There has been rapid growth of interest over the past decade in the use of artificial intelligence (AI) in the field of surgery to perform data-driven tasks efficiently and ultimately improve patient care (1). Machine learning (ML) is a division of AI which learns from large datasets and algorithms to provide personalized analysis and predictions. In visceral surgery, applications of ML in surgery include optimization of patients and resources, intraoperative analysis and feedback, and prediction of postoperative complications.
Despite the increase in research and demonstration of application on ML in visceral surgery, there remains a challenge in implementation. Numerous factors play a role in this dilemma including ML training, validating and testing of quality data, model selection, implementation of appropriate resources and infrastructure for ML, along with ethical and professional acceptance (2).
In this mini review, we will assess current literature on application of ML in visceral surgery in the preoperative, intraoperative, and postoperative settings (Table 1).
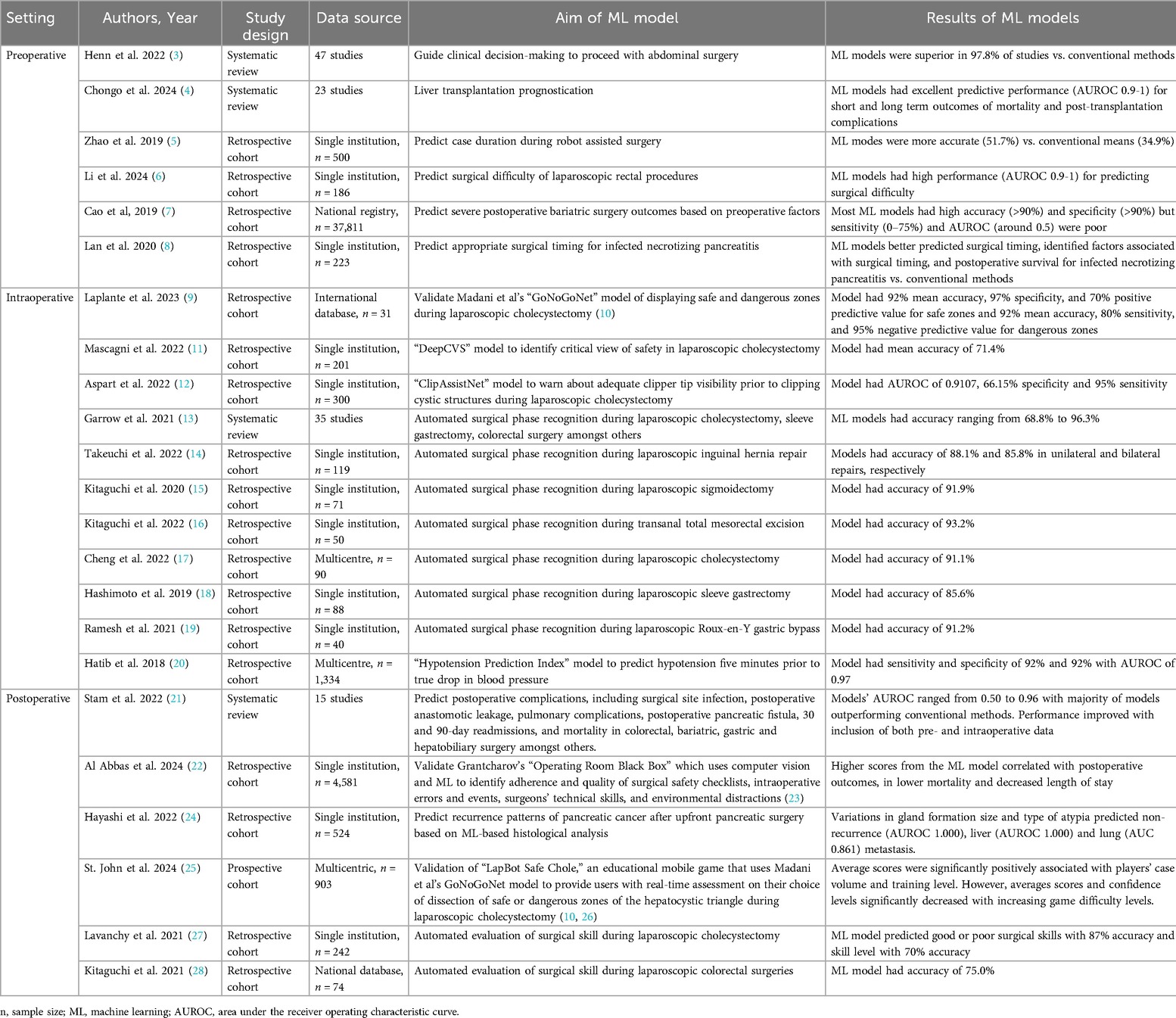
Table 1. Summary of studies on application of machine learning in visceral surgery in the preoperative, intraoperative, and postoperative settings.
Preoperative applicationsPrior to a patient's operation, numerous complex factors are deliberated by surgeons to deliver safe, quality and evidence-based care, in an efficient manner. Information such as indication for and timing of surgery, risk calculation, prognostication, time and resources required are some of these factors. Use of ML has the potential to more efficiently and effectively process the data required to advise about these factors.
Henn et al. evaluated the value of ML on guiding clinical decision-making to proceed with abdominal surgery, by conducting a systematic review of 47 articles with mean number of 55,843 patients (3). Surgical domains included the breadth of visceral general surgery including colorectal, surgical oncology, bariatric, and hepatobiliary surgery. Studies looked at predicting risk or benefit of procedures using ML vs. conventional decision-making. Standard measures of clinical decision making such as scores and tests, logistic regression, expert opinion and Cox regression were used to compare ML to traditional decision-making. 97.8% of the studies demonstrated ML to be superior to conventional methods in guiding clinical decision-making to offer surgery. They suggest that ML can be used to offer more personalized care, decrease costs by targeting high risk patients for prehabilitation and focused perioperative care, and that area under the receiver operating characteristic (AUROC) be used for ML model evaluation.
Another application of ML in the preoperative setting is prognostication. Chongo et al. studied ML use for prognostication of liver transplantation in a systematic review (4). 23 articles were included in which the ML models, using pre-transplant data, outperformed traditional scoring systems such as the Model for End-stage Liver Disease (MELD) and Child-Turcotte-Pugh scores consistently. Primary outcomes were mortality and post-transplant complications, in which AUROC demonstrated ML models’ excellent predictive performance (AUROC 0.9-1) for both short and long term outcomes. They conclude with highlighting the potential of ML models to optimize organ allocation, improve patient outcomes and decrease healthcare costs.
Furthermore, Zhao et al. used ML to predict case duration during robot assisted surgery (5). They performed a retrospective cohort analysis of 500 elective robot assisted surgeries of primarily abdominal visceral procedures at a single institution. 28 variables were selected for model building including patient age, obesity, tumor location, time of day, and surgeon postgraduate year, amongst others. Primary outcome was scheduled case duration as predicted by the ML model vs. the conventional system. All ML models including multivariable linear regression, ridge regression, lasso regression, random forest, boosted regression tree, and neural network decreased the average root-mean-squared error when compared to the baseline model. ML predicted durations were accurate 51.7% of the time compared to the traditional system's 34.9%. They highlight that ML can improve the accuracy of robot assisted case length predictions, to help improve utilization of scarce resources.
Li et al. explored ML in the preoperative setting by developing models for predicting surgical difficulty of laparoscopic rectal cancer resection (6). Surgical difficulty was scored based on duration of surgery, conversion to open procedure, postoperative stay >14 days, visceral fat area, body surface area and morbidities. 186 patients were included from a single centre institution. Four ML models were developed and validated, utilizing support vector machine, random forest, logistic regression, and decision tree. All models showed high performance using AUROC (0.9-1). Thus, the authors propose ML models can assist surgeons evaluate surgical difficulty preoperatively and accordingly make treatment decisions and resource allocations.
Cao et al. compared supervised ML models to predict severe postoperative bariatric surgery complications (7) based on preoperative factors. The ML algorithms were trained using 37,811 bariatric surgery patients from the Scandinavian Obesity Surgery Registry from 2010 to 2014, and validated on 6,250 patients in 2015. Preoperative data points such as age, BMI, year of operation, HbA1c, and obesity-related comorbidities were included in the ML models. 29 ML algorithms were studied including deep learning neural network, k-nearest neighbor, support vector machine, random forest, and adaptive boosting logistic regression to name a few. Synthetic minority oversampling technique was used to tackle the imbalanced data as only 3.2% of patients experienced severe postoperative bariatric surgery complications. Performance of the ML models were assessed using accuracy, sensitivity, specificity, and AUROC. Most of the ML algorithms demonstrated high accuracy (>90%) and specificity (>90%) but sensitivity (0%–75%) and AUROC (around 0.5) were poor for all models. They postulate this to the low incidence of postoperative bariatric surgery severe complications. They also note that studies that include information following the preoperative setting, such as intraoperative complications, show improved model performance. However, this is not useful for utilizing ML for preoperative risk calculations. Therefore, while potential is shown for the use of ML models in the preoperative setting in predicting severe postoperative complications, it is not clinically beneficial yet.
Lastly, ML has also shown potential in guiding appropriate timing of surgery. Lan et al. developed a ML model to predict timing of surgical intervention for infected necrotizing pancreatitis (8). A retrospective analysis was conducted on 223 patients in a single centre hospital. The ML models were logistic regression, support vector machine and random forest. Generative adversarial networks were used to generate simulated samples to overcome the small sample size. Compared to traditional models, the ML models better predicted surgical timing, as well as identified factors associated with surgical timing, and postoperative survival for infected necrotizing pancreatitis. They conclude stating that ML can provide good references for surgeons to make personalized surgical plans for infected necrotizing pancreatitis patients.
Intraoperative applicationsAlong with providing valuable and personalized data in the preoperative setting, ML has also been demonstrated to have application in the intraoperative setting, which is particularly significant as the source of most adverse events for surgical patients can be traced back to intraoperative events (10). Areas that have shown promise are visual annotation of the surgical field, automated surgical phase recognition and prediction of intraoperative patient decompensation. This information can provide the surgical team with important information to optimize procedures, surgical training and ultimately provide more effective and safe care to patients.
Madani et al. created an AI model, GoNoGoNet, to visually display safe “Go” and dangerous “No Go” zones during laparoscopic cholecystectomy. This was generated using deep neural networks, a subset of AI algorithms that use machine learning (10). Laplante et al. validated GoNoGoNet with expert high volume surgeons from the Society of American Gastrointestinal and Endoscopic Surgeons’ (SAGES) Safe Cholecystectomy Task Force (9). They found the AI model to have 92% mean accuracy, 97% specificity, and 70% positive predictive value for safe zones and 92% mean accuracy, 80% sensitivity, and 95% negative predictive value for dangerous zones. Deep neural network modeling has also been used by Mascagni et al. who created a model, DeepCVS, to identify the critical view of safety in laparoscopic cholecystectomy with a mean accuracy of 71.4% (11). Another intraoperative use of machine learning in laparoscopic cholecystectomy is warning surgeons about adequate clipper tip visibility prior to clipping the cystic duct and artery. This model, ClipAssistNet, was also generated using deep neural networking by Aspart et al, which had a AUROC of 0.9107, 66.15% specificity and 95% sensitivity (12). Thus, these models may eventually be implemented for real-time intraoperative guidance to decrease the risk of bile duct injury during laparoscopic cholecystectomy.
Moreover, Garrow et al. assessed ML models for automated surgical phase recognition through a systematic review with 35 articles including laparoscopic cholecystectomy, sleeve gastrectomy, and colorectal surgery (13). The most common ML models used were Hidden Markov Model and Artificial Neural Network. Accuracy of the models ranged from 68.8% to 96.3%. Despite the majority of studies focusing on laparoscopic cholecystectomy, there was significant heterogeneity in the number of phases and definitions of phases, along with ML models used, precluding accurate comparison of studies. Interestingly, complexity of models and those used in more recent studies did not affect accuracy. Takeuchi et al. similarly looked at surgical phase recognition of laparoscopic inguinal hernia repair using deep learning models, displaying accuracy of 88.1% and 85.8% in unilateral and bilateral repairs, respectively (14). Kitaguchi and his team specifically studied automated phase recognition in laparoscopic sigmoidectomy and transanal total mesorectal excision using deep learning models with accuracies of 91.9% and 93.2%, respectively (15, 16). Cheng et al. likewise explored surgical phase recognition of laparoscopic cholecystectomy using deep learning models, and showed accuracy of 91.1% (17). Bariatric surgery automatic surgical phase recognition has also been studied. Hashimoto et al.'s deep neural based model showed 85.6% accuracy in laparoscopic sleeve gastrectomy (18). Ramesh et al. used multitask multi-stage temporal convolutional networks to automate phase recognition in laparoscopic Roux-en-Y gastric bypass with accuracy up to 91.2% (19). These studies conclude that automated surgical phase recognition is an emerging field and recommend data collection be compatible for ML analysis and standardized surgical phase definitions.
Another use of ML for intraoperative application is prediction of intraoperative decompensation. Intraoperative hypotension is associated with postoperative complications including higher mortality, acute kidney injury and myocardial infarction (29–33). Consequently, Hatib et al. developed an algorithm, the Hypotension Prediction Index, using machine learning to predict hypotension five minutes prior to actual drop in blood pressure. This was created using arterial waveform data of 1,344 patients, with a sensitivity and specificity of 92% and 92% with AUROC of 0.97 (20). The algorithm was validated in a randomized control trial along with a treatment protocol by Wijnberge et al. (34). Gastrointestinal, pancreas, esophagus, and gynecology procedures were included. They demonstrated a median of 16.7 min less of intraoperative hypotension for patients in the intervention group that used the Hypotension Prediction Index and treatment protocol compared to the control group. Therefore, this ML based model for prediction and prevention of intraoperative hypotension has the potential to decrease postoperative complications.
Postoperative applicationsLastly, ML has been studied the most in the postoperative environment. Areas of interest are prediction of postoperative complications, recurrence patterns of malignancy, surgical education and automated surgical skill assessment. These applications have the prospect of providing patients with prevention and earlier management of complications, enhanced treatment after oncologic surgeries, and improved training and feedback for surgical trainees.
Numerous prediction models using ML have been developed for postoperative complications, particularly in the last few years (2). The range of visceral surgery including colorectal, bariatric, gastric and hepatobiliary has been studied. Surgical site infection, postoperative anastomotic leakage, pulmonary complications, postoperative pancreatic fistula, 30 and 90-day readmissions, and mortality are some of the complications that have been analyzed (1, 2, 21, 35–39). ML generally outperformed conventional logistic regression models (2). Performance can be improved by inclusion of both pre- and intraoperative data, instead of just one, along with both structured and unstructured data. One such intraoperative platform is Grantcharov's Operating Room Black Box platform which uses multimodal audiovisual data that is analyzed through computer vision and machine learning to identify adherence and quality of surgical safety checklists, intraoperative errors and events, surgeons’ technical skills, and environmental distractions (23). The ML-driven analysis from this platform has been demonstrated to correlate with postoperative outcomes. For example, Al Abbas et al. assessed surgical teams’ quality of surgical safety checklist performance, based on the scores output by the Operating Room Black Box, to postoperative outcomes (22). Higher scoring teams’ patients correlated with lower mortality and decreased length of stay. Therefore, these ML-based prediction models can foster improved and individualized management of patients’ complications in the postoperative period.
Hayashi et al. assessed a different aspect of postoperative care, in predicting recurrence patterns of pancreatic cancer after upfront pancreatic surgery (24). They presented a retrospective, single-centre study, with 524 patients in which histology-based supervised ML was used to predict recurrence patterns of pancreatic cancer. Variations in gland formation size and type of atypia predicted non-recurrence (AUROC 1.000), liver (AUROC 1.000) and lung (AUROC 0.861) metastasis. This information may lead to earlier, personalized chemotherapy for these patients.
ML has also been used to promote surgical education as demonstrated by Noroozi et al.'s LapBot Safe Chole, an educational mobile game that uses AI to provide users with real-time assessment on their choice of dissection of safe or dangerous zones of the hepatocystic triangle during laparoscopic cholecystectomy (26). The application uses Madani et al.'s deep neural network based GoNoGoNet model to provide feedback (10). St John et al. validated the study with 903 participants from 64 countries (25). Average scores were significantly positively associated with players’ case volume and training level. Meanwhile, averages scores and confidence levels significantly decreased with increasing game difficulty levels. This suggests that surgical education games can be an effective adjunct tool to offer practice and coaching for trainees.
Another potential of ML postoperatively is automated evaluation of surgical skill, which Lavanchy et al. assessed with laparoscopic cholecystectomy (27). 242 videos were used for annotation in the study. They developed a three-stage ML model to assess surgeon ability, based on surgical instrument handling. The premise being more skilled surgeons handle instruments in a focused area while less skilled surgeons have slow, trembling motions with repetitive direction changes and greater areas of movement. The three stages are instrument detection and localization, tracking motion, and skill prediction based on calculated motion metrics. Prediction skills ratings were correlated with expert ratings. The ML model was able to predict good or poor surgical skills with 87% accuracy and skill level with 70% accuracy. Similarly, Kitaguchi et al. also developed an automated surgical skill assessment tool for laparoscopic colorectal surgeries. They used 3-dimensional convolutional neural networking to create the model which demonstrated mean accuracy of 75.0% (28). The authors of these studies advocate that with a larger training database and model refinement, an improved automated surgical skill evaluation is possible to ultimately help provide continuous objective feedback on surgical skills for surgeons.
Limitations and challenges going forwardDespite the scale of emerging data, there remains limitations and challenges in clinical implementation of ML in visceral surgery. The majority of this review's studies’ ML models were trained and validated internally with local institutional or organizational datasets as opposed to being validated externally. This can lead to potential biases and generalizability concerns to other patient populations. As well, most of the studies are observational (see Table 1), and thus, more statistically robust designs such as randomized control trials can be explored to validate the ML models in future studies. ML models in the studies that performed poorly were generally due to fewer modality sources of data and unstandardized and varying definitions of surgical steps or phases. As a result, there are less preoperative ML models compared to intraoperative and postoperative applications which can extract from more data points.
Quality and quantity of data affect ML performance. Vast amounts of data are required which may involve interdisciplinary regional, national, and international collaboration (40). The data is generally not standardized and requires immense time commitment to organize and annotate.
To help resolve this, the Global Surgical AI Collaborative has been established to offer surgeons access to large, international shared databases and an infrastructure to utilize this data (41). Federated learning, an encrypted and decentralized form of machine learning that allows data processing at remote physical locations to train local models which are then amalgamated into a final model. This increases data privacy and the possibility of greater data accessibility. Thus, models can be created collaboratively with other centres, mitigating the immense data annotation workload often required to create a single centre based ML model. Another method of decreasing the demand of data annotation is using coarser labels in which the content of a video sequence is categorized or qualitatively described instead of segmenting each frame (42). This method of labelling is less demanding, however may lead to less clinically relevant applications. Coarse labelling is suited for tasks such as navigation to specific points of videos as well as education by describing the contents of videos.
Secondly, appropriate resources and infrastructure to implement ML are necessary. Modifications to current electronic medical record systems are required to allow safe and real-time interaction between patient files and machine learning models (2). The ML models must also be trained and validated in the proposed healthcare facilities of installation to ensure accurate performance for that specific hospital. As well, incentivizing surgeons that participate in providing and annotating videos to standardized databases for AI video-based assessment, with compensation such as continuing medical education (CME) points, may increase the available data pool and improve ML models’ performances.
Lastly, ethical considerations and acceptance by patients and the surgical team are another barrier in the application of ML. To help address the ethical issues of using vast patient datasets, de Almeida et al. published a review and developed a framework for AI regulation, from legislation to research and development, with 21 guidelines (43). Moreover, in a systematic review of public perception on AI, patients generally viewed AI in positive light but still preferred to receive healthcare with human physician supervision over AI (44). Greater acceptance of AI was found if patients were given a choice between AI and provider, AI was applied in a low risk setting, AI was proven to be more accurate than providers, if physicians recommended AI, and if AI matched societal and cultural norms. While the evidence of ML in surgery is growing, surgeons are also weary of ML implementation without institution-specific validated models. It's important for surgeons to understand how ML works, help develop it, and then, push for its clinical application.
In summary, there is abundant potential of ML application in visceral surgery in the preoperative, intraoperative, and postoperative settings with the benefit of safer, more effective and higher quality patient care. Some challenges have created a gap between research findings and clinical implementation of ML which future studies should further address.
Author contributionsIH: Formal Analysis, Writing – original draft, Writing – review & editing. AM: Writing – original draft, Writing – review & editing. SL: Conceptualization, Writing – original draft, Writing – review & editing, Supervision.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestAM is a consultant for J&J.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Ravenel M, Joliat GR, Demartines N, Uldry E, Melloul E, Labgaa I. Machine learning to predict postoperative complications after digestive surgery: a scoping review. Br J Surg. (2023) 110(12):1646–9. doi: 10.1093/bjs/znad229
PubMed Abstract | Crossref Full Text | Google Scholar
2. Stam WT, Ingwersen EW, Ali M, Spijkerman JT, Kazemier G, Bruns ERJ, et al. Machine learning models in clinical practice for the prediction of postoperative complications after major abdominal surgery. Surg Today. (2023) 53(10):1209–15. doi: 10.1007/s00595-023-02662-4
PubMed Abstract | Crossref Full Text | Google Scholar
3. Henn J, Buness A, Schmid M, Kalff JC, Matthaei H. Machine learning to guide clinical decision-making in abdominal surgery—a systematic literature review. Langenbecks Arch Surg. (2022) 407(1):51–61. doi: 10.1007/s00423-021-02348-w
PubMed Abstract | Crossref Full Text | Google Scholar
4. Chongo G, Soldera J. Use of machine learning models for the prognostication of liver transplantation: a systematic review. World J Transplant. (2024) 14(1):88891. doi: 10.5500/wjt.v14.i1.88891
PubMed Abstract | Crossref Full Text | Google Scholar
5. Zhao B, Waterman RS, Urman RD, Gabriel RA. A machine learning approach to predicting case duration for robot-assisted surgery. J Med Syst. (2019) 43(2):32. doi: 10.1007/s10916-018-1151-y
PubMed Abstract | Crossref Full Text | Google Scholar
6. Li X, Zhou Z, Zhu B, Wu Y, Xing C. Development and validation of machine learning models and nomograms for predicting the surgical difficulty of laparoscopic resection in rectal cancer. World J Surg Oncol. (2024) 22(1):111. doi: 10.1186/s12957-024-03389-3
PubMed Abstract | Crossref Full Text | Google Scholar
7. Cao Y, Fang X, Ottosson J, Näslund E, Stenberg E. A comparative study of machine learning algorithms in predicting severe complications after bariatric surgery. J Clin Med. (2019) 8(5):668. doi: 10.3390/jcm8050668
PubMed Abstract | Crossref Full Text | Google Scholar
8. Lan L, Guo Q, Zhang Z, Zhao W, Yang X, Lu H, et al. Classification of infected necrotizing pancreatitis for surgery within or beyond 4 weeks using machine learning. Front Bioeng Biotechnol. (2020) 8:1–8. doi: 10.3389/fbioe.2020.00541
PubMed Abstract | Crossref Full Text | Google Scholar
9. Laplante S, Namazi B, Kiani P, Hashimoto DA, Alseidi A, Pasten M, et al. Validation of an artificial intelligence platform for the guidance of safe laparoscopic cholecystectomy. Surg Endosc. (2023) 37(3):2260–8. doi: 10.1007/s00464-022-09439-9
PubMed Abstract | Crossref Full Text | Google Scholar
10. Madani A, Namazi B, Altieri MS, Hashimoto DA, Rivera AM, Pucher PH, et al. Artificial intelligence for intraoperative guidance: using semantic segmentation to identify surgical anatomy during laparoscopic cholecystectomy. Ann Surg. (2022) 276(2):363–9. doi: 10.1097/SLA.0000000000004594
PubMed Abstract | Crossref Full Text | Google Scholar
11. Mascagni P, Vardazaryan A, Alapatt D, Urade T, Emre T, Fiorillo C, et al. Artificial intelligence for surgical safety: automatic assessment of the critical view of safety in laparoscopic cholecystectomy using deep learning. Ann Surg. (2022) 275(5):955–61. doi: 10.1097/SLA.0000000000004351
PubMed Abstract | Crossref Full Text | Google Scholar
12. Aspart F, Bolmgren JL, Lavanchy JL, Beldi G, Woods MS, Padoy N, et al. Clipassistnet: bringing real-time safety feedback to operating rooms. Int J Comput Assist Radiol Surg. (2022) 17(1):5–13. doi: 10.1007/s11548-021-02441-x
PubMed Abstract | Crossref Full Text | Google Scholar
13. Garrow CR, Kowalewski KF, Li L, Wagner M, Schmidt MW, Engelhardt S, et al. Machine learning for surgical phase recognition: a systematic review. Ann Surg. (2021) 273(4):684–93. doi: 10.1097/SLA.0000000000004425
PubMed Abstract | Crossref Full Text | Google Scholar
14. Takeuchi M, Collins T, Ndagijimana A, Kawakubo H, Kitagawa Y, Marescaux J, et al. Automatic surgical phase recognition in laparoscopic inguinal hernia repair with artificial intelligence. Hernia. (2022) 26(6):1669–78. doi: 10.1007/s10029-022-02621-x
PubMed Abstract | Crossref Full Text | Google Scholar
15. Kitaguchi D, Takeshita N, Matsuzaki H, Takano H, Owada Y, Enomoto T, et al. Real-time automatic surgical phase recognition in laparoscopic sigmoidectomy using the convolutional neural network-based deep learning approach. Surg Endosc. (2020) 34(11):4924–31. doi: 10.1007/s00464-019-07281-0
PubMed Abstract | Crossref Full Text | Google Scholar
16. Kitaguchi D, Takeshita N, Matsuzaki H, Hasegawa H, Igaki T, Oda T, et al. Deep learning-based automatic surgical step recognition in intraoperative videos for transanal total mesorectal excision. Surg Endosc. (2022) 36(2):1143–51. doi: 10.1007/s00464-021-08381-6
PubMed Abstract | Crossref Full Text | Google Scholar
17. Cheng K, You J, Wu S, Chen Z, Zhou Z, Guan J, et al. Artificial intelligence-based automated laparoscopic cholecystectomy surgical phase recognition and analysis. Surg Endosc. (2022) 36(5):3160–8. doi: 10.1007/s00464-021-08619-3
PubMed Abstract | Crossref Full Text | Google Scholar
18. Hashimoto DA, Rosman G, Witkowski ER, Stafford C, Navarette-Welton AJ, Rattner DW, et al. Computer vision analysis of intraoperative video: automated recognition of operative steps in laparoscopic sleeve gastrectomy. Ann Surg. (2019) 270(3):414–21. doi: 10.1097/SLA.0000000000003460
PubMed Abstract | Crossref Full Text | Google Scholar
19. Ramesh S, Dall’Alba D, Gonzalez C, Yu T, Mascagni P, Mutter D, et al. Multi-task temporal convolutional networks for joint recognition of surgical phases and steps in gastric bypass procedures. Int J CARS. (2021) 16(7):1111–9. doi: 10.1007/s11548-021-02388-z
PubMed Abstract | Crossref Full Text | Google Scholar
20. Hatib F, Jian Z, Buddi S, Lee C, Settels J, Sibert K, et al. Machine-learning algorithm to predict hypotension based on high-fidelity arterial pressure waveform analysis. Anesthesiology. (2018) 129(4):663–74. doi: 10.1097/ALN.0000000000002300
PubMed Abstract | Crossref Full Text | Google Scholar
21. Stam WT, Goedknegt LK, Ingwersen EW, Schoonmade LJ, Bruns ERJ, Daams F. The prediction of surgical complications using artificial intelligence in patients undergoing major abdominal surgery: a systematic review. Surgery. (2022) 171(4):1014–21. doi: 10.1016/j.surg.2021.10.002
PubMed Abstract | Crossref Full Text | Google Scholar
22. Al Abbas AI, Meier J, Daniel W, Cadeddu JA, Bartolome S, Willett DL, et al. Impact of team performance on the surgical safety checklist on patient outcomes: an operating room black box analysis. Surg Endosc. (2024) 38:5613–22. doi: 10.1007/s00464-024-11064-7
PubMed Abstract | Crossref Full Text | Google Scholar
24. Hayashi K, Ono Y, Takamatsu M, Oba A, Ito H, Sato T, et al. Prediction of recurrence pattern of pancreatic cancer post-pancreatic surgery using histology-based supervised machine learning algorithms: a single-center retrospective study. Ann Surg Oncol. (2022) 29:4624–34. doi: 10.1245/s10434-022-11471-x
Crossref Full Text | Google Scholar
25. John A S, Khalid MU, Masino C, Noroozi M, Alseidi A, Hashimoto DA, et al. LapBot-Safe chole: validation of an artificial intelligence-powered mobile game app to teach safe cholecystectomy. Surg Endosc. (2024) 38:5274–84. doi: 10.1007/s00464-024-11068-3
PubMed Abstract | Crossref Full Text | Google Scholar
26. Noroozi M, St John A, Masino C, Laplante S, Hunter J, Brudno M, et al. Education in laparoscopic cholecystectomy: design and feasibility study of the LapBot safe chole Mobile game. JMIR Form Res. (2024) 8:e52878. doi: 10.2196/52878
PubMed Abstract | Crossref Full Text | Google Scholar
27. Lavanchy JL, Zindel J, Kirtac K, Twick I, Hosgor E, Candinas D, et al. Automation of surgical skill assessment using a three-stage machine learning algorithm. Sci Rep. (2021) 11(1):5197. doi: 10.1038/s41598-021-84295-6
PubMed Abstract | Crossref Full Text | Google Scholar
28. Kitaguchi D, Takeshita N, Matsuzaki H, Igaki T, Hasegawa H, Ito M. Development and validation of a 3-dimensional convolutional neural network for automatic surgical skill assessment based on spatiotemporal video analysis. JAMA Network Open. (2021) 4(8):e2120786. doi: 10.1001/jamanetworkopen.2021.20786
PubMed Abstract | Crossref Full Text | Google Scholar
29. Bijker JB, van Klei WA, Vergouwe Y, Eleveld DJ, van Wolfswinkel L, Moons KGM, et al. Intraoperative hypotension and 1-year mortality after noncardiac surgery. Anesthesiology. (2009) 111(6):1217–26. doi: 10.1097/ALN.0b013e3181c14930
PubMed Abstract | Crossref Full Text | Google Scholar
30. Monk TG, Bronsert MR, Henderson WG, Mangione MP, Sum-Ping STJ, Bentt DR, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. (2015) 123(2):307–19. doi: 10.1097/ALN.0000000000000756
PubMed Abstract | Crossref Full Text | Google Scholar
31. Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. (2015) 123(3):515–23. doi: 10.1097/ALN.0000000000000765
PubMed Abstract | Crossref Full Text | Google Scholar
32. Hallqvist L, Granath F, Huldt E, Bell M. Intraoperative hypotension is associated with acute kidney injury in noncardiac surgery: an observational study. Eur J Anaesthesiol. (2018) 35(4):273. doi: 10.1097/EJA.0000000000000735
PubMed Abstract | Crossref Full Text | Google Scholar
33. Xu L, Yu C, Jiang J, Zheng H, Yao S, Pei L, et al. Major adverse cardiac events in elderly patients with coronary artery disease undergoing noncardiac surgery: a multicenter prospective study in China. Arch Gerontol Geriatr. (2015) 61(3):503–9. doi: 10.1016/j.archger.2015.07.006
PubMed Abstract | Crossref Full Text | Google Scholar
34. Wijnberge M, Geerts BF, Hol L, Lemmers N, Mulder MP, Berge P, et al. Effect of a machine learning–derived early warning system for intraoperative hypotension vs standard care on depth and duration of intraoperative hypotension during elective noncardiac surgery: the HYPE randomized clinical trial. JAMA. (2020) 323(11):1052–60. doi: 10.1001/jama.2020.0592
PubMed Abstract | Crossref Full Text | Google Scholar
35. Merath K, Hyer JM, Mehta R, Farooq A, Bagante F, Sahara K, et al. Use of machine learning for prediction of patient risk of postoperative complications after liver, pancreatic, and colorectal surgery. J Gastrointest Surg. (2020) 24(8):1843–51. doi: 10.1007/s11605-019-04338-2
PubMed Abstract | Crossref Full Text | Google Scholar
36. Hyer JM, White S, Cloyd J, Dillhoff M, Tsung A, Pawlik TM, et al. Can we improve prediction of adverse surgical outcomes? Development of a surgical complexity score using a novel machine learning technique. J Am Coll Surg. (2020) 230(1):43–52.e1. doi: 10.1016/j.jamcollsurg.2019.09.015
PubMed Abstract | Crossref Full Text | Google Scholar
37. Xue Q, Wen D, Ji MH, Tong J, Yang JJ, Zhou CM. Developing machine learning algorithms to predict pulmonary complications after emergency gastrointestinal surgery. Front Med. (2021) 8:1–6. doi: 10.3389/fmed.2021.655686
Crossref Full Text | Google Scholar
38. Chen B, Sheng W, Wu Z, Ma B, Cao N, Li X, et al. Machine learning based peri-surgical risk calculator for abdominal related emergency general surgery: a multicenter retrospective study. Int J Surg. (2024) 110(6):3527–35. doi: 10.1097/JS9.0000000000001276
PubMed Abstract | Crossref Full Text | Google Scholar
39. Bihorac A, Ozrazgat-Baslanti T, Ebadi A, Motaei A, Madkour M, Pardalos PM, et al. Mysurgeryrisk: development and validation of a machine-learning risk algorithm for Major complications and death after surgery. Ann Surg. (2019) 269(4):652–62. doi: 10.1097/SLA.0000000000002706
PubMed Abstract | Crossref Full Text | Google Scholar
40. Khalid MU, Laplante S, Madani A. Machines with vision for intraoperative guidance during gastrointestinal cancer surgery. Front Med (Lausanne). (2022) 9:1025382. doi: 10.3389/fmed.2022.1025382
PubMed Abstract | Crossref Full Text | Google Scholar
41. Madani A, Hashimoto DA, Mascagni P, Alseidi A, Watanabe Y, Dingemans F, et al. Global Surgical AI Collaboration. Global Surgical AI Collaboration. Available online at: https://www.surgicalai.org (cited July 19, 2024).
留言 (0)