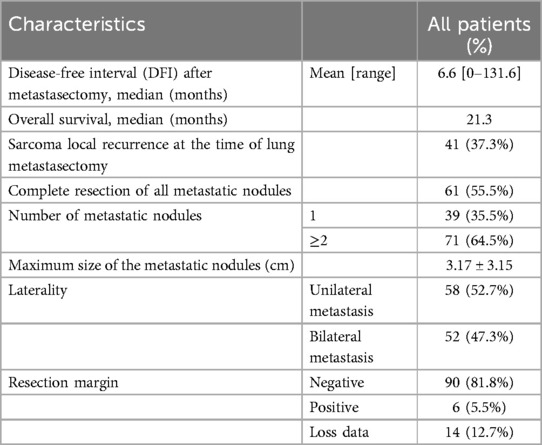
Table 3. Clinical features at the time of lung metastasectomy and pathological features of sarcoma patients with lung metastasis patients who underwent metastasectomy.
During the resection of pulmonary metastases, some important pathological and clinical features were revealed. A significant 64.5% of patients had multiple (more than two) lung metastases, emphasizing the aggressive nature of the disease. Bilateral metastases occur in approximately half of patients, indicating the systemic nature of sarcoma metastasis. Consistent with contemporary trends in thoracic surgery, the majority of patients (84.6%) underwent sublobar resection and the majority (83.6%) underwent VATS. One-third of VATS procedures are single-port VATS procedures. According to the postoperative pathology report of our hospital, 81.8% of the surgeries achieved R0 resection, with ideal oncological results.
In addition, the surgical methods performed on the patients are summarized in Table 4. Among them, 23.2% of the patients underwent sequential resection of bilateral pulmonary metastases. The average postoperative hospital stay was 8.5 days, and the average ICU stay was 1.1 days. The rate of serious complications among the 110 patients after surgery was low at 4.5%, and there was no death record among all patients within 30 days after surgery.
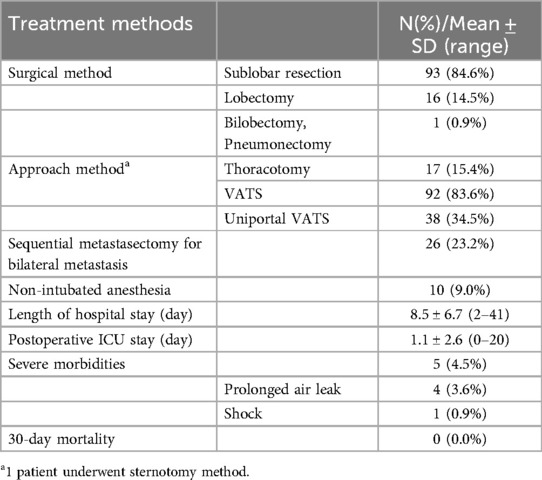
Table 4. Perioperative outcomes of sarcoma patients with lung metastasis patients who underwent metastasectomy.
3.2 Factors correlated with overall survivalAn important component of this study is the assessment of overall survival and potential influencing factors. The 5-year survival rate of surgical patients was observed to be 62.9% (Figure 2), which is a significant improvement compared with the results of previous studies. Additionally, this study used a comprehensive Kaplan-Meier analysis to identify several factors associated with improved overall survival, including a disease-free interval of more than 12 months after lung surgery, the absence of local sarcoma recurrence at the time of lung metastasectomy, and the presence of Single pulmonary metastasis tuberculosis. The results of univariate analysis of long-term prognosis are shown in Table 5. Among surgical patients, including factors such as DFI >12 months (P = 0.01), whether the sarcoma recurred during lung metastasis resection (P = 0.009), and whether the number of metastatic nodules exceeded 2 (P = 0.037), in this study Both are considered to have a significant impact on patient prognosis and OS. However, we further conducted multivariate analysis on the medical data of 110 patients. The results of the analysis are detailed in Table 6. We confirmed that only a disease-free interval of more than 12 months after lung surgery and the absence of local sarcoma recurrence during lung metastasis resection were significantly associated with overall survival (P < 0.05), this result is the same as the result of univariate analysis.
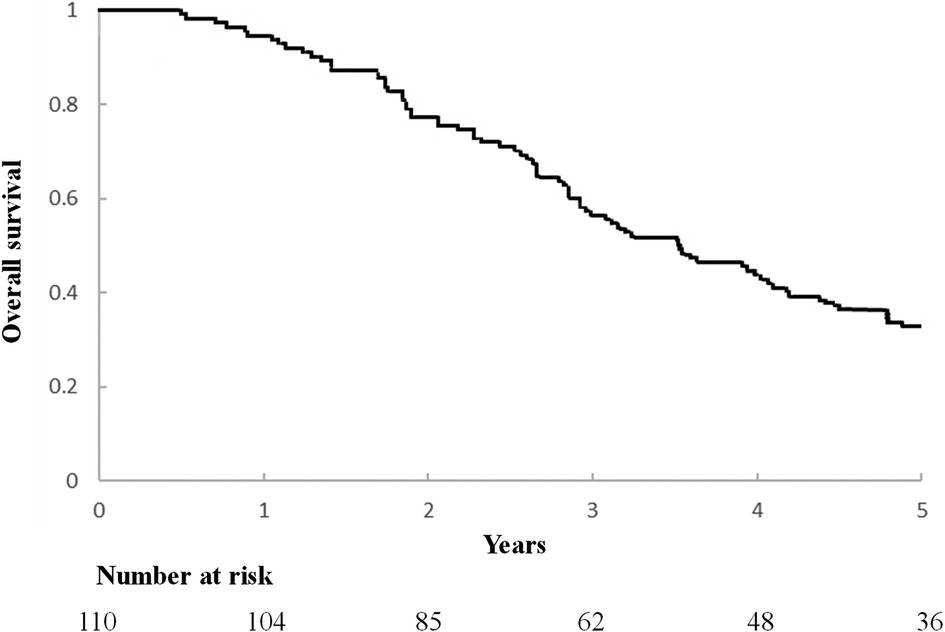
Figure 2. Kaplan-Meier curve of overall survival after lung metastasis surgery.
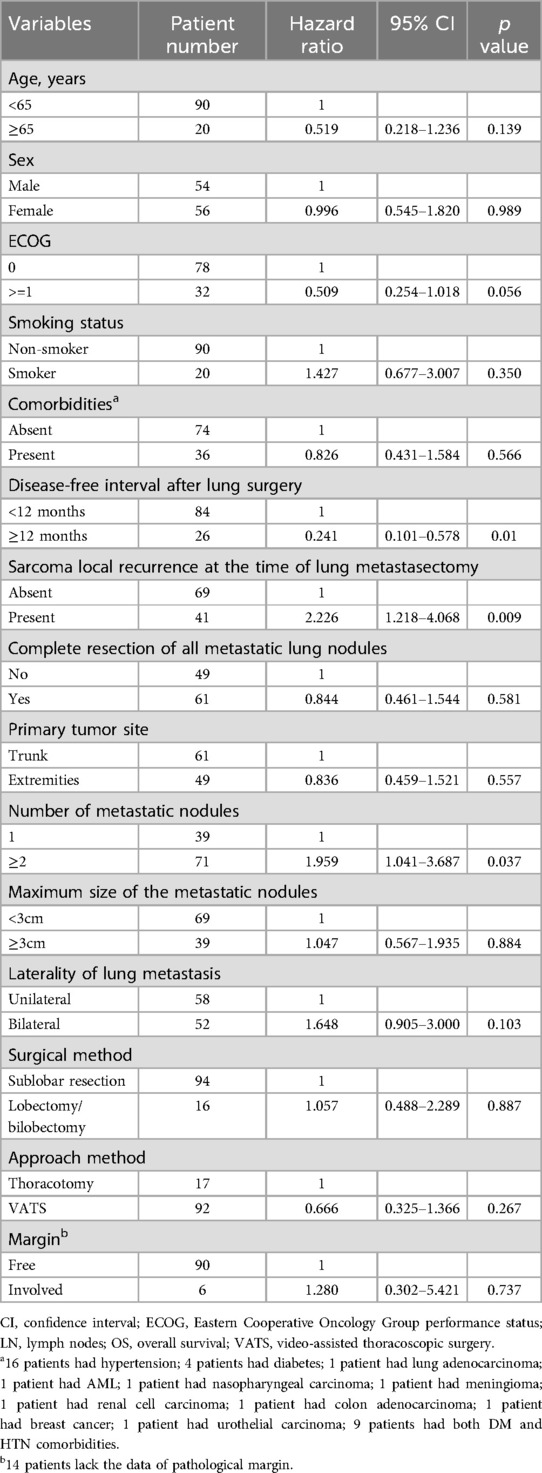
Table 5. Univariate analysis of correlations between clinicopathological features and OS for sarcoma patients with lung metastasis patients who underwent metastasectomy.
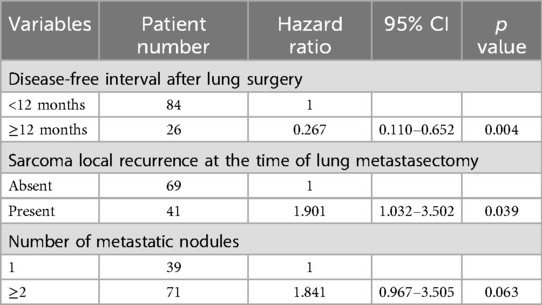
Table 6. Multivariate analysis of correlations between clinicopathological features and OS for sarcoma patients with lung metastasis patients who underwent metastasectomy.
The histological subtypes of metastatic sarcomas in this study are shown in Table 7. Consistent with the results of past research (10), the most common histological subtype was leiomyosarcoma (23.6%), followed by osteosarcoma (20.0%). These findings provide important insights into treatment strategies for patients with lung metastatic sarcoma and lay the foundation for further research.
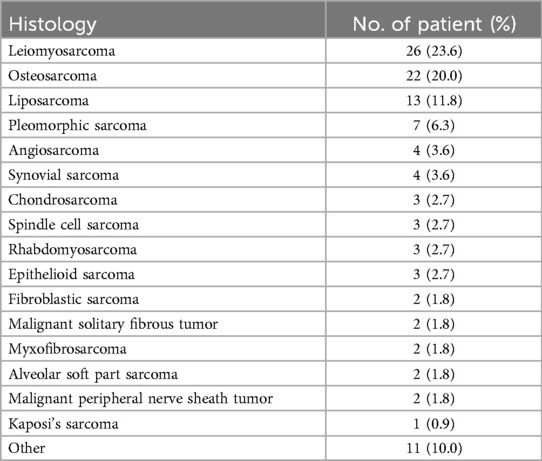
Table 7. Histological subtype of metastatic sarcoma patients.
Among the 110 patients, 17 had bilateral metastases and underwent secondary surgeries within three months. In addition, another important finding was that a total of 92 of the 110 patients in this study underwent VATS minimally invasive surgery. After treatment, compared with the other 18 patients (one patient underwent sternotomy) who used traditional thoracotomy surgery, they had a higher survival rate within one year, and their survival time was also relatively longer.
Taken together, the comprehensive analysis of this study provides a comprehensive understanding of the patient experience, from demographic and clinical characteristics to overall survival. The presented tables also provide an organized and comprehensive view of the analyzed data, helping to understand correlations and findings more easily.
4 DiscussionThis study provides a comprehensive analysis of clinical characteristics and outcomes in patients diagnosed with pulmonary metastatic sarcoma who underwent metastasectomy. The results of this study indicate a higher 5-year survival rate of 62.9% compared to prior reports, which ranged from 15% to 50.9% (10, 32–34). This difference in survival rates may be due to several factors, including advances in surgical techniques, perioperative care, and patient selection. It is worth noting that the results are based on patients treated in a single institute, which might have implications for the generalizability of the findings.
Our study is unique in its focus on an Asian population, a demographic that is often underrepresented in sarcoma research. The distribution of sarcoma subtypes varies between different ethnic and geographical groups, which can influence survival outcomes. Our analysis of Asian patients demonstrated that a disease-free interval over 12 months and the absence of local sarcoma recurrence at the time of lung metastasectomy significantly improved overall survival outcomes. Patients with a single lung metastatic nodule tended to have better overall survival outcomes compared to those with more than one metastatic nodule. This finding is noteworthy, although it did not reach statistical significance in the multivariate analysis in our study.
The selection of VATS or thoracotomy in our study correlates with the tumor's location, size, and the era of surgical advancements. We focused on smaller, peripherally located tumors for VATS, which coincides with the period when thoracic surgery was transitioning to the minimally invasive technique of VATS. This change overlaps with the time our study was conducted, reflecting the growing proficiency and preference for VATS among surgeons as their familiarity with the technique increased. In our practice, all patients undergo preoperative pulmonary function testing. The surgical indications for bilateral pulmonary metastasectomy are as follows: When the physician assesses that both sides of the pulmonary lesions can potentially be completely resected through staged surgeries, and the patient's lung function is evaluated to be sufficient to undergo surgeries on both sides. Our data indicate that the overall survival (OS) of patients who undergo bilateral pulmonary resections does not show significant differences compared to those with unilateral pulmonary metastases, except in cases of single-lung metastases, where a statistically significant difference was observed. However, since most of the procedures were performed using VATS, there was no notable difference in postoperative recovery time (hospital stay) or the patients’ ability to return to daily activities. All patients were able to walk and return to outpatient follow-ups after discharge.
In line with previous research, the most common histological subtype in our study was leiomyosarcoma (23.6%) (10, 35, 36). However, the frequency of this subtype was lower than that reported by some previous studies, possibly owing to the different study populations and geographical differences in sarcoma subtypes (37).
An important finding from this study was that a disease-free interval of >12 months post-lung surgery and the absence of local sarcoma recurrence at the time of lung metastasectomy were significantly associated with overall survival. These results are consistent with previous research, underscoring the importance of early detection and resection of lung metastases in improving the prognosis of sarcoma patients (35).
Our study further showed that 81.8% of surgeries achieved R0 resection, an ideal oncological outcome. This high rate of R0 resection may partly explain the higher survival rate observed in our study compared to previous reports, as complete resection of metastatic lesions has been associated with long-term survival (35). The majority of our surgeries were performed using minimal invasive surgery (VATS), which resulted in a shorter hospital stay and better postoperative recovery for most patients (38).
With the advancements in immunotherapy, particularly immune checkpoint inhibitors, there is emerging evidence supporting its use in specific sarcoma subtypes (39). While our study primarily focuses on surgical treatment, we acknowledge the potential for combining surgery with immunotherapy to enhance outcomes for sarcoma patients.
However, our study has several limitations. It is a single-arm, single-center retrospective study, which may limit the generalizability of our findings. The retrospective nature of the study may have led to some data loss, and the lack of randomization could have introduced selection bias. Furthermore, the study was conducted in a single center, which may have limited the diversity of the patient population and the applicability of the results to other settings.
5 ConclusionsPatients who underwent VATS minimally invasive pulmonary metastasectomy showed good long-term survival rates. In addition, statistical results on 110 samples showed that several important factors including disease-free interval after lung surgery exceeding 12 months, no local sarcoma recurrence during lung metastasis resection, and two or more tuberculosis metastases to the lungs significantly affect the survival rate of patients undergoing surgery.
Furthermore, while this study contributes valuable insights into the clinical characteristics and outcomes of patients with pulmonary metastatic sarcoma undergoing metastasectomy, further multi-center studies with larger sample sizes are needed to validate these findings. Moreover, future research should investigate the potential role of adjuvant therapies and personalized medicine approaches in improving the outcomes of these patients.
Data availability statementThe datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statementThe studies involving humans were approved by The Research Ethics Committee of National Taiwan University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the study involves retrospective medical record analysis, and we have applied for an exemption from informed consent, so signing a consent form is not required.
Author contributionsC-HC: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. X-HC: Formal Analysis, Methodology, Resources, Software, Writing – review & editing. M-WL: Data curation, Methodology, Resources, Writing – review & editing. S-WK: Data curation, Methodology, Resources, Writing – review & editing. P-MH: Data curation, Methodology, Resources, Writing – review & editing. H-HH: Conceptualization, Data curation, Investigation, Methodology, Resources, Supervision, Validation, Writing – review & editing. J-SC: Conceptualization, Methodology, Resources, Supervision, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from National Taiwan University Hospital (MS441) to HHH.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Wigge S, Heißner K, Steger V, Ladurner R, Traub F, Sipos B, et al. Impact of surgery in patients with metastatic soft tissue sarcoma: a monocentric retrospective analysis. J Surg Oncol. (2018) 118:167–76. doi: 10.1002/jso.25115
PubMed Abstract | Crossref Full Text | Google Scholar
2. Harati K, Goertz O, Pieper A, Daigeler A, Joneidi-Jafari H, Niggemann H, et al. Soft tissue sarcomas of the extremities: surgical margins can be close as long as the resected tumor has no ink on it. Oncologist. (2017) 22:1400–10. doi: 10.1634/theoncologist.2016-0498
PubMed Abstract | Crossref Full Text | Google Scholar
3. Callegaro D, Miceli R, Bonvalot S, Ferguson PC, Strauss DC, van Praag VV, et al. Development and external validation of a dynamic prognostic nomogram for primary extremity soft tissue sarcoma survivors. EClinicalmedicine. (2019) 17:100215. doi: 10.1016/j.eclinm.2019.11.008
PubMed Abstract | Crossref Full Text | Google Scholar
4. Brennan MF, Antonescu CR, Moraco N, Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. (2014) 260:416–21. doi: 10.1097/SLA.0000000000000869
PubMed Abstract | Crossref Full Text | Google Scholar
5. Gamboa AC, Ethun CG, Switchenko JM, Lipscomb J, Poultsides GA, Grignol V, et al. Lung surveillance strategy for high-grade soft tissue sarcomas: chest x-ray or CT scan? J Am Coll Surg. (2019) 229:449–57. doi: 10.1016/j.jamcollsurg.2019.07.010
PubMed Abstract | Crossref Full Text | Google Scholar
6. Tierney JF, Stewart LA, Parmar MKB. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma meta-analysis collaboration. Lancet. (1997) 350:1647–54. doi: 10.1016/S0140-6736(97)08165-8
PubMed Abstract | Crossref Full Text | Google Scholar
7. Iqbal N, Shukla NK, Deo SV, Agarwala S, Sharma DN, Sharma MC, et al. Prognostic factors affecting survival in metastatic soft tissue sarcoma: an analysis of 110 patients. Clin Transl Oncol. (2016) 18:310–6. doi: 10.1007/s12094-015-1369-9
PubMed Abstract | Crossref Full Text | Google Scholar
8. Bedi M, King DM, Charlson J, Whitfield R, Hackbarth DA, Zambrano EV, et al. Multimodality management of metastatic patients with soft tissue sarcomas may prolong survival. Am J Clin Oncol. (2014) 37:272–7. doi: 10.1097/COC.0b013e318277d7e5
PubMed Abstract | Crossref Full Text | Google Scholar
9. van der Graaf WT, Orbach D, Judson IR, Ferrari A. Soft tissue sarcomas in adolescents and young adults: a comparison with their paediatric and adult counterparts. Lancet Oncol. (2017) 18:e166–75. doi: 10.1016/S1470-2045(17)30099-2
PubMed Abstract | Crossref Full Text | Google Scholar
10. Marulli G, Mammana M, Comacchio G, Rea F. Survival and prognostic factors following pulmonary metastasectomy for sarcoma. J Thorac Dis. (2017) 9:S1305–15. doi: 10.21037/jtd.2017.03.177
PubMed Abstract | Crossref Full Text | Google Scholar
11. Stojadinovic A, Leung DH, Hoos A, Jaques DP, Lewis JJ, Brennan MF. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg. (2002) 235:424–34. doi: 10.1097/00000658-200203000-00015
PubMed Abstract | Crossref Full Text | Google Scholar
12. Ueda T, Uchida A, Kodama K, Doi O, Nakahara K, Fujii Y, et al. Aggressive pulmonary metastasectomy for soft tissue sarcomas. Cancer. (1993) 72:1919–25.<1919::AID-CNCR2820720621>3.0.CO;2-D8364869
PubMed Abstract | Google Scholar
13. Espat NJ, Bilsky M, Lewis JJ, Leung D, Brennan MF. Soft tissue sarcoma brain metastases. Prevalence in a cohort of 3829 patients. Cancer. (2002) 94:2706–11. doi: 10.1002/cncr.10554
PubMed Abstract | Crossref Full Text | Google Scholar
14. Judson I, Verweij J, Gelderblom H, Hartmann JT, Schöffski P, Blay JY, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. (2014) 15:415–23. doi: 10.1016/S1470-2045(14)70063-4
PubMed Abstract | Crossref Full Text | Google Scholar
15. Yamamoto Y, Kanzaki R, Kanou T, Ose N, Funaki S, Shintani Y, et al. Long-term outcomes and prognostic factors of pulmonary metastasectomy for osteosarcoma and soft tissue sarcoma. Int J Clin Oncol. (2019) 24:863–70. doi: 10.1007/s10147-019-01422-0
PubMed Abstract | Crossref Full Text | Google Scholar
16. Mizuno T, Taniguchi T, Ishikawa Y, Kawaguchi K, Fukui T, Ishiguro F, et al. Pulmonary metastasectomy for osteogenic and soft tissue sarcoma: who really benefits from surgical treatment? Eur J Cardiothorac Surg. (2013) 43:795–9. doi: 10.1093/ejcts/ezs419
PubMed Abstract | Crossref Full Text | Google Scholar
17. Dear RF, Kelly PJ, Wright GM, Stalley P, McCaughan BC, Tattersall MH. Pulmonary metastasectomy for bone and soft tissue sarcoma in Australia: 114 patients from 1978 to 2008. Asia Pac J Clin Oncol. (2012) 8:292–302. doi: 10.1111/j.1743-7563.2012.01521.x
PubMed Abstract | Crossref Full Text | Google Scholar
18. Matsuoka M, Onodera T, Yokota I, Iwasaki K, Matsubara S, Hishimura R, et al. Surgical resection of primary tumor in the extremities improves survival for metastatic soft-tissue sarcoma patients: a population-based study of the SEER database. Clin Transl Oncol. (2021) 23:2474–81. doi: 10.1007/s12094-021-02646-1
PubMed Abstract | Crossref Full Text | Google Scholar
19. Lindner LH, Litière S, Sleijfer S, Benson C, Italiano A, Kasper B, et al. Prognostic factors for soft tissue sarcoma patients with lung metastases only who are receiving first-line chemotherapy: an exploratory, retrospective analysis of the European organization for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC-STBSG). Int J Cancer. (2018) 142:2610–20. doi: 10.1002/ijc.31286
PubMed Abstract | Crossref Full Text | Google Scholar
20. Italiano A, Mathoulin-Pelissier S, Cesne AL, Terrier P, Bonvalot S, Collin F, et al. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer. (2011) 117:1049–54. doi: 10.1002/cncr.25538
PubMed Abstract | Crossref Full Text | Google Scholar
21. Bölükbas S, Sponholz S, Kudelin N, Eberlein M, Schirren J. Risk factors for lymph node metastases and prognosticators of survival in patients undergoing pulmonary metastasectomy for colorectal cancer. Ann Thorac Surg. (2014) 97:1926–32. doi: 10.1016/j.athoracsur.2014.02.026
PubMed Abstract | Crossref Full Text | Google Scholar
22. Renaud S, Alifano M, Falcoz PE, Magdeleinat P, Santelmo N, Pagès O, et al. Does nodal status influence survival? Results of a 19-year systematic lymphadenectomy experience during lung metastasectomy of colorectal cancer. Interact Cardiovasc Thorac Surg. (2014) 18:482–7. doi: 10.1093/icvts/ivt554
PubMed Abstract | Crossref Full Text | Google Scholar
23. Zampino MG, Maisonneuve P, Ravenda PS, Magni E, Casiraghi M, Solli P, et al. Lung metastases from colorectal cancer: analysis of prognostic factors in a single institution study. Ann Thorac Surg. (2014) 98:1238–45. doi: 10.1016/j.athoracsur.2014.05.048
PubMed Abstract | Crossref Full Text | Google Scholar
24. Sun F, Chen L, Shi M, Yang X, Li M, Yang X, et al. Prognosis of video-assisted thoracoscopic pulmonary metastasectomy in patients with colorectal cancer lung metastases: an analysis of 154 cases. Int J Colorectal Dis. (2017) 32:897–905. doi: 10.1007/s00384-017-2768-x
留言 (0)