Blood-based MCED tests have the potential to detect multiple types of cancer, including uncommon cancers and those that lack standard-of-care screening options. Similar to standard-of-care cancer screening tests, a positive MCED test result provides a suspicion of cancer that requires a diagnostic evaluation to establish a definitive diagnosis. Several prospective studies investigating the clinical performance of MCED testing are underway. However, there are limited published data describing the diagnostic journey and outcomes of patients with positive MCED tests and pre-malignant findings following a clinical workup.
The DETECT-A study was the first prospective interventional trial to assess an MCED test, CancerSEEK, a multi-analyte assay that evaluated circulating proteins and cell-free DNA (cfDNA) mutations in the blood (1).
DETECT-A participants with positive baseline and confirmatory CancerSEEK tests underwent subsequent PET-CT with follow-up procedures, when clinically indicated, to confirm the presence and location of cancer.
Out of 134 participants with a positive CancerSEEK test, 26 were diagnosed with cancer. Of the 108 false-positive cases, 7 were adjudicated as false positive without PET-CT imaging, 98 were classified as false positive following PET-CT imaging, and three were diagnosed with premalignant conditions and are the focus of this report.
2 MethodsDETECT-A was an ongoing prospective, interventional study to evaluate the performance of CancerSEEK, an early version of the Exact Sciences MCED test currently in development. The study included 10,006 women 65 to 75 years of age with no personal history of cancer (1). The study was approved by the Institutional Review Boards (IRBs) for Human Research at the Geisinger Health System (Geisinger; #2017-0268) and the Johns Hopkins Medical Institutions (#00119844) and was compliant with U.S. Common Rule and The Health Insurance Portability and Accountability Act. Written, informed consent was obtained from all participants. Women with a current or previously known cancer were excluded from the study.
The DETECT-A study utilized a two-step MCED testing process for determining CancerSEEK positivity prior to a subsequent diagnostic evaluation. The multi-analyte CancerSEEK test incorporated the analysis of specific somatic mutations in cell free DNA (cfDNA) and the detection of nine cancer-associated protein biomarkers.
The cfDNA mutations were assessed using a sequencing error reduction technology (2) that was comprised of a panel designed to cover common oncogenic hot spot regions of 16 genes (represented by 61 amplicons). While specific mutations were considered when determining positive test results, there were no strict criteria established to differentiate between pathogenic variants and variants of uncertain significance (VUS). Therefore, the panel had the capacity to detect both known pathogenic variants and those of uncertain significance. The nine protein biomarkers included CA15-3, CEA, CA125, CA19-9, HGF, AFP, OPN, TIMP-1, and Prolactin.
A CancerSEEK confirmation test was performed only on participants with a positive baseline test. It employed the same sequencing error reduction technology and the high cfDNA mutation and protein biomarker thresholds used in the baseline test, but assessed only the specific cfDNA mutations or proteins that were abnormal in the baseline test. It also rigorously excluded cfDNA mutations attributed to clonal hematopoiesis of indeterminate potential (CHIP) through more thorough examination of a larger amount of white blood cell (WBC) cfDNA than what was used in the baseline CancerSEEK test. Technical details of the baseline and confirmation test components are provided in the Lennon, et al., study (1). A multidisciplinary review committee then reviewed the medical histories of cases with positive baseline and confirmatory CancerSEEK tests to rule out potential non-cancer-related conditions that could potentially result in a positive Cancer SEEK result.
3 Results3.1 Participant 1A 71-year-old asymptomatic participant with no identified comorbidities tested positive on the baseline and confirmatory CancerSEEK tests based on a substitution in the PIK3CA gene, NM_006218.4(PIK3CA):c.1030G>A (p.Val344Met) (Table 1). The Variant Allele Frequency (VAF) for the PIK3CA substitution was 1.146% in the baseline test and 1.36% in the confirmatory test. PET-CT imaging identified multiple pulmonary nodules accompanied by calcified granulomas and mild bronchiectasis, and a pelvic cystic mass. Bilateral lung nodules measuring 16, 11, 8, and 5 mm in greatest diameter were noted. The lung nodules remained stable on CT monitoring and were assessed to be inflammatory, rather than malignant. The pelvic mass, measuring 10.3 x 9.8 x 7.8 cm (Figure 1), was similarly monitored, with the patient eventually undergoing a laparoscopic left oophorectomy and salpingectomy 15.7 months after initial CancerSEEK testing, revealing a mucinous cystadenoma. The tissue was not available for mutational analysis. The participant remained alive and cancer-free 3 years and 5 months after enrollment, which marks the conclusion of participation due to voluntary withdrawal from study.
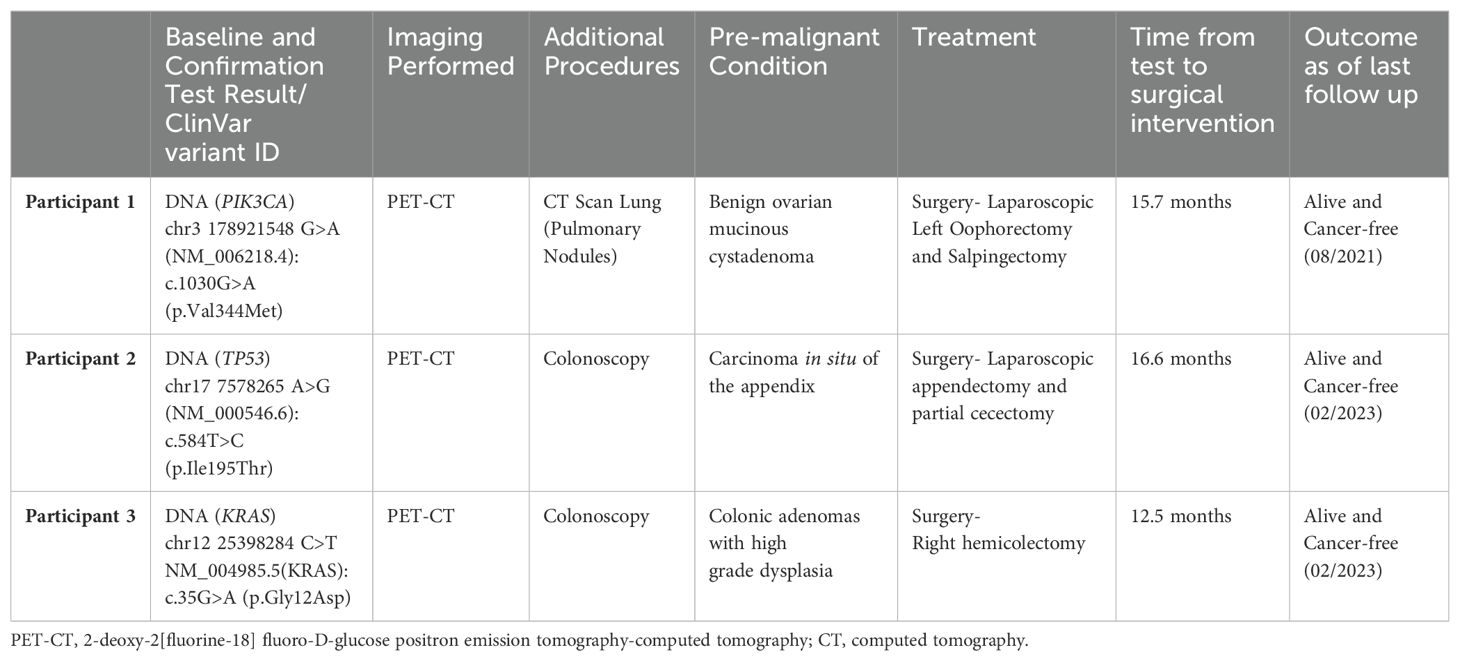
Table 1. Details of pre-cancerous findings detected by the CancerSEEK MCED blood test.
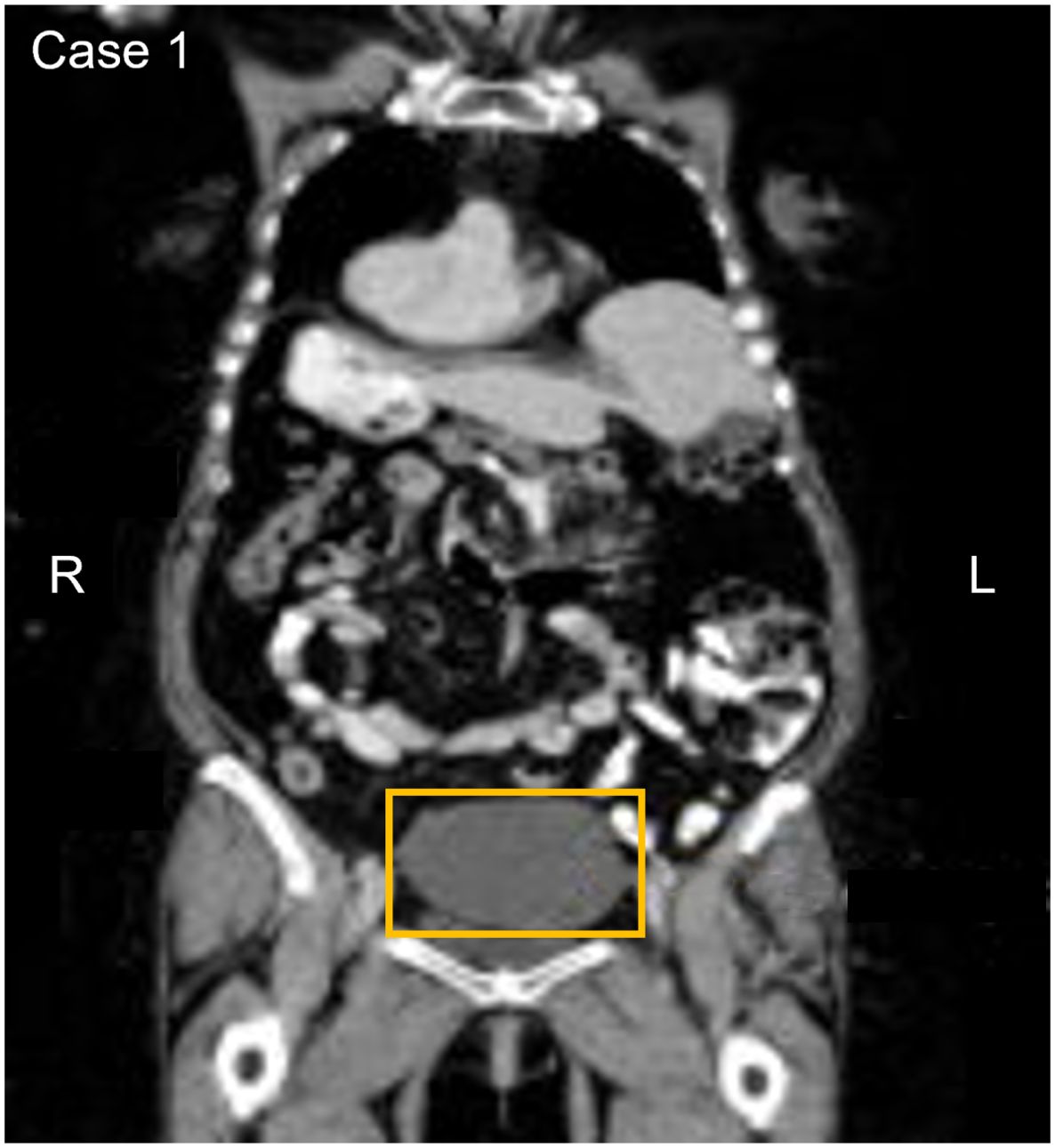
Figure 1. Participant one CT image. The area in the yellow box shows a large cystic mass in the pelvis measuring 10.3 x 9.8 x 7.8 cm.
3.2 Participant 2An asymptomatic 67-year-old participant with a history of connective tissue disease, tested positive on the baseline and confirmatory CancerSEEK tests based on a substitution in the TP53 gene, NM_000546.6(TP53):c.584T>C (p.Ile195Thr) (Table 1). The variant allele frequency for the TP53 substitution was 0.06% in the baseline test and 0.018% in the confirmatory test. PET-CT imaging revealed an appendix measuring 1.3 cm in diameter and displaying internal fluid density without surrounding inflammatory changes, peripheral calcification, associated 18F-FDG uptake, or lymphadenopathy (Figure 2). Based on the PET-CT findings, a colonoscopy was performed, revealing a 3 mm benign sessile polyp, which was excised. 16.6 months after enrollment, the participant underwent laparoscopic appendectomy and partial cecectomy, revealing a 7.9 x 1.8 cm appendix. Histopathological assessment revealed the presence of a non-invasive low-grade appendiceal mucinous neoplasm (LAMN). This participant was adherent to standard of care (SOC) colorectal cancer screening (FOBT test) within 12 months after their first blood draw, and the FOBT test was negative. The tissue sample was negative for the TP53 variant. The participant remains alive and cancer-free at last follow-up, 5 years and 6 months after enrollment.

Figure 2. Participant two CT image. The area in the yellow box shows an appendix measuring 1.3 cm and displaying internal fluid density without surrounding inflammatory changes.
3.3 Participant 3An asymptomatic 70-year-old participant with a history of moderate to severe renal disease, tested positive on the baseline and confirmatory CancerSEEK tests based on a substitution in the KRAS gene, NM_004985.5(KRAS):c.35G>A (p.Gly12Asp) (Table 1). The baseline test detected a KRAS substitution at a VAF of 0.078% and 0.047% in the confirmatory test. PET-CT imaging identified a filling defect and increased metabolic activity in the cecum and ascending colon (Figure 3). Colonoscopy identified 2 masses, one at the ileocecal valve and the other in the ascending colon. Additionally, 5 small polyps (tubulovillous adenomas and hyperplastic polyps, including one with mucosal Schwann cell hamartoma) (3) were removed at colonoscopy from the rectum and sigmoid colon. The hamartoma represents an exceedingly rare neurogenic tumor (4), with only 35 cases reported to date, all of which were incidentally found during colonoscopy (5). The patient was evaluated for endoscopic resection, which was not deemed feasible. A right hemicolectomy was therefore performed 12.5 months following enrollment, revealing a 4.5 cm tubulovillous adenoma with focal high-grade dysplasia at the ileocecal valve, a 5 cm tubulovillous adenoma with similar dysplasia in the ascending colon, and a 0.7 cm tubular adenoma in the ascending colon. This participant was adherent to SOC colorectal cancer screening; however, the details of the SOC testing were not available. The adenoma tissue was not available for mutational analysis. The participant remains alive and cancer-free at last follow-up, 5 years and 1 month after enrollment.
Figure 3. Participant three CT image. The area in the yellow box shows increased metabolic activity in the cecum and ascending colon with a filing defect suggesting a possible anomaly or irregularity in this region.
4 DiscussionUnderstanding non-cancer findings that may become evident in the course of a diagnostic evaluation of a positive MCED result will aid clinicians evaluating such patients. Fewer than 3% of participants with positive CancerSEEK results in DETECT-A had pre-cancerous lesions identified. In all three cases, the triggering biomarker was a DNA variant, the diagnoses represented clinically significant conditions, and were judged by clinicians to warrant therapeutic intervention to remove the lesions.
The diagnosis of the ovarian mucinous cystadenoma was prompted by the detection of a PIK3CA variant, an alteration present in about 12% of ovarian tumors across various histological types (3). Mucinous cystadenomas, considered precursors of ovarian cancer, are primarily benign neoplasms characterized by mucin-producing epithelial cells (4). The progression from benign mucinous ovarian tumors to carcinomas is thought to occur due to the accumulation of multiple genetic abnormalities, resulting in the progression from benign to borderline to carcinoma (5). We were not able to determine if the PIK3CA variant identified by CancerSEEK originated from the ovarian mucinous cystadenoma. However, intratumor genetic heterogeneity is common in mucinous ovarian tumors. BRAF and p53 mutations are frequently identified within mucinous borderline tumor components, while KRAS and PIK3CA mutations are more commonly identified within the mucinous ovarian carcinoma components of mucinous ovarian tumors (5, 6). Among post-menopausal women, the preferred treatment options are salpingo-oophorectomy, as was the approach in this patient, or ovarian cystectomy (4).
The diagnosis of appendiceal carcinoma in situ followed the detection of a variant in the TP53 gene. The inactivation of tumor suppressor genes, such as TP53 and SMAD4, along with the activation of oncogenes, such as KRAS, GNAS, and BRAF, are thought to drive appendiceal tumor progression though proliferation, angiogenesis, and evasion of apoptosis (7). However, molecular analysis of this participant’s paraffin embedded tissue sample did not confirm the presence of the TP53 variant, possibly due to intratumoral heterogeneity. Low-grade appendiceal mucinous neoplasm (LAMN) is a rare appendiceal tumor identified in fewer than 0.3% of appendectomy specimens and generally presents as an incidental finding (8). While the risk of invasion with a LAMN is low, it may rupture and seed mucin and neoplastic cells into the peritoneum.
In the third case, the CancerSEEK test identified a variant in the KRAS gene. KRAS variants are common in both colorectal pre-cancer and cancer (9–11), with reported KRAS mutation frequencies of 32.4% in adenomas and 45.5% of colorectal carcinomas (12). The diagnostic evaluation revealed large (4.5 cm and 5.0 cm) colon adenomas with high-grade dysplasia, which have high likelihood of progressing to invasive colorectal cancer (13).
The DETECT-A study was the first prospective interventional trial to evaluate an MCED blood test, and the objectives included investigating the study feasibility and participant safety. Several safety measures were implemented that may have contributed to the turnaround time for study results. Initially, a baseline CancerSEEK test was performed, followed by a confirmatory test for those participants with positive baseline tests to rule out mutations attributed to CHIP. The median time from the 1st blood draw to result was 4.8 months and from the 2nd blood draw to return of result was 2.3 months. Subsequently, the multidisciplinary review committee assessment took place, and PET-CT imaging was carried out. Additional procedures, including a lung CT scan for the first participant and colonoscopies for the other two, also contributed to the delay before surgery. The details of these additional procedures may be found in Lennon, et al. Science, 2020) (1). Future tests implemented into clinical practice will require much more rapid turnaround times with optimized clinical workflows.
5 ConclusionThese three cases illustrate examples of pre-cancerous conditions that may be diagnosed consequent to a work-up following a positive cancer signal from an MCED test. While the known biology of such lesions suggests that they could have plausibly been the source of the somatic variants that triggered the MCED results, we were not able to confirm the relationship in these 3 cases and therefore cannot rule out the possibility that these were incidental findings on PET-CT imaging. Future studies should examine these questions further by evaluating the biology of excised lesions and performing MCED tests after resection to determine if the signal had resolved. Investigators planning MCED studies should consider these uncommon outcomes in their study plans, and patients and clinicians need to be prepared to encounter some cases with pre-cancerous lesions.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Geisinger Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. All participant data have been anonymized and de-identified to the fullest extent possible to protect confidentiality. Any potentially identifiable information has been masked or removed to prevent re-identification.
Author contributionsOC: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. AK: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. SR: Methodology, Writing – original draft, Writing – review & editing. PE: Investigation, Writing – original draft, Writing – review & editing. AB: Project administration, Writing – original draft, Writing – review & editing. AL: Writing – original draft, Writing – review & editing. NP: Writing – original draft, Writing – review & editing. FD: Writing – original draft, Writing – review & editing. TB: Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsThe authors are grateful for the project administration contributions of the Geisinger team: Ashley Honushefsky, Kathleen Sheridan, Eric Wagner, and Carroll Walter; and for the Johns Hopkins University team that developed the CancerSEEK test: Joshua Cohen, Chrisopher Douville, Isaac Kinde, Allison Klein, Cristian Tomasetti, Ralph Hruban, Kenneth Kinzler, Elliot Fishman, and Bert Vogelstein. Writing and editorial assistance was provided by Carolyn Hall and Feyza Sancar (Exact Sciences). The authors would also like to thank the participants who generously participated in the original sample collection studies.
Conflict of interestAuthors OC, AK, SR, PE, and FD are employees of and own stock in Exact Sciences Corp., and report travel expense reimbursement from Exact Sciences Corp. Author AB reports research funding from Exact Sciences Corp. and Freenome Holdings, Inc. outside the submitted work, and intellectual property interests at MeTree and You, Inc. outside the submitted work. Author AL reports consulting services to Exact Sciences Corp., and intellectual property interests including PCT/US2018/045669 and US9476095B2 owned and managed by Johns Hopkins University. Author NP reports a leadership role in and compensation from Haystack Oncology, outside the submitted work, and stock/ownership interests in ManaT Bio, Haystack Oncology Personal Genome Diagnostics, NeoPhore, and CAGE Pharma, outside the submitted work. Author NP reports consulting services and compensation from Personal Genome Diagnostics, Neophore, CAGE Pharma and Vidium outside the submitted work. Author NP reports intellectual property interests including PCT/US2018/045669 and US9476095B2 owned and managed by Johns Hopkins University. Author TB is an employee of and owns stock in Exact Sciences Corp. Outside the submitted work, he reports owning stock/ownership interests in Arvinas and Salarius Pharmaceuticals, and consulting services and compensation from Abbvie, Amgen, Arvinas, Astellas Pharma, AstraZeneca, Bayer, Bristol-Myers Squibb, Constellation Pharmaceuticals, Dantari Pharmaceuticals, GlaxoSmithKline, GRAIL, Janssen, Pfizer, Sanofi, and Sapience Therapeutics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from Exact Sciences, Madison. The funder was involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Lennon AM, Buchanan AH, Kinde I, Warren A, Honushefsky A, Cohain AT, et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science. (2020) 369(6499):eabb9601. doi: 10.1126/science.abb9601
PubMed Abstract | Crossref Full Text | Google Scholar
2. Kinde I, Wu J, Papadopoulos N, Kinzler KW, Vogelstein B. Detection and quantification of rare mutations with massively parallel sequencing. Proc Natl Acad Sci U S A. (2011) 108:9530–5. doi: 10.1073/pnas.1105422108
PubMed Abstract | Crossref Full Text | Google Scholar
3. Levine DA, Bogomolniy F, Yee CJ, Lash A, Barakat RR, Borgen PI, et al. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin Cancer Res. (2005) 11:2875–8. doi: 10.1158/1078-0432.CCR-04-2142
PubMed Abstract | Crossref Full Text | Google Scholar
5. Razia S, Nakayama K, Yamashita H, Ishibashi T, Ishikawa M, Kanno K, et al. Histological and genetic diversity in ovarian mucinous carcinomas: A pilot study. Curr Oncol. (2023) 30:4052–9. doi: 10.3390/curroncol30040307
PubMed Abstract | Crossref Full Text | Google Scholar
6. Wamunyokoli FW, Bonome T, Lee JY, Feltmate CM, Welch WR, Radonovich M, et al. Expression profiling of mucinous tumors of the ovary identifies genes of clinicopathologic importance. Clin Cancer Res. (2006) 12:690–700. doi: 10.1158/1078-0432.CCR-05-1110
PubMed Abstract | Crossref Full Text | Google Scholar
7. Constantin M, Matanie C, Petrescu L, Bolocan A, Andronic O, Bleotu C, et al. Landscape of genetic mutations in appendiceal cancers. Cancers (Basel). (2023) 15(14):3591. doi: 10.3390/cancers15143591
PubMed Abstract | Crossref Full Text | Google Scholar
8. Tirumani SH, Fraser-Hill M, Auer R, Shabana W, Walsh C, Lee F, et al. Mucinous neoplasms of the appendix: a current comprehensive clinicopathologic and imaging review. Cancer Imaging. (2013) 13:14–25. doi: 10.1102/1470-7330.2013.0003
PubMed Abstract | Crossref Full Text | Google Scholar
9. Castagnola P, Giaretti W. Mutant KRAS, chromosomal instability and prognosis in colorectal cancer. Biochim Biophys Acta (BBA) - Rev Cancer. (2005) 1756:115–25. doi: 10.1016/j.bbcan.2005.06.003
Crossref Full Text | Google Scholar
11. Juárez M, Egoavil C, Rodríguez-Soler M, Hernández-Illán E, Guarinos C, García-Martínez A, et al. KRAS and BRAF somatic mutations in colonic polyps and the risk of metachronous neoplasia. PLoS One. (2017) 12:e0184937. doi: 10.1371/journal.pone.0184937
PubMed Abstract | Crossref Full Text | Google Scholar
12. Jungwirth J, Urbanova M, Boot A, Hosek P, Bendova P, Siskova A, et al. Mutational analysis of driver genes defines the colorectal adenoma: in situ carcinoma transition. Sci Rep. (2022) 12:2570. doi: 10.1038/s41598-022-06498-9
留言 (0)