Malnutrition has been recognized as a major health problem in pediatric intensive care unit (PICU), which affects 37.19% of critically ill children (1). On PICU admission, 18% to 47% (2, 3) of critically ill children are already malnourished. During the hospitalization in PICU, critically ill children often go through a catabolic stress state and altered inflammatory response due to trauma of infection (4, 5), and 74% of them may experience iatrogenic underfeeding encouraged by prolonged fasting and frequent feeding pauses (6). Meanwhile, taking the lower percentage of muscle mass and fat, higher resting energy expenditure (REE) and great nutritional requirements for growth and development (7) into consideration, nutritional deterioration is frequent and often intense. In critically ill children, malnutrition is associated with deterioration of muscle strength, multiorgan dysfunction, increased risk of infection, greater length of mechanical ventilation (MV) and PICU stay, and increased mortality (7–10). The relationship between nutrition support therapy and the improvement in clinically relevant outcomes in pediatric critical care has currently demonstrated by advances in published scientific literature (11). Optimal nutritional support therapy can avoid under or overfeeding, showing advantages in attenuating the morbidity rate, decreasing the length of PICU stay, and improving patient outcomes (12).
Among several ways to provide nutritional support therapy, enteral nutrition (EN) is recommended as the preferred nutrition mode in critically ill children by Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN) (13). EN is proved to have many advantages, such as inducing gastrointestinal mucosa trophism to avoid bacterial translocation, costing less hospitalization expenses and decreasing the risk of infection than parenteral nutrition (PN) (14). Additionally, achievement of up to two thirds of the nutrient goal (15) and withholding PN (16) in the first week of critical illness is associated with improved outcomes. Thus, European Society of Pediatric and Neonatal Intensive Care (ESPNIC) recommends using EN protocols to initiate nutrition delivery in 24 h and improve nutritional intake to reduce the risk of malnutrition and promote recovery (15). However, more than half of children cannot receive adequate energy or protein intake through EN at their first week of admission (17, 18). Among the obstacles inhibiting the delivery of optimal EN, enteral feeding intolerance (EFI) is one of the main reasons (19), and the detecting of EFI should be included in the EN protocol (13).
EFI can result in feed interruptions, leading to insufficient energy delivery and delayed achievement of EN (20), and is also associated with skin integrity, pulmonary infections, and sepsis (21). However, the concept of EFI in critically ill children remains nebulous and inconsistent. It is usually characterized by clinical symptoms, with a 20% incidence in PICU (22). The most common clinical symptoms include increased gastric residual volume (GRV) and gastrointestinal (GI) discomfort signs, such as epigastric discomfort, vomiting, diarrhea, reflux, abdominal distension and pain/discomfort (23). However, the measurements of EFI are different across different PICUs, and sometimes even across the same PICU. The time point, frequency and threshold of GI symptom monitoring vary among different studies (23). The difference in measurements can affect the detection of EFI, and it is likely that the subjective measurements, which lack an evidence base, are conservative and define a lower threshold for withholding of EN than necessary (24). This may cause unnecessary nutritional interruption, and contribute to increased risk of inadequate nutrient intake.
Therefore, a comprehensive review summarizing the measurements of EFI in critically ill children is essential from a clinical and scientific perspective. Given the heterogeneity of EFI definitions in studies, we cannot draw any firm conclusions on measurements, prevalence or outcomes through a systematic review (22). Under this circumstance, a scoping review can be suitable to investigate research involving the measurements of EFI. Thus, the objective of the present scoping review was to systematically map out the body of existing literatures on measurements of EFI in critically ill children to identify knowledge gaps and opportunities for further research.
2 Materials and methods 2.1 Protocol and registration information sourcesThis review was conducted in accordance with the Joanna Briggs Institute methodology for scoping reviews, and has been registered with Open Science Framework (osf.io/5hbe7). The review was completed in a systematic, rigorous, and transparent way to minimize bias. We performed this scoping review of literature to summarize the measurements of EFI in pediatric critically ill population.
2.2 Search strategiesA comprehensive search of the literature was conducted through 7 English electronic databases and 4 Chinese electronic databases: MEDLINE, EMBAS, PubMed, Web of Science, Cochrane Library, JBI EBP, CINAHL, CNKI Citation, VIP, Wanfang Medical Network, and Sinomed. As a first step, a limited research combing MeSH terms and keywords was conducted in the databases of PubMed and CNKI, we piloted the searching strategy to check the appropriateness of the keywords and databases. The initial search terms in PubMed included (child OR adolescent OR pediatrics) AND (critical care nursing OR critical illness) AND (enteral nutrition OR enteral feeding) AND (symptom assessment OR measurements). Suitable articles were examined, with keywords and index terms identified from titles and abstracts (e.g., preschool child, teenager, pediatric intensive care unit, artificial feeding, symptom evaluation, etc.) used to develop a full search strategy for PubMed and CNKI databases, which can be modified and adapted to suit a range of databases in English and Chinese. Thereafter, a second search using all the identified MeSH terms, keywords and index terms was done across all databases. We searched the PubMed database with the following strategy: (((([child OR children OR preschool child* OR adolescent* OR adolescence OR teenager* OR youth* OR teen* OR pediatric* OR paediatric*(Title/Abstract)] OR [child OR child, preschool OR adolescent OR pediatrics(MeSH Terms)]) AND ((critical care nursing OR intensive care units, pediatric OR intensive care units OR critical illness OR intensive care nursing OR critical care OR intensive care unit OR pediatric intensive care unit* OR Pediatric ICU OR PICU OR ICU OR critically ill OR critical illnesses[Title/Abstract]) OR (critical care nursing OR intensive care units OR intensive care units, pediatric OR critical illness[MeSH Terms]))) AND ((enteral nutrition OR nutritional support[MeSH Terms]) OR (enteral nutrition OR nutritional support OR enteral feeding OR tube feeding OR gastric feeding tube OR nutrition support OR artificial feeding [Title/Abstract]))) AND (enteral feeding intolerance OR complication OR feeding intolerance OR enteral nutrition intolerance[Title/Abstract])) AND ((symptom assessment OR nursing assessment OR symptom assessments OR symptom evaluation OR nursing assessments OR assess OR measurements[Title/Abstract]) OR (symptom assessment OR nursing assessment[MeSH Terms])). Our research was limited to articles published from 1 January 2004 until 31 December 2023, as the first review paper of feeding intolerance in children was published in 2004. We performed the searches on April 12, 2024. The full search, as executed, is available in Supplementary File 1. Additionally, the third step included screening of the reference lists of all studies selected for this scoping review to look for additional sources.
2.3 Inclusion and exclusion criteriaThe inclusion criteria for our study were: (1) the population consists of pediatric patients (28 days to 18 years old (13), with critical illness; (2) the article includes detailed methods for measuring EFI, including delayed gastric emptying (GE) and GI symptoms (nausea, vomiting, diarrhea, abdominal discomfort, etc.); (3) the types of studies include systematic reviews, experimental studies, observational studies, and qualitative study designs; (4) literature published in Chinese or English between 1 January 2004 and 31 December 2023. The exclusion criteria were (1) full-text vision unavailable; (2) opinion pieces and conference abstracts (e.g., editorials). Final results of the evidence search and selection progress were presented in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
2.4 Data screening and extractionAll reference identified were imported into the reference manager software, EndNote X9. The references from different databases were combined and any duplicate records were removed. A two-steps screening process to select the study was adopted. Firstly, the title and abstract of study were screened, followed by a full-text review. Two reviewers screened the articles against the eligibility criteria independently. All the disagreements were discussed, and a third reviewer was consulted if no consensus can be reached. Relevant data were extracted from all included studies by two reviewers independently. A structured data recording form developed by the group was used and the information was recorded on Microsoft Excel. The following data were extracted: study design, study objective, study population, and measurements of EFI. Any disagreement between reviewers was resolved through discussion and a third review author acted as an arbiter when disagreements could not be resolved.
2.5 Data analysis and synthesisIn keeping with the primary intention of this scoping review to investigate and describe the available literature, analysis of the data gathered were descriptive in nature. The categorical elements of the data were counted and tabulated into groupings, such as the study design and country of origin. Qualitative data was organized into categories, and simple descriptive statistics, such as frequency counts and basic coding was used to collate quantitative data.
We divided the indicators of EFI into 2 categories: delayed gastric emptying (GE) and GI symptoms. Given the different descriptions in different studies, we combined terms with the same connotation. For example, vomiting also stood for emesis, and high GRV also stood for gastric retention. With each section, a narrative summary was completed to highlight the trends, gaps, and areas that warrant further study.
3 Results 3.1 Search resultsIn total, 627 results were obtained from the preliminary search. After removing duplicates, 433 articles were left for the screening of title and abstract. As a result, 55 articles met the inclusion criteria were retrieved to screen the full papers. 32 articles were included in the final data analysis. Figure 1 shows the screening process and results as a flowchart using the PRISMA template.
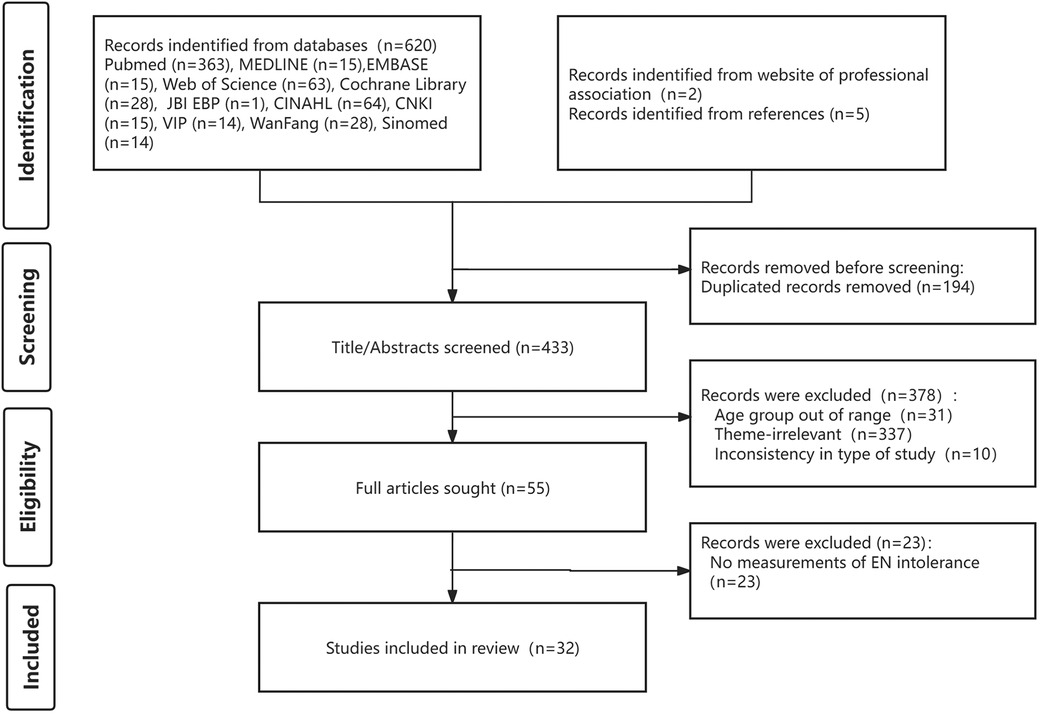
Figure 1. Flow diagram of the study selection.
3.2 Study characteristicsAmong the 32 included articles, 13 articles took the observational-analytic design, followed by experimental design (8/32) and quasi-experimental design (6/32). Seven studies were conducted in children with MV. Over half of the studies (17/32) were published in the last five years. The countries with the most publications are the United States (9/32), followed by China (8/32) and Spain (6/32).
3.3 Measurements of EFIDifferent measurements were used in different studies. According to the characteristics of the indicators, we divided them into delayed GE and GI symptoms. The most common indicators included were high GRV, diarrhea, and vomiting. The summaries of the measurements were shown in Table 1. In the 32 included studies, a total of 7 indicators of feeding intolerance were identified, with measurements mentioned 69 times. The frequency of mentions for each indicator is shown in Figure 2.
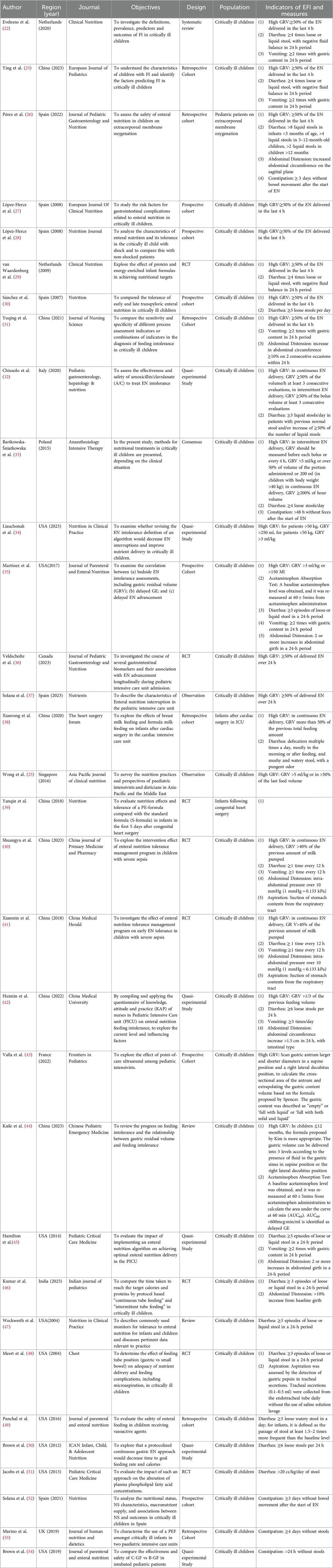
Table 1. Indicators and corresponding measures of EFI in critically ill children.
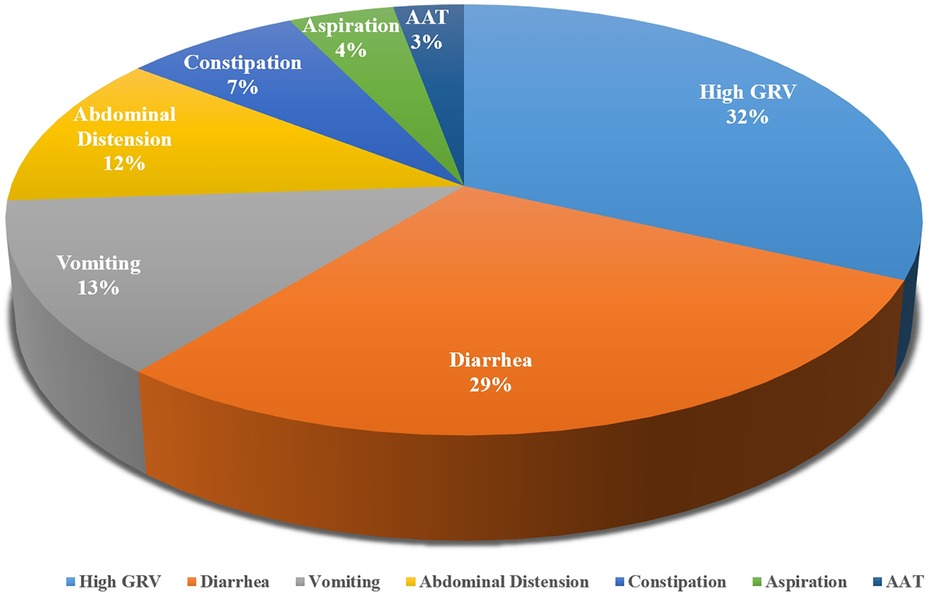
Figure 2. Indicators of EFI in critically ill children.
3.3.1 Delayed GEOur review found that GE is measured by evaluating GRV and acetaminophen absorption test (AAT). 68.75% (22/32) of the included studies incorporated high GRV as an important indicator of EFI in critically ill children. The most common method to assess GRV is to use a syringe to draw back from the feeding device. However, although a high GRV is stated as the most important sign of gastro-intestinal intolerance (25), there is no common standard for the cut-off values. In 8 studies, a high GRV was commonly considered as ≥50% of the EN delivered in last 4 h (22, 26–31, 55). In other studies, the cut-off value ranges from 1/3 to 300% of the administered volume (34, 39, 42). Some scholars also developed specifical standards for continuous EN delivery, including 50% (32) or 200% (33) of the hour volume in continuous feeding, and 50% (38) of the previous total feeding amount. Another commonly used calculation of the cut-off value is based on body weight, which ranges from 3 ml/kg (34, 35) to 5 ml/kg (25, 33). Another measurement of GE is gastric ultrasound. By scanning gastric antrum diameters in a supine position and a right lateral decubitus position, the cross-sectional area of the antrum can be calculated. Then, different formula was chosen to calculate the gastric content volume according to the age of the child. In children ≤12 months, the formula proposed by Kim was suggested (44), and in children >11 months, the formula proposed by Spencer was used (43).
In addition, acetaminophen absorption test (AAT) can also be used to monitor GE. Since acetaminophen cannot be absorbed in stomach, the rise of blood acetaminophen concentration reflects gastric emptying and small intestinal absorption of acetaminophen (35, 56). A baseline acetaminophen level was obtained, and it was re-measured at 60mins from drug administration to calculate the area under the curve at 60 min (AUC60). AUC60 < 600 mcg min/ml is identified as delayed GE (35).
3.3.2 GI symptomsAlthough many GI symptoms were listed in the included studies as indicators of EFI, there were no detailed measurements for some of the symptoms, such as nausea, abdominal pain, increased bowl sounds, and gastrointestinal hemorrhage. Therefore, we only focused on five symptoms with specific measurements.
Diarrhea is another commonly used indictor of EFI, which was mentioned in 20 included studies. Majority (14/20) of the studies assessed diarrhea by measuring the frequency and nature of stools, and the other 6 studies also took the weight of stools and accompanying consequences into account. Loose and liquid stools were signs of diarrhea, and the threshold of frequency ranged from 3 to 6 times in a 24-h period. 5 studies considered 3 episodes as the threshold of diarrhea in critically ill children (35, 45–48). Only two studies (26, 57) considered the criteria for diarrhea according to the age of the child, and one of them suggested 8 liquid stools could be normal in infants younger than 3 months of age (26). When taking the weight of stools into consideration, 10 g/kg/day (39) and 20 cc/kg/day (51) was mentioned in two studies. Besides, negative fluid balance and inefficient use of antidiarrheal drugs were also considered as measurements of diarrhea in 4 studies (22, 29, 39, 55).
Vomiting was measured in 9 included studies. 88.89% (8/9) of the studies consider vomiting as 2–3 episodes with gastric content in 24 h (22, 31, 35, 40–42, 45, 55). Abdominal distension was measured in 7 included studies as an indicator of EFI. Abdominal grith and intra-abdominal pressure were assessed. Abdominal distension was considered when the abdominal grith increases 1.5–2 cm in 24 h or by more than 10% of the baseline girth (35, 42, 45, 46). It was also considered abdominal distension when intra-abdominal pressure was greater than 10 mmHg (40, 41). Another study argued that failure to improve vomiting symptom with GI motility drugs and the need to suspend feeding was an indicator of EFI (39). Constipation was also included in 5 studies, measuring by the days without stools. 2 of them set the threshold at 3 days (26, 52), while the others ranged from 24 h to 4 days (33, 53, 54). Besides, three articles described the measurements of aspiratory, two of them determined aspiratory according to the presence of stomach contents in the respiratory tract (40, 41). The other study assessed aspiration daily by the detection of gastric pepsin in tracheal secretions (48).
4 Discussion 4.1 Current EFI measurements are numerous and complexIn this article, we sought to synthesize and summarize major measurements of EFI in critically ill children. The articles included had identified multiple measurements of the indicators of EFI, and thus highlights the complexity of the measurements of EFI in pediatric critical illness care. We found that the EFI was commonly defined as anyone or a combination of the two aspects mentioned above: delayed GE and GI symptoms. However, different GI symptoms were considered as predictive indicators in different studies. We finally included 32 studies and there were 9 GI symptoms were covered in total. None of the studies included all of the GI symptoms and most of them only included 3–4 of them. Among the total 9 GI symptoms, only 5 of them was described in detail, which were summarize above. Besides, even the measurement of one same indicator varied from each other. In the 7 indicators summarized in this article, only one of them (AAT) was in agreement between two studies, and the other indicators were measured by different methods. High GRV was the most commonly used indicator of EFI, which was mentioned in 22 studies. Only 8 of the studies agreed on the same measurement of high GRV, and the remaining 14 studies listed a total of 12 additional measurements. Thus, we found it quite difficult to reach a uniform standard in this topic. Given the consensus on the benefits of adequate energy intake (14, 58), proper measurements of EFI to avoid unnecessary feeding interruptions is the focus of current research (37). The various measurements of EFI contributes to the difficulty in comparing the incidence of EFI, and also hinders the development of nutrition promotion research in critically ill children.
4.2 Current EFI measurements lack adaptability and necessityAlthough current EFI measurements are numerous and various, they cannot provide targeted guidance in complex clinical situations. Although we had limited the age of critically ill children to 28 days—18 years, there were still differences in the gastrointestinal function development in children at different growth stages (59). Diarrhea was listed as an indicator over half of the included studies (20/34), while only 4 of them take the age or weight of children into consideration during the measurement (26, 33, 39, 49). The absolute limits to the number of normal bowel movements in children of different ages are difficult to define (60), so the World Health Organization also defines diarrhea as more frequently than normal situation for a person (61). Corresponding to that, 2 studies included the baseline level of bowel movements into the measurement of diarrhea (32, 49). Setting a low threshold for diarrhea may increase the risk of feeding interruptions and decrease the adequacy of nutrition intake. There is still a need to refine the criteria of diarrhea, taking full account of the age of children, bowel habits, and the influence of diarrhea.
Another controversial measurement is GRV. Similar to the results of this study, GRV is widely used as a surrogate marker of gastric emptying in a majority of PICUs (32, 36, 37). In a large multi-country studies conducted in Asia-Pacific and the Middle East (25), GRV was measured in 77% (36/47) of respondents from 18 different countries. However, despite being widely used, its clinical value has not been confirmed by the evidence. In a prospective cohort study in critically ill children, GRV failed to predict delayed gastric emptying, and related to slow EN advancement (35). Routine measurement of GRV in critically ill children is not recommended by the European Society of Pediatric and Neonatal Intensive Care (ESPNIC) as well (15). The measurement of GRV is frequently inaccurate due to the position of the feeding tube in the stomach, the feeding method, patient position, syringe sizes used, and the technique of aspiration (62, 63). Consequently, some researchers also begin to explore the feasibility of not routinely monitoring GRV. It is showed that routinely measure GRV was of questionable benefit (64), and not measuring GRV did not increase vomiting, ventilator-acquired pneumonia or necrotizing enterocoltis (65). When trying to change the long-standing and embedded practice of routinely measuring GRV, PICU nurse had significant fear around pulmonary aspiration (63). Therefore, future studies should explore individualized measures for different age stages and disease characteristics to accurately assess EFI, and advance EN intake to promote recovery in critically ill children.
4.3 More education and teamwork are neededThe management of EN delivery should be the responsibility of a nutrition support team (NST), including doctors, nurses, dietitians and pharmacists (66). The NST should use a stepwise algorithmic approach to advance EN in critically ill children, which must include the detection of EFI by bedside support (60). Due to the uniform measurements of each indicator, multiple indicators involved, and the loose definition of EFI, there are challenges in the measurements of EFI among this population (59). Thus, more focused nutrition education for NST should be highlighted (19). The understanding of EN measurements can be unified and further collaboration can be developed by written guidelines, multiprofessional nutrition rounds, and the continual auditing of practices (67). In the context of the continuous development of EFI measurements and the need to change clinical practice, the NST should work together to develop a viable protocol, which takes the opinions of parents into consideration (68). Currently, nurses play a vital role in the measure of EFI and the delivery of EN in PICU, and their knowledge can impact EN notably (63). A simple flow chart and education package were suggested to address concerns of junior nurses (65).
On the other hand, accurate measurement should be applied in critically ill children to assess delayed GE, which is the most common manifestation of gastric dysmotility in this cohort (56). Ultrasound is a non-invasive, non-interrupted measurement of EN. In a prospective observational study involving 64 critically ill children, gastric ultrasound improved that GRV appeared unreliable as a measure of gastric emptiness (43). But the performance of ultrasonography is positionally dependent and is not appropriate in children with special positioning, such as the prone position. AAT was also reported in previous studies, while it can only be administered to eligible children (35). The need for additional blood samples may also increase the pain and economic burden of children. Electrical impedance method is another measurement of GE, which showed encourage effect in the monitoring of EFI in non-invasive critically ill patients (69). Developing accurate bedside measures for gastric emptying are highly desirable and need to be further investigated.
5 Strengths and limitationsThe literature was systematically searched and screened following an a priori protocol, and guidelines published by the Joanna Briggs institute. In highlight these findings, we also acknowledged some limitations of this review and across the studies examined. The definition of EFI was various in included articles, which was not summarized in detail as it was beyond the scope of this review. It may add the difficulty of the understanding of EFI. Another limitation was that only 5 GI symptoms were summarized in this article, due to the lack of specific information of other GI symptoms. Future researchers can develop further studies of EFI measurements by providing more detailed information.
6 ConclusionsThe current paper reviewed the numerous measurements of EFI in critically ill children, which was categorized by 7 indicators of EFI. The summarized data provided insight into how EFI was assessed, and highlighted the complexity of the measurements. Importantly, the various measurements applied in different studies increased the difficulty of integrating the results. It highlighted the necessary to develop individual measurements of EFI. We suggested that the age of children and disease condition to be taken into consideration. Further studies can also investigate accurate and objective physiological measurements of EFI to advance EN and improve outcomes in critically ill children.
Author contributionsYL: Conceptualization, Funding acquisition, Methodology, Validation, Writing – original draft. C-HF: Methodology, Validation, Writing – original draft. M-JJ: Validation, Visualization, Writing – original draft. JL: Formal Analysis, Project administration, Writing – original draft. X-YY: Formal Analysis, Project administration, Writing – original draft. T-TX: Conceptualization, Funding acquisition, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work was supported by research grants from Scientific Research Program of Shanghai Nursing Association (Grant 2022QN-B01) and Shanghai Jiao Tong University School of Medicine: Nursing Development Program (Grant [2023] No. 3). The study sponsor was not involved in the design of the study, the collection, analysis and interpretation of data, writing the report, or the decision to submit the report for publication.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1441171/full#supplementary-material
References1. Abera EG, Sime H. The prevalence of malnutrition among critically ill children: a systematic review and meta-analysis. BMC Pediatr. (2023) 23(1):583. doi: 10.1186/s12887-023-04419-x
PubMed Abstract | Crossref Full Text | Google Scholar
2. Campos-Miño S, Figueiredo-Delgado A, Zárate P, Zamberlan P, Muñoz-Benavides E, Coss-Bu JA, et al. Malnutrition and nutrition support in Latin American PICUs: the nutrition in PICU (NutriPIC) study. Pediatr Crit Care Med. (2023) 24(12):1033–42. doi: 10.1097/PCC.0000000000003337
PubMed Abstract | Crossref Full Text | Google Scholar
3. Ventura JC, Hauschild DB, Barbosa E, Bresolin NL, Kawai K, Mehta NM, et al. Undernutrition at PICU admission is predictor of 60-day mortality and PICU length of stay in critically ill children. J Acad Nutr Diet. (2020) 120(2):219–29. doi: 10.1016/j.jand.2019.06.250
PubMed Abstract | Crossref Full Text | Google Scholar
4. Singer P, Blaser AR, Berger MM, Calder PC, Casaer M, Hiesmayr M, et al. ESPEN practical and partially revised guideline: clinical nutrition in the intensive care unit. Clin Nutr. (2023) 42(9):1671–89. doi: 10.1016/j.clnu.2023.07.011
PubMed Abstract | Crossref Full Text | Google Scholar
5. Rahman A, Agarwala R, Martin C, Nagpal D, Teitelbaum M, Heyland DK. Nutrition therapy in critically ill patients following cardiac surgery: defining and improving practice. J Parenter Enteral Nutr. (2017) 41(7):1188–94. doi: 10.1177/0148607116661839
PubMed Abstract | Crossref Full Text | Google Scholar
6. Heyland DK, Dhaliwal R, Wang M, Day AG. The prevalence of iatrogenic underfeeding in the nutritionally “at-risk” critically ill patient: results of an international, multicenter, prospective study. Clin Nutr. (2015) 34(4):659–66. doi: 10.1016/j.clnu.2014.07.008
PubMed Abstract | Crossref Full Text | Google Scholar
8. Agarwal E, Ferguson M, Banks M, Batterham M, Bauer J, Capra S, et al. Malnutrition and poor food intake are associated with prolonged hospital stay, frequent readmissions, and greater in-hospital mortality: results from the nutrition care day survey 2010. Clin Nutr. (2013) 32(5):737–45. doi: 10.1016/j.clnu.2012.11.021
PubMed Abstract | Crossref Full Text | Google Scholar
10. Wong JJ, Han WM, Sultana R, Loh TF, Lee JH. Nutrition delivery affects outcomes in pediatric acute respiratory distress syndrome. J Parenter Enteral Nutr. (2017) 41(6):1007–13. doi: 10.1177/0148607116637937
Crossref Full Text | Google Scholar
11. Albert BD, Spolidoro GC, Mehta NM. Metabolism and energy prescription in critically ill children. Minerva Anestesiol. (2021) 87(9):1025–33. doi: 10.23736/S0375-9393.21.14825-4
PubMed Abstract | Crossref Full Text | Google Scholar
12. Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. (2019) 38(1):48–79. doi: 10.1016/j.clnu.2018.08.037
PubMed Abstract | Crossref Full Text | Google Scholar
13. Mehta NM, Skillman HE, Irving SY, Coss-Bu JA, Vermilyea S, Farrington EA, et al. Guidelines for the provision and assessment of nutrition support therapy in the pediatric critically ill patient: society of critical care medicine and American Society for Parenteral and Enteral Nutrition. Pediatr Crit Care Med. (2017) 18(7):675–715. doi: 10.1097/PCC.0000000000001134
PubMed Abstract | Crossref Full Text | Google Scholar
14. Mehta NM, Bechard LJ, Cahill N, Wang M, Day A, Duggan CP, et al. Nutritional practices and their relationship to clinical outcomes in critically ill children—an international multicenter cohort study. Crit Care Med. (2012) 40(7):2204–11. doi: 10.1097/CCM.0b013e31824e18a8
PubMed Abstract | Crossref Full Text | Google Scholar
15. Tume LN, Valla FV, Joosten K, Jotterand Chaparro C, Latten L, Marino LV, et al. Nutritional support for children during critical illness: European Society of Pediatric and Neonatal Intensive Care (ESPNIC) metabolism, endocrine and nutrition section position statement and clinical recommendations. Intensive Care Med. (2020) 46(3):411–25. doi: 10.1007/s00134-019-05922-5
PubMed Abstract | Crossref Full Text | Google Scholar
16. Fivez T, Kerklaan D, Mesotten D, Verbruggen S, Wouters PJ, Vanhorebeek I, et al. Early versus late parenteral nutrition in critically ill children. N Engl J Med. (2016) 374(12):1111–22. doi: 10.1056/NEJMoa1514762
PubMed Abstract | Crossref Full Text | Google Scholar
17. Campos-Miño S, López-Herce Cid J, Figueiredo Delgado A, Muñoz Benavides E, Coss-Bu JA. The Latin American and Spanish survey on nutrition in pediatric intensive care (ELAN-CIP2). Pediatr Crit Care Med. (2019) 20(1):e23–9. doi: 10.1097/PCC.0000000000001761
Crossref Full Text | Google Scholar
18. Li J, Li B, Qian J, Zhang J, Ren H, Ning B, et al. Nutritional survey in critically ill children: a single center study in China. Transl Pediatr. (2020) 9(3):221–30. doi: 10.21037/tp-19-173
PubMed Abstract | Crossref Full Text | Google Scholar
19. Tume LN, Eveleens RD, Verbruggen SCAT, Harrison G, Latour JM, Valla FV, et al. Barriers to delivery of enteral nutrition in pediatric intensive care: a world survey. Pediatr Crit Care Med. (2020) 21(9):e661–71. doi: 10.1097/PCC.0000000000002382
PubMed Abstract | Crossref Full Text | Google Scholar
20. Canarie MF, Barry S, Carroll CL, Hassinger A, Kandil S, Li S, et al. Risk factors for delayed enteral nutrition in critically ill children. Pediatr Crit Care Med. (2015) 16(8):e283–9. doi: 10.1097/PCC.0000000000000527
PubMed Abstract | Crossref Full Text | Google Scholar
21. Sánchez C, López-Herce J, de Guerra M, Carrillo A, Moral R, Sancho L. The use of transpyloric enteral nutrition in the critically ill child. J Intensive Care Med. (2000) 15(5):247–54. doi: 10.1046/j.1525-1489.2000.00247.x
Crossref Full Text | Google Scholar
22. Eveleens RD, Joosten KFM, de Koning BAE, Hulst JM, Verbruggen SCAT. Definitions, predictors and outcomes of feeding intolerance in critically ill children: a systematic review. Clin Nutr. (2020) 39(3):685–93. doi: 10.1016/j.clnu.2019.03.026
PubMed Abstract | Crossref Full Text | Google Scholar
24. Mehta NM, McAleer D, Hamilton S, Naples E, Leavitt K, Mitchell P, et al. Challenges to optimal enteral nutrition in a multidisciplinary pediatric intensive care unit. J Parenter Enteral Nutr. (2010) 34(1):38–45. doi: 10.1177/0148607109348065
PubMed Abstract | Crossref Full Text | Google Scholar
25. Wong JJM, Ong C, Han WM, Mehta NM, Lee JH. Survey of contemporary feeding practices in critically ill children in the Asia-pacific and the Middle East. Asia Pac J Clin Nutr. (2016) 25(1):118–25. doi: 10.6133/apjcn.2016.25.1.07
PubMed Abstract | Crossref Full Text | Google Scholar
26. Pérez G, González E, Zamora L, Fernández SN, Sánchez A, Bellón JM, et al. Early enteral nutrition and gastrointestinal complications in pediatric patients on extracorporeal membrane oxygenation. J Pediatr Gastroenterol Nutr. (2022) 74(1):110–5. doi: 10.1097/MPG.0000000000003317
PubMed Abstract | Crossref Full Text | Google Scholar
27. López-Herce J, Santiago MJ, Sánchez C, Mencía S, Carrillo A, Vigil D. Risk factors for gastrointestinal complications in critically ill children with transpyloric enteral nutrition. Eur J Clin Nutr. (2008) 62(3):395–400. doi: 10.1038/sj.ejcn.1602710
Crossref Full Text | Google Scholar
28. López-Herce J, Mencía S, Sánchez C, Santiago MJ, Bustinza A, Vigil D. Postpyloric enteral nutrition in the critically ill child with shock: a prospective observational study. Nutr J. (2008) 7(1):6. doi: 10.1186/1475-2891-7-6
PubMed Abstract | Crossref Full Text | Google Scholar
29. Van Waardenburg DA, De Betue CT, Goudoever JBV, Zimmermann LJ, Joosten KF. Critically ill infants benefit from early administration of protein and energy-enriched formula: a randomized controlled trial. Clin Nutr. (2009) 28(3):249–55. doi: 10.1016/j.clnu.009.03.005
PubMed Abstract | Crossref Full Text | Google Scholar
30. Sánchez C, López-Herce J, Carrillo A, Mencía S, Vigil D. Early transpyloric enteral nutrition in critically ill children. Nutrition. (2007) 23(1):16–22. doi: 10.1016/j.nut.2006.10.002
PubMed Abstract | Crossref Full Text | Google Scholar
31. Yu-qing W, Yin G. Sensitivity and specificity of feeding intolerance process evaluation indexed in critically ill children. J Nurs Sci. (2021) 36(23):42–5. doi: 10.3870/j.issn.1001-4152.2021.23.042
Crossref Full Text | Google Scholar
32. Chiusolo F, Capriati T, Erba I, Bianchi R, Ciofi Degli Atti ML, Picardo S, et al. Management of enteral nutrition in the pediatric intensive care unit: prokinetic effects of amoxicillin/clavulanate in real life conditions. Pediatr Gastroenterol Hepatol Nutr. (2020) 23(6):521. doi: 10.5223/pghn.2020.23.6.521
PubMed Abstract | Crossref Full Text | Google Scholar
33. Bartkowska-Śniatkowska A, Zielińska M, Świder M, Bittner G, Sarnowska-Wroczyńska I, Witulska K, et al. Nutrition therapy in paediatric intensive care units: a consensus statement of the section of paediatric anaesthesia and intensive therapy of the Polish society of anarsthesiology and intensive therapy, Polish society of neonatology and Polish Society for Clinical Nutrition of Children. Anaesthesiol Intensive Ther. (2015) 47(4):267–83. doi: 10.5603/AIT.2015.0056
Crossref Full Text | Google Scholar
34. Liauchonak S, Hamilton S, Franks JD, Callif C, Akhondi-Asl A, Ariagno K, et al. Impact of implementing an evidence-based definition of enteral nutrition intolerance on nutrition delivery: a prospective, cross-sectional cohort study. Nutr Clin Pract. (2023) 38(2):376–85. doi: 10.1002/ncp.10941
留言 (0)