First, a strikingly large number of medical conditions are accompanied by olfactory dysfunction (Tables 1–3). The remarkably long and diverse list of medical conditions that co-occur with olfactory loss raises the possibility that there is something deeper to these relationships.
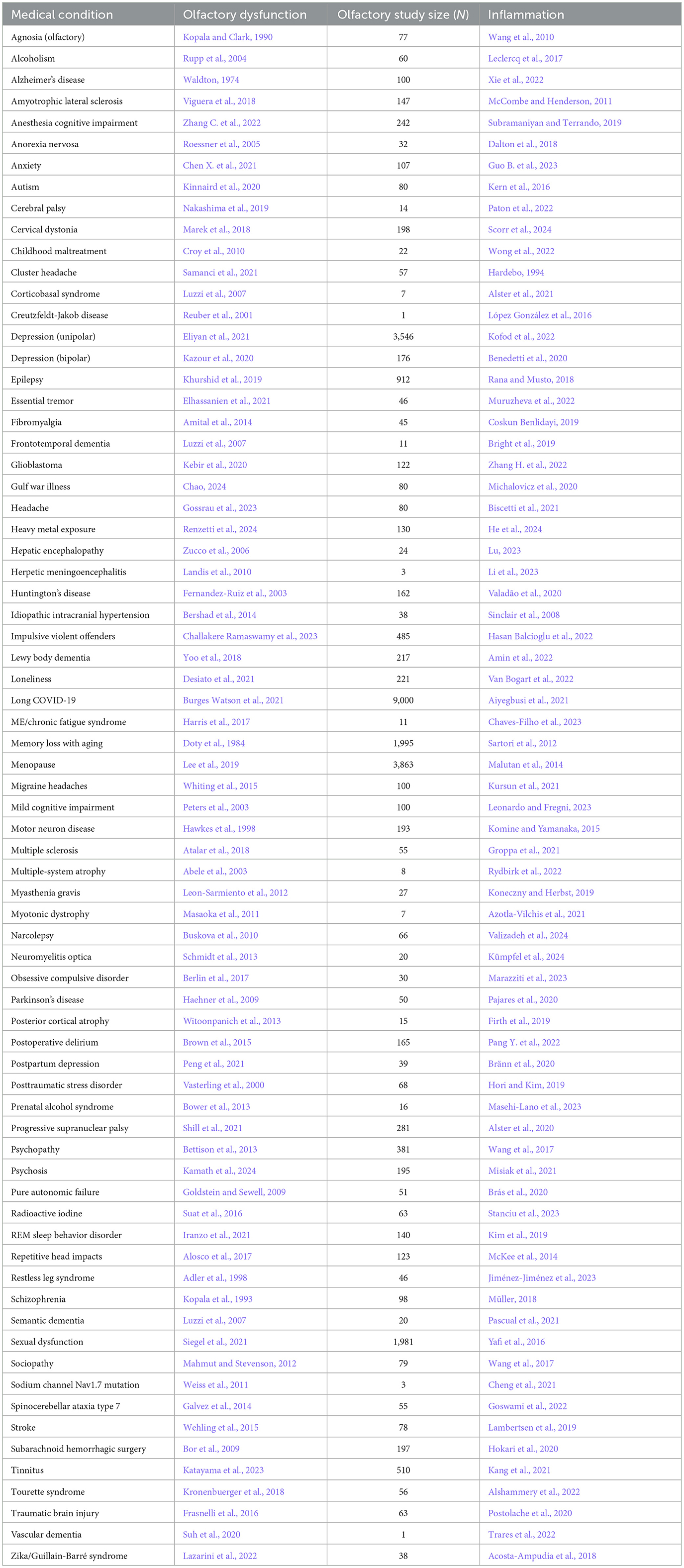
Table 1. Neurological condition/disorder, the reference for accompanying olfactory dysfunction, study size of olfactory study, and reference for inflammation.
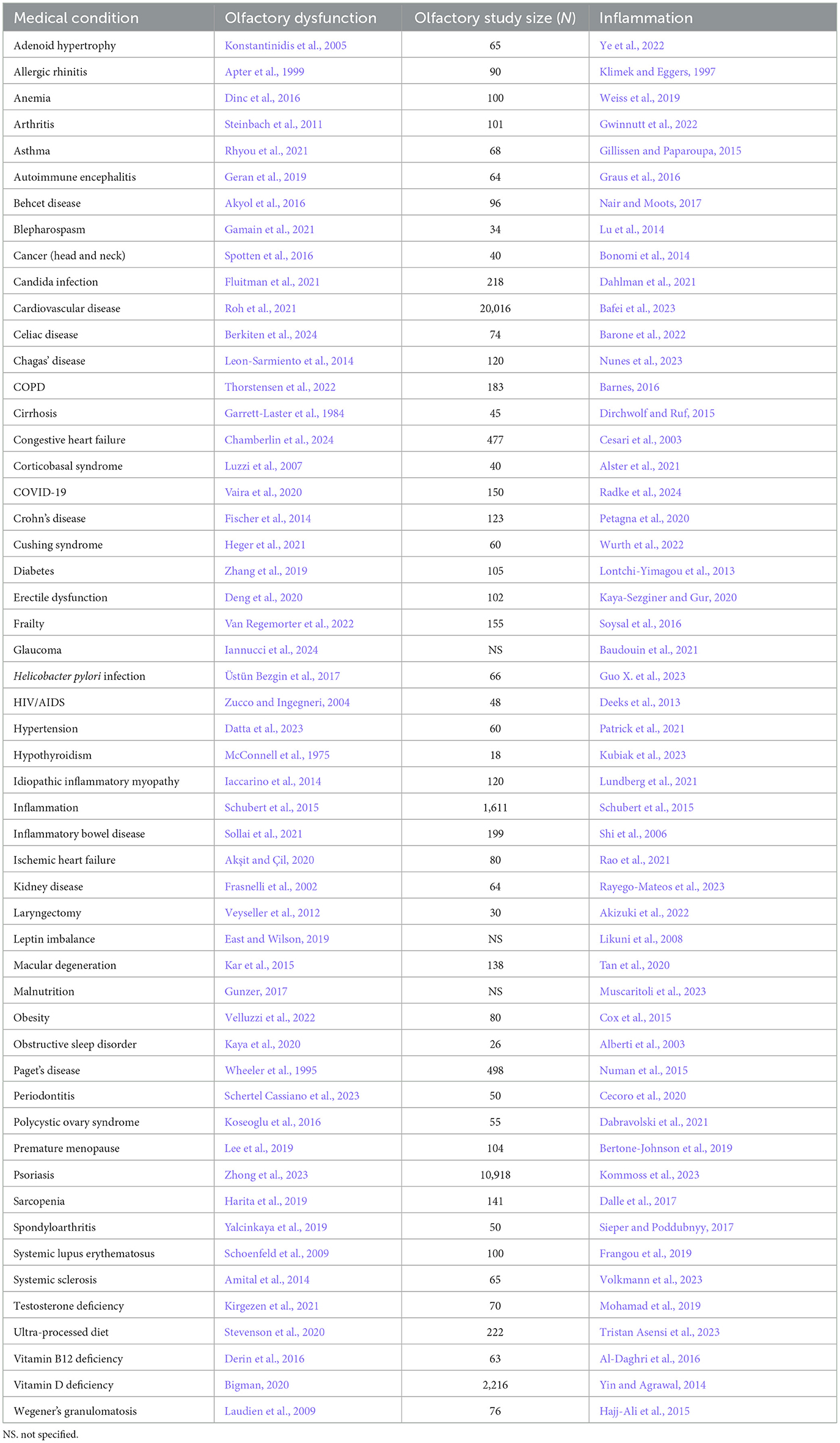
Table 2. Somatic condition/disorder, the reference for accompanying olfactory dysfunction, study size of olfactory study, and reference for inflammation.
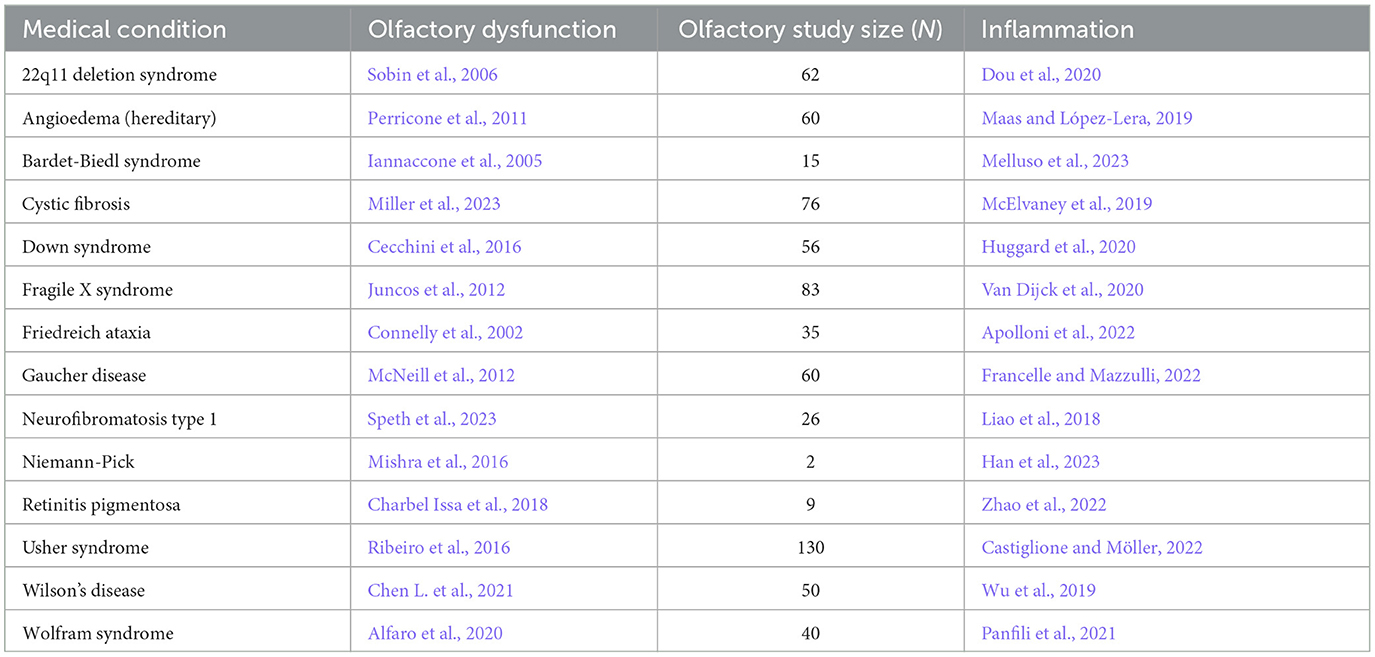
Table 3. Congenital/hereditary disorder, the reference for accompanying olfactory dysfunction, study size of olfactory study, and reference for inflammation.
Many of the associations between olfactory loss and medical conditions are supported by a single study. However, there are several conditions that have been studied extensively and there is strong support that has been reviewed for the relationship between these conditions and olfactory dysfunction: COVID-19 (Las Casas Lima et al., 2022), Alzheimer's disease (McLaren and Kawaja, 2024), Parkinson's disease (Bagherieh et al., 2023), depression (Kohli et al., 2016), and rhinitis (Ahmed and Rowan, 2020).
1.1.2 Olfactory dysfunction occurs early in the development of some medical conditionsTo show that olfactory loss increases the risk of developing symptoms of medical conditions, one would need to show that olfactory dysfunction arises before the medical condition. The relevant experiments are quite difficult to do because one must evaluate the olfactory ability of many individuals and then follow them for years to determine whether poor olfactory ability precedes the medical condition. Despite the challenge, several such studies have been conducted. Olfactory loss appears well before any other Parkinson's symptoms (Walker et al., 2021), and similarly, an early symptom of Alzheimer's disease is the loss of olfaction (Serby et al., 1991), with the first part of the brain to deteriorate in that disease being the olfactory pathway (Peters et al., 2003). Schizophrenia is associated with olfactory dysfunction and such dysfunction can be seen in youths who eventually develop schizophrenia (Kamath et al., 2012). Olfactory loss also precedes depression (Kamath et al., 2024), major cardiac events (Chamberlin et al., 2024), and multiple sclerosis (Constantinescu et al., 1994); olfactory dysfunction therefore appears to be a prodromal symptom of these conditions.
1.1.3 Olfactory dysfunction prospectively predicts cognitive loss and all-cause mortalityIn men, significant correlations are found in measurements of olfactory thresholds and language index score, along with correlations with executive function. On the other hand, women had correlations for olfactory discrimination and olfactory identification with a visuospatial index score (Masala et al., 2024). In young adults, olfactory ability is correlated with cognitive performance as assessed by verbal fluency, word list learning, word list recall, and the Trail Making Tests, even when the outcomes were adjusted for age, sex, education, and depression symptoms (Yahiaoui-Doktor et al., 2019). Challakere Ramaswamy and Schofield (2022) reviewed 54 studies and found a variety of cognitive abilities that correlated with olfactory ability, including: impulsivity, processing speed, inhibitory control, verbal fluency, working memory, mental flexibility, decision-making, visuospatial processing, planning, and executive function.
If olfactory loss has a causal relationship with at least some medical conditions, one might expect that the loss of olfaction would predict the incidence of those conditions. Indeed, one can predict the probability that older adults will later develop mild cognitive impairment (MCI) based on their olfactory ability (Wheeler and Murphy, 2021). Furthermore, of those individuals who have MCI, one can predict which individuals will develop Alzheimer's disease, as well as which individuals will descend rapidly into their dementia, based on their olfactory ability (Wheeler and Murphy, 2021). Parkinson's patients have both a loss of olfactory function and a loss of executive function (Solla et al., 2023). There are now a number of large prospective cohort studies showing that olfactory ability is a strong predictive factor for all-cause mortality up to 17 years later (Wilson et al., 2011; Gopinath et al., 2012; Pinto et al., 2014; Devanand et al., 2015; Ekström et al., 2017; Schubert et al., 2017; Fuller-Thomson and Fuller-Thomson, 2019; Kamath and Leff, 2019; Liu et al., 2019; Choi et al., 2021; Pinto, 2021; Xiao et al., 2021; Pang N. Y. et al., 2022), with higher accuracy than predictions based on heart disease (Pinto et al., 2014).
1.2 Mechanisms linking olfactory loss and medical conditions: inflammation, neuroanatomy, environmental stressors 1.2.1 Mechanism for triggering olfactory system damageThere are several possibilities for the mechanism underlying the many associations between olfaction and disease. One possibility is that there is a common mechanism that affects both the olfactory system and various neurological and somatic targets. Another possibility is that the neurological and somatic conditions produce something that degrades the olfactory system. A third possibility is that the olfactory system produces something that puts the brain and the body at risk either for contracting diseases or for expressing the symptoms of those diseases. One common product of disease is inflammation, and there is a strong relationship between olfactory dysfunction and elevated inflammation. As can be seen in Tables 1–3, at least 139 conditions that are associated with olfactory loss are also associated with increased inflammatory responses. These conditions have been subdivided into three separate categories: neurological, somatic, and congenital/hereditary conditions (Tables 1–3, respectively). Although the conditions could have been further subdivided into many other more specific categories, and some of the conditions may fall under two different categories, for simplicity, each medical condition was included in only one of the three categories.
1.2.2 Inflammation may be causing the olfactory dysfunctionPerhaps the olfactory system is particularly sensitive to inflammation that reaches it either from other parts of the brain or through the peripheral bloodstream. Alternatively, inflammation in the olfactory system may be triggered by agents that enter through the nose, such as air pollution (Ajmani et al., 2017) or unpleasant odors (Anja Juran et al., 2022). In addition, olfactory dysfunction associated with SARS-CoV-2 (COVID-19) infection is thought to be mediated in part via inflammation (Chang et al., 2024). The olfactory system may be uniquely sensitive to damage inflicted by other sources of inflammation (brain or body) that arise from various diseases because it is already sustaining high levels of inflammation from exposure to volatile agents from the air.
Poor ability to sniff contributes to the olfactory dysfunction of Parkinson's patients (Sobel et al., 2001). The ability to sniff predicted performance on olfactory tasks and increasing sniff vigor improved olfactory ability. The problems with sniffing may be due to increased inflammation that may prevent the respiratory system from compensating for the olfactory dysfunction (Huxtable et al., 2011).
Murphy et al. (2024) found that the efficacy of olfactory training for those individuals who had lost their olfactory ability after a COVID-19 infection was quite variable, with large differences in outcomes for different age groups. They surveyed more than 5,500 patients who had olfactory dysfunction following COVID-19 and compared the efficacy of various treatments including steroids and olfactory training. They found that nasal steroid use, given to reduce inflammation, was most effective for those 25–39 years old, with their effectiveness at about 25%, while oral steroid use was most effective for 18–24-year-olds, nearing 50%. Nasal steroids were most effective for treating hyposmia (poor olfactory ability), while oral steroids were most effective for phantosmia (imagined odors). Olfactory training was most effective for 18–24-year-olds, with effectiveness nearing 50%, while 40–60-year-olds had very poor effectiveness scores. Olfactory training was most effective for hyposmia.
Interestingly, several scents have been shown to have anti-inflammatory action in animal models, including: eucalyptol (Juergens et al., 2003), 1,8-cineol (Pries et al., 2023), lavender (Ueno-Iio et al., 2014), ginger (Aimbire et al., 2007), carvacrol (Alavinezhad et al., 2018), Shirazi thyme (Alavinezhad et al., 2017), farnesol (Ku and Lin, 2016), thymoquinone (El Gazzar et al., 2006, thymol (Gholijani et al., 2016), limonene (Hirota et al., 2012), citronellol (Pina et al., 2019), α-terpineol (Pina et al., 2019), Mentha piperita (Hudz et al., 2023), and mango (Rivera et al., 2011; see Ramsey et al., 2020 and Gandhi et al., 2020 for reviews).
The links between olfaction and inflammation seem also to be mediated by diet. Transgenic mice with high levels of the apolipoprotein E gene APOE4 (a risk factor for Alzheimer's disease) and given a diet with low docosahexaenoic acid (an omega-3 fatty acid) had olfactory loss and memory loss along with an increase in IBA-1, an inflammatory factor, in the olfactory bulb. The mice given a diet high in docosahexaenoic acid experienced no olfactory loss, cognitive loss, or elevated inflammation (González et al., 2023). Humans who have a diet low in monosaturated and polyunsaturated fats have an increased risk of both cognitive loss and olfactory loss (Vohra et al., 2023).
Although the list of conditions in which olfactory loss and inflammation co-occur is long, there do exist medical conditions that involve olfactory loss, without reports of inflammation. One example is Kallmann syndrome, in which olfactory bulb development is disordered. Individuals with this condition have olfactory loss as well as deterioration in various brain areas, but it is unclear whether the neurological differences arise from olfactory dysfunction or from the other aspects of the syndrome (Manara et al., 2014; Ottaviano et al., 2015). It certainly is possible that this condition involves an increase in inflammation, even though no one has reported it.
1.2.3 Olfactory loss results in damage to brain regions central to memory functionGiven the predictive nature of olfactory loss for memory impairment in dementia, the question arises as to how olfactory loss could play a role in memory loss specifically. In fact, the olfactory system is anatomically unique among the senses, in that it has a “superhighway” that bypasses the thalamus and projects directly to regions of the brain involved in memory processing (Gottfried, 2006). Multiple studies now show that loss of olfaction is associated with deterioration of several brain regions (Bitter et al., 2010a,b; Eckert et al., 2024; Han et al., 2023; Kovalová et al., 2024; Peter et al., 2023; Seubert et al., 2020; Whitcroft et al., 2023; Yao et al., 2018), including the regions of the brain integral to memory acquisition and processing. While the deterioration of brain areas may be due to olfactory loss, it is also possible that the factor that produced the olfactory dysfunction also produced the damage in the other brain areas.
1.2.4 Environmental challenges compromise both olfaction and memoryHaving identified inflammation as a possible global mediating factor in the links between olfactory loss and medical conditions and mortality, as well as neuroanatomical factors creating a tighter fit between olfactory loss and memory loss specifically, we can proceed to ask whether specific life experiences may activate such connections. There are indeed experiences that are known to cause both loss of olfactory ability and loss of memory, as well as the more diffuse impairments often referred to as “brain fog”. These include: smoking (Ajmani et al., 2017; Lewis et al., 2021), air pollution (Calderón-Garcidueñas and Ayala, 2022; Wang X. et al., 2021), a wide range of medications (Schiffman, 2018; Chavant et al., 2011), stress (Hoenen et al., 2017; Shields et al., 2017), childhood maltreatment (Maier et al., 2020; O'Shea et al., 2021), illiteracy (Dong et al., 2021; Arce Rentería et al., 2019), menopause (Lee et al., 2019; Maki, 2015), toxins (Upadhyay and Holbrook, 2004; Guan et al., 2022), alcoholism (Maurage et al., 2014; Pitel et al., 2014), respiratory infections (Potter et al., 2020; Matsui et al., 2003), nasal passage blockage (Mohamed et al., 2019; Arslan et al., 2018), head trauma (Lötsch et al., 2016; McInnes et al., 2017), highly processed food (Makhlouf et al., 2024; Gomes Gonçalves et al., 2023), and COVID-19 (Doty, 2022).
In one longitudinal study (Douaud et al., 2022), imaging was used to examine the effects of COVID-19 on the brain for individuals who had contracted a mild case of COVID-19 during the time between two brain scans. The second scan was completed approximately 141 days after testing positive for COVID-19, with an average time of 3 years between scans. Comparisons were made with brain scans from individuals who had not tested positive between scans. In the group who had contracted COVID-19, the researchers found significant damage in the regions of the brain involved in olfaction and memory, including the anterior cingulate cortex, orbitofrontal cortex, ventral striatum, amygdala, hippocampus, and parahippocampal gyrus, and the extent of olfactory loss predicted the extent of the brain damage (Campabadal et al., 2023). These individuals also continued to experience cognitive loss.
1.2.5 Olfactory dysfunction and cognitive lossCompared to our ancestors, most humans in the affluent world experience a narrower range of evolutionarily relevant odors. In addition, people typically have experiences that damage their olfactory system: air pollution, stress, toxins, anatomical blockage, smoking, various medications, adverse childhood experiences, menopause, and even chronic sinusitis, all of which also trigger memory loss (Eimer and Vassar, 2013). As people age, the deterioration of their olfactory ability accompanies the deterioration of their cognitive ability (Leon and Woo, 2022; Doty et al., 1984), perhaps because olfactory loss results in a significant loss of both gray matter and white matter in the cognitive areas of human brains (Schaie et al., 2004; Bitter et al., 2010a,b).
1.2.5.1 Olfactory loss accompanies dementiaOlfactory dysfunction predicts cognitive dysfunction in humans (Schubert et al., 2008) and the loss of olfactory function precedes or parallels the initiation of a wide variety of cognitive disorders such as: AD, MCI, Parkinson's disease, Lewy body dementia, frontotemporal dementia, Creutzfeldt-Jakob disease, alcoholism, and schizophrenia (Wang Q. et al., 2021; Conti et al., 2013; Adams et al., 2018; Ponsen et al., 2004; Birte-Antina et al., 2018).
1.2.5.2 COVID-19 links olfactory loss and dementiaCOVID-19 typically produces olfactory loss, and comparisons of MRI scans from individuals both pre-infection and post-infection have revealed neural deterioration that resembles a decade of aging in the cognitive brain regions that receive olfactory-system projections, along with damage to those areas involved in olfaction (Kollndorfer et al., 2015; Segura et al., 2013). Kay (2022) made the case that COVID-19 infections that produce olfactory loss may foster the cognitive loss that is seen in Alzheimer's disease. In fact, Wang et al. (2022) did a retrospective study of 6,245,282 older adults and showed that people with COVID-19 were at significantly increased risk for new diagnosis of Alzheimer's disease within 360 days after the initial COVID-19 diagnosis. Rahmati et al. (2023) went on to do a meta-analysis of twelve studies tracking over 33 million individuals who either had contracted COVID-19 or did not contract the virus. The pooled analyses showed a significant association between COVID-19 infection and subsequent increased risk for new-onset Alzheimer's disease. Given the remarkable number of physiological systems that were affected by the disease (Nasserie et al., 2021), there is no reason to believe that the olfactory loss was the sole factor in increasing the risk of Alzheimer's, but it may be that the loss of olfaction contributed to the degradation of regions in the brain integral to normal memory functioning, as mentioned previously (Kovalová et al., 2024).
1.3 Efficacy of olfactory enrichment 1.3.1 Olfactory enrichment improves symptoms of cognitive impairmentShi et al. (2023) reviewed a number of studies examining the effects of exposure to essential oils and found a wide range of benefits to the brain and behavior. The benefits included normalizing neurotransmitter levels, decreasing inflammatory factors, decreasing oxidation, increasing neuroprotective factors, improving memory, decreasing neuronal loss, and suppressing beta amyloid levels.
1.3.2 Olfactory enrichment results in memory benefits for healthy adultsFrom a preventive perspective, about 20 studies have now been performed showing that increasing olfactory stimulation can improve memory (Vance et al., 2024).
For example, olfactory enrichment improves cognition in older adults. Birte-Antina et al. (2018) provided olfactory enrichment with 4 essential-oil odorants twice a day for 5 months, while controls solved daily Sudoku puzzles. The olfactory-enriched group had a significant improvement of olfactory function, improved verbal function, and decreased depression symptoms. Oleszkiewicz et al. (2022) exposed 68 older adults either to 9 odorants twice a day or to no new odorants for 3–6 months, and found the enriched olfactory experience produced improvements in cognitive abilities, dementia status, and olfactory function relative to controls. Specifically, the Montreal Cognitive Assessment revealed a significant improvement in the olfactory-enriched group relative to controls, and the AD8 Dementia Screening Interview showed that enriched participants had no increase in dementia symptoms over the trial period, while control participants had an increase in symptoms.
Increased complexity of olfactory enrichment also improves dementia symptoms. Cha et al. (2022) exposed 34 older adults with dementia to 40 odorants twice a day for 15 days. The control group consisting of 31 individuals with dementia received no such stimulation. There were no initial differences between groups, and all had a Mini-Mental Status Examination score of at least 10. The results were remarkable, as the olfactory-enriched group showed highly significant improvements in memory, olfactory identification, depression symptoms, attention, and language skills. Olfactory-enriched individuals improved their olfactory identification, while controls did not. The Verbal Fluency Test also showed significant improvements for the enriched group relative to the controls. Similarly, the Boston Naming Test revealed a significant improvement in the enriched subjects relative to controls. The Word-List Memory Test, the Word-List Recall Test, the Word List Recognition Test, and the Geriatric Depression Scale all improved in the enriched group relative to controls.
Lin and Li (2022) exposed older adults with mild-to-moderate dementia to 15 essential oils/essences twice a week for 30-min sessions over a 12-week randomized clinical trial. Participants in the olfactory enrichment group also were asked to relate each scent to a matching photo of the scent source. The olfactory enrichment group showed significant cognitive improvement on the Loewenstein Occupational Therapy Cognitive Assessment-Geriatric test. In addition, olfactory enrichment prevented the increase in plasma beta amyloid seen in the control group.
In an effort to minimize burden and increase compliance, we tested the idea that we could get enhanced neural and cognitive outcomes after even minimal olfactory enrichment at night (Woo et al., 2023). The limitations of the available diffusion devices at the time forced us to use this minimal level of olfactory enrichment. Therefore, we gave olfactory enrichment or control exposures to older adults (60–85 years old) for 2 h every night for 6 months, using a single odorant each night, rotating through seven scents a week (Woo et al., 2023). There were statistically significant differences between enriched and control older adults in their cognitive ability using the Rey Auditory Verbal Learning Test, with enriched individuals scoring 226% better than controls. We also found a statistically significant change in mean diffusivity in the uncinate fasciculus of the enriched group compared to controls.
1.4 Mechanisms of olfactory enrichment: inflammation, neuroanatomy, and cognitive reserve 1.4.1 Reduction of inflammation may be the mechanism by which olfactory enrichment improves neurological symptomsA range of correlational and causal relationships connect inflammation with olfactory loss. Olfactory loss is associated with an increase in Interleukin-6 (IL-6), which increases both inflammation and the maturation of B cells (Henkin et al., 2013) and is also correlated with an increase in C-reactive protein, which increases in the presence of inflammation as indicated by IL-6 (Ekström et al., 2021). Chronic inflammation is associated with olfactory dysfunction (LaFever and Imamura, 2022). As noted earlier, a proinflammatory diet for older adults with low levels of polyunsaturated fatty acids and monosaturated fatty acids is associated with elevated inflammation, olfactory dysfunction, and cognitive decline (Vohra et al., 2023). Moreover, such a diet increases the risk of dementia (Simopoulos, 2002). The association between olfactory dysfunction and frailty varies with the level of inflammation, as measured by circulating levels of the pro-inflammatory cytokine IL-6 (Laudisio et al., 2019). Hahad et al. (2020) found that inflammation mediated the loss of cognition in those exposed to high levels of pollution.
Turning to causal links, unpleasant odors activate the inflammatory response by increasing tumor necrosis factor alpha (TNFα) and decreasing secretory immunoglobulin A (slgA) in saliva (Anja Juran et al., 2022). Imamura and Hasegawa-Ishii (2016) found that toxins can activate the immune response in the olfactory mucosa. Conversely, smelling pleasant odors suppresses immune activity, and more strikingly, even the act of imagining pleasant odors suppresses the immune response, specifically circulating interleukin-2 (IL-2; Matsunaga et al., 2013; Shibata et al., 1991). Casares et al. (2023) found that 6 months of exposure to menthol odor improved both the memory of young mice and the memory of mice that were modified to model Alzheimer's disease. This odor exposure also suppressed inflammation (IL-1β; Casares et al., 2023). Equally, pharmaceutical suppression of inflammation in those mice improved their memory (Casares et al., 2023).
The suppression of the inflammatory response may therefore underlie the finding that olfactory enrichment can improve memory (Cha et al., 2022; Woo et al., 2023). In addition, olfactory enrichment may improve symptoms of other neurological conditions through a similar mechanism.
1.4.2 Olfactory enrichment creates functional and structural changes in the brainIncreased olfactory stimulation, as experienced daily by master perfumers and sommeliers, who sample many odors each day for months and years, results in increased volume of brain regions that receive olfactory projections (Royet et al., 2013; Filiz et al., 2022). A longitudinal study showed that after a year and a half of olfactory training, sommeliers in training, who sampled dozens of odors every day for months to be able to identify those odors in fine wines, increased the thickness of their entorhinal cortex, a brain region critical for memory formation and consolidation (Filiz et al., 2022; Takehara-Nishiuchi, 2014). This structural change may well have functional benefits. Daily olfactory training for 6 weeks resulted in improved olfactory functioning as well as increased cortical thickness of olfactory processing regions of the brain (Al Aïn et al., 2019), and multiple scents presented daily improved cognition in both adults and older adults (Oleszkiewicz et al., 2021, 2022). Additionally, reversal of some medical issues, such as removing an anatomical blockade in the nasal passages, can result in improved cognition and attention, as measured using neuropsychological assessments and event-related auditory evoked potentials (P300; Arslan et al., 2018). In the memory study with healthy older adults described above (Woo et al., 2023), the enriched group that showed improvement in memory performance also had a statistically significant change in mean diffusivity in the uncinate fasciculus, a brain pathway involved with maintaining cognitive processes.
1.4.3 Electrical stimulation of the olfactory systemOne mechanism by which olfactory enrichment may be working is by stimulating specific brain areas. Beta amyloid (Aβ) is elevated in Alzheimer's disease (Pignataro and Middei, 2017). In a rat model of Alzheimer's disease, electrical stimulation of the olfactory bulb reversed the accumulation of beta amyloid (Aβ) plaque formation in the prefrontal cortex, the entorhinal cortex, the dorsal hippocampus, and the ventral hippocampus. It also blocked the impairments in working memory in these rats (Salimi et al., 2024). In addition, electrical stimulation of the olfactory bulb also increased functional connectivity in the brain during a working memory task. It should be noted that transethmoid electrical stimulation of the human olfactory bulb induced olfactory perceptions (Holbrook et al., 2019). Olfactory enrichment may therefore act to stimulate the areas to which the olfactory input projects (Gottfried, 2006). Conversely, intrabulbar injections of Aβ in rats decreased olfactory function, a phenomenon that was more easily triggered in older rats (Alvarado-Martínez et al., 2013).
1.4.4 Making a distinction between contracting a disease vs. experiencing symptoms of a diseaseIt is important in a discussion regarding causality to consider whether something can differentially change the risk of contracting a disease or the risk of experiencing the symptoms of the disorder. This distinction may be important for our understanding of the relationships between olfaction, cognition, and disease. Typically, the symptoms of the disease accompany the disease itself, but there are exceptions. Some people who contracted the COVID-19 virus, for instance, did not show any symptoms of the disease (Rasmussen and Popescu, 2021). In the phenomenon called cognitive reserve, an individual can develop the neuropathology of Alzheimer's disease, indicating that they had contracted the disease, but show none of the symptoms of severe memory loss (Stern, 2012).
1.4.5 Olfactory ability and cognitive reserveIn mice, long-term olfactory enrichment improves olfactory ability, and it also improves learning and memory for tasks that do not involve odors (Terrier et al., 2024). This effect may represent a form of cognitive reserve in mice, here mediated by an increase in noradrenergic innervation and resulting in the remodeling of brain connectivity in older mice. These data suggest a causal association between olfactory enrichment and cognition. In humans, odor threshold correlates with a measure of cognitive reserve that involves education, while odor discrimination ability correlates with career experiences and leisure experiences. Women had significant correlations between odor threshold, discrimination and identification, and leisure experiences, while men had a significant association between odor threshold and educational experiences (Masala et al., 2023).
1.4.6 Olfactory enrichment may induce cognitive reserve in humansCognitive reserve in humans comes from leading a life filled with environmental enrichment, with a high level of education, a cognitively engaging career, and a high level of socializing (Stern, 2012). Conversely, illiterate individuals have the highest probability of developing Alzheimer's disease (Dong et al., 2021), and they have little of the enrichment that seems to protect those with cognitive reserve (Brucki, 2010). Perhaps the uniquely direct connections of the olfactory system to the regions of the brain that are critical for memory functioning allow the olfactory system to rapidly induce what may be called cognitive reserve in humans.
2 DiscussionThere is reason to believe that the relationship between olfactory loss and medical conditions may be more than coincidental. First, there are many instances where both are present, with at least 139 medical conditions showing associations with olfactory dysfunction. Second, olfactory loss precedes the expression of the medical condition, raising the possibility that olfactory loss makes the brain or body vulnerable to expressing the symptoms of these medical conditions. Third, olfactory loss prospectively predicts both memory loss and all-cause mortality.
Inflammation could be a key mechanism underlying a causal relationship between olfaction and memory; neuroanatomical and environmental factors also play a role. While the causal arrow may go either way, it is possible that for some conditions, it is the olfactory loss that raises the risk of expressing the symptoms of those conditions.
If olfactory loss increases the risk of either developing these medical conditions or having the symptoms of the conditions, then it may be possible to prevent the onset of symptoms from these conditions. Studies show that olfactory enrichment improves memory performance in healthy adults and there are even greater improvements found for adults with dementia. These benefits may be mediated via reduction of inflammation.
A suggestive notion underlying many of these observations is that neuropathology is not always symptomatic, thanks to phenomena such as cognitive reserve. For instance, people with cognitive reserve have the neuropathology of Alzheimer's disease, but they don't have the memory-loss symptoms. The olfactory system may be involved in generating protective cognitive reserve especially for memory-related conditions. More widely, since pleasant scents can decrease harmful inflammation, it seems possible that olfactory enrichment may reduce the symptoms of other medical conditions.
Future directions for research in this area would include simultaneously studying both olfaction and inflammation in specific medical conditions, studying more conditions in individuals who have olfactory dysfunction, and studying these variables over time. It also would be interesting to block inflammation in specific medical conditions to determine the effects on olfaction.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributionsML: Writing – original draft. ET: Conceptualization, Writing – review & editing. CW: Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsWe thank Dr. Tom Lane for his insightful comments on the manuscript.
Conflict of interestML holds equity in Science Lab 3, which is developing Memory Air®, a system that automatically delivers olfactory enrichment.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesAbele, M., Riet, A., Hummel, T., Klockgether, T., and Wüllner, U. (2003). Olfactory dysfunction in cerebellar ataxia and multiple system atrophy. J. Neurol. 250, 1453–1455. doi: 10.1007/s00415-003-0248-4
PubMed Abstract | Crossref Full Text | Google Scholar
Acosta-Ampudia, Y., Monsalve, D. M., Castillo-Medina, L. F., Rodríguez, Y., Pacheco, Y., Halstead, S., et al. (2018). Autoimmune neurological conditions associated with Zika virus infection. Front. Mol. Neurosci. 11:116. doi: 10.3389/fnmol.2018.00116
PubMed Abstract | Crossref Full Text | Google Scholar
Adams, D. R., Kern, D. W., Wroblewski, K. E., McClintock, M. K., Dale, W., and Pinto, J. M. (2018). Olfactory dysfunction predicts subsequent dementia in older U.S. Adults. J. Am. Geriatr. Soc. 66, 140–144. doi: 10.1111/jgs.15048
PubMed Abstract | Crossref Full Text | Google Scholar
Aimbire, F., Penna, S. C., Rodrigues, M., Rodrigues, K. C., Lopes-Martins, R. A., and Sertié, J. A. (2007). Effect of hydroalcoholic extract of Zingiber officinalis rhizomes on LPS-induced rat airway hyperreactivity and lung inflammation. Prostagland. Leukot. Essent. Fatty Acids 77, 129–138. doi: 10.1016/j.plefa.2007.08.008
PubMed Abstract | Crossref Full Text | Google Scholar
Aiyegbusi, O. L., Hughes, S. E., Turner, G., Rivera, S. C., McMullan, C., Chandan, J. S., et al. (2021). Symptoms, complications and management of long COVID: a review. J. R. Soc. Med. 114, 428–442. doi: 10.1177/01410768211032850
PubMed Abstract | Crossref Full Text | Google Scholar
Ajmani, G. S., Suh, H. H., Wroblewski, K. E., and Pinto, J. M. (2017). Smoking and olfactory dysfunction: a systematic literature review and meta-analysis. Laryngoscope 127, 1753–1761. doi: 10.1002/lary.26558
PubMed Abstract | Crossref Full Text | Google Scholar
Akizuki, H., Wada, T., and Tabuchi, K. (2022). Inflammation-based score (combination of platelet count and neutrophil-to-lymphocyte ratio) predicts pharyngocutaneous fistula after total laryngectomy. Laryngoscope 132, 1582–1587. doi: 10.1002/lary.29970
PubMed Abstract | Crossref Full Text | Google Scholar
Akyol, L., Günbey, E., Karlı, R., Önem, S., Özgen, M., and Sayarlioglu, M. (2016). Evaluation of olfactory function in Behçet's disease. Eur. J. Rheumatol. 3, 153–156. doi: 10.5152/eurjrheum.2016.017
PubMed Abstract | Crossref Full Text | Google Scholar
Al Aïn, S., Poupon, D., Hétu, S., Mercier, N., Steffener, J., and Frasnelli, J. (2019). Smell training improves olfactory function and alters brain structure. Neuroimage 189, 45–54. doi: 10.1016/j.neuroimage.2019.01.008
PubMed Abstract | Crossref Full Text | Google Scholar
Alavinezhad, A., Hedayati, M., and Boskabady, M. H. (2017). The effect of Zataria multiflora and carvacrol on wheezing, FEV1 and plasma levels of nitrite in asthmatic patients. Avicenna J. Phytomed. 7, 531–541.
PubMed Abstract | Google Scholar
Alavinezhad, A., Khazdair, M. R., and Boskabady, M. H. (2018). Possible therapeutic effect of carvacrol on asthmatic patients: a randomized, double blind, placebo-controlled, Phase II clinical trial. Phytother. Res. 32, 151–159. doi: 10.1002/ptr.5967
PubMed Abstract | Crossref Full Text | Google Scholar
Alberti, A., Sarchielli, P., Gallinella, E., Floridi, A., Floridi, A., Mazzotta, et al. (2003). Plasma cytokine levels in patients with obstructive sleep apnea syndrome: a preliminary study. J. Sleep Res. 12, 305–311. doi: 10.1111/j.1365-2869.2003.00361.x
PubMed Abstract | Crossref Full Text | Google Scholar
Al-Daghri, N. M., Rahman, S., Sabico, S., Yakout, S., Wani, K., Al-Attas, O. S., et al. (2016). Association of vitamin B12 with pro-inflammatory cytokines and biochemical markers related to cardiometabolic risk in Saudi subjects. Nutrients 8:460. doi: 10.3390/nu8090460
PubMed Abstract | Crossref Full Text | Google Scholar
Alfaro, R., Doty, R. T., Narayanan, A., Lugar, H., Hershey, T., and Pepino, M. Y. (2020). Taste and smell function in Wolfram syndrome. Orphanet J. Rare Dis. 15:57. doi: 10.1186/s13023-020-1335-7
PubMed Abstract | Crossref Full Text | Google Scholar
Alosco, M. L., Jarnagin, J., Tripodis, Y., Platt, M., Martin, B., Chaisson, C. E., et al. (2017). Olfactory function and associated clinical correlates in former National Football League players. J. Neurotrauma 34, 772–780. doi: 10.1089/neu.2016.4536
PubMed Abstract | Crossref Full Text | Google Scholar
Alshammery, S., Patel, S., Jones, H. F., Han, V. X., Gloss, B. S., Gold, W. A., et al. (2022). Common targetable inflammatory pathways in brain transcriptome of autism spectrum disorders and Tourette syndrome. Front. Neurosci. 16:999346. doi: 10.3389/fnins.2022.999346
PubMed Abstract | Crossref Full Text | Google Scholar
Alster, P., Madetko, N., and Friedman, A. (2021). Neutrophil-to-lymphocyte ratio (NLR) at boundaries of progressive supranuclear palsy syndrome (PSPS). and corticobasal syndrome (CBS). Neurol. Neurochir. Pol. 55, 97–101. doi: 10.5603/PJNNS.a2020.0097
PubMed Abstract | Crossref Full Text | Google Scholar
Alster, P., Madetko, N., Koziorowski, D., and Friedman, A. (2020). microglial activation and inflammation as a factor in the pathogenesis of progressive supranuclear palsy (PSP). Front. Neurosci. 14:893. doi: 10.3389/fnins.2020.00893
PubMed Abstract | Crossref Full Text | Google Scholar
Alvarado-Martínez, R., Salgado-Puga, K., and Peña-Ortega, F. (2013). Amyloid beta inhibits olfactory bulb activity and the ability to smell. PLoS ONE 8:e75745. doi: 10.1371/journal.pone.0075745
PubMed Abstract | Crossref Full Text | Google Scholar
Amin, J., Erskine, D., Donaghy, P. C., Surendranathan, A., Swann, P., Kunicki, A. P., et al. (2022). Inflammation in dementia with Lewy bodies. Neurobiol. Dis. 168:105698. doi: 10.1016/j.nbd.2022.105698
PubMed Abstract | Crossref Full Text | Google Scholar
Amital, H., Agmon-Levin, N., Shoenfeld, N., Arnson, Y., Amital, D., Langevitz, P., et al. (2014). Olfactory impairment in patients with the fibromyalgia syndrome and systemic sclerosis. Immunol. Res. 60, 201–207. doi: 10.1007/s12026-014-8573-5
PubMed Abstract | Crossref Full Text | Google Scholar
Anja Juran, S., Tognetti, A., Lundström, J. N., Kumar, L., Stevenson, R. J., Lekander, M., et al. (2022). Disgusting odors trigger the oral immune system. Evol. Med. Public Health 11, 8–17. doi: 10.1093/emph/eoac042
PubMed Abstract | Crossref Full Text | Google Scholar
Apter, A. J., Gent, J. F., and Frank, M. (1999). Fluctuating olfactory sensitivity and distorted odor perception in allergic rhinitis. Arch. Otolaryngol. Head Neck Surg. 125, 1005–1010. doi: 10.1001/archotol.125.9.1005
PubMed Abstract | Crossref Full Text | Google Scholar
Arce Rentería, M., Vonk, J. M. J., Felix, G., Avila, J. F., Zahodne, L. B., Dalchand, E., et al. (2019). Illiteracy, dementia risk, and cognitive trajectories among older adults with low education. Neurology 93, e2247–e2256. doi: 10.1212/WNL.0000000000008587
PubMed Abstract | Crossref Full Text | Google Scholar
Arslan, F., Tasdemir, S., Durmaz, A., and Tosun, F. (2018). The effect of nasal polyposis related nasal obstruction on cognitive functions. Cogn. Neurodyn. 12, 385–390. doi: 10.1007/s11571-018-9482-4
留言 (0)