Neuropsychiatric disorders affect up to 88% of traumatic brain injury (TBI) survivors (1). The symptomatology of such brain damage manifests in various psychopathological conditions, including personality changes, impulsivity, severe irritability, affective instability, and delusions (2).
Pharmacotherapy and cognitive behavioral therapy are considered first-line treatments for TBI patients (1). However, approximately half of these patients are refractory to medical treatment and require augmentation strategies or advanced treatments (3). Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive and outpatient therapy, which is gaining traction in the field of neurology and psychiatry (4).
Inhibitory rTMS protocols targeting the right prefrontal cortex (PFC) have shown efficacy in reducing obsessive-compulsive disorder (OCD) symptoms (5, 6) and depression post-TBI (7, 8). Moreover, excitatory rTMS protocols on the dorsolateral prefrontal cortex (DLPFC), bilaterally, have shown promise in managing a variety of psychiatric conditions, including depression (9), borderline personality disorders (BPD) (10, 11), and post-traumatic stress disorder (12). A recent study (13) compared two types of rTMS frequencies (inhibitory and excitatory) applied to the DLPFC, showing that both contribute to reducing impulsiveness, affective instability, and anger in patients with BPD.
Neuroimaging studies highlight the amygdala and PFC as two critical components of the brain’s circuitry that regulate personality and emotions (14, 15). Moreover, each amygdala has unique connections with different brain areas—the right amygdala with the contralateral area, basal ganglia, and frontal cortex, and the left amygdala with the anterior cingulate, right occipital, and left middle temporal gyrus. Disrupted connectivity in the amygdala is linked to various psychiatric disorders or populations who are at genetic risk for such illnesses (16, 17).
The PFC also plays a crucial role in regulating emotions throughout the brain (18). Damage to the right PFC can exacerbate negative emotions such as sadness or irritability, while damage to the left PFC can diminish positive emotions and motivation, which are closely associated with depression (19, 20).
Based on this evidence, the present study explores a dual-site rTMS treatment that combines inhibitory and excitatory stimulation on the DLPFC to modulate the frontolimbic network and improve symptoms in a TBI patient with complex psychopathology.
The primary outcomes measured were changes in impulsivity, OCD symptoms, and emotion regulation at 2, 4 and 8 weeks after the beginning of rTMS treatment, compared to baseline performance.
2 Materials and methods 2.1 ParticipantThe patient is a 34-year-old right-handed woman with 12 years of education, currently unemployed due to professional incapacity. At the age of 18, she met with a severe road traffic accident, resulting in polytrauma and a complex psychopathological condition. Prior to the accident, the patient was in good physical and psychological health.
The polytrauma led to a multifaceted clinical scenario, including penetrating injuries at the thoracic level, which caused a hypertensive pneumothorax, and at the abdominal level, leading to pneumoperitoneum. Additionally, the patient sustained severe contusions to both the head and the spine. The cranial trauma was particularly severe, inducing a coma due to bilateral subdural hematomas that required an emergency craniotomy. There was also widespread damage to the axons within the brain’s subcortical white matter.
Post-recovery, the patient exhibited a range of neurological symptoms, including cerebellar ataxia and right pyramidal syndrome, alongside cognitive impairments (deficits in executive function and attention) and behavioral issues (frontal disinhibition). In January 2008, an MRI of the brain revealed hemosiderin deposits, which are indicative of axonal damage at the subcortical level, primarily in the frontotemporal and temporobasal regions (predominantly on the left), along the trunk of the corpus callosum, and in the right upper paravermial region (Figure 1; upper row).
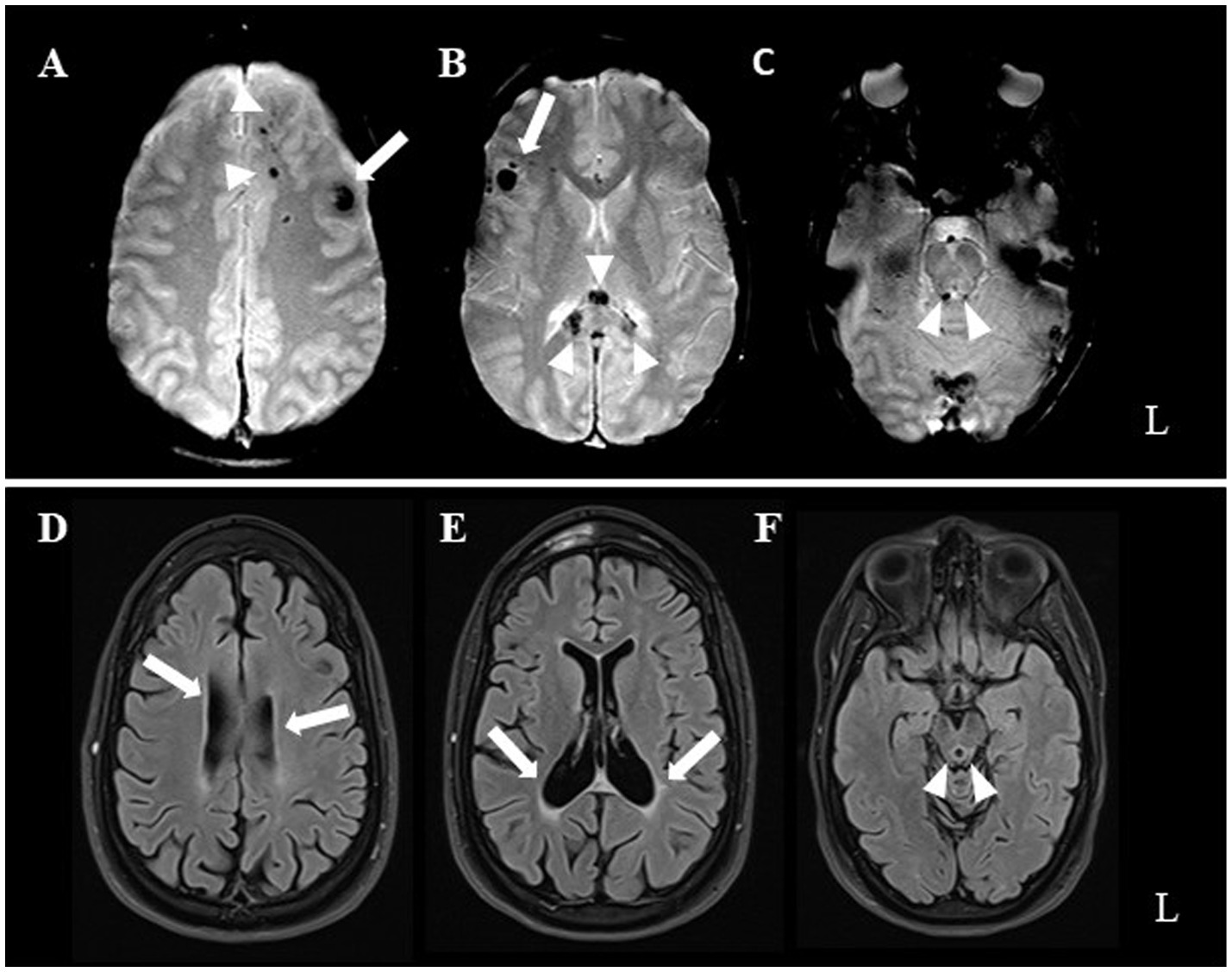
Figure 1. Upper row illustrates early post-injury axial T2*-weighted MRI. Hemorrhagic hypointense foci mark brain contusions at the level of the left forceps minor (arrowheads in A), left dorsolateral prefrontal cortex (arrow in A), right inferior frontal gyrus (arrow in B), splenium of corpus callosum (arrowheads in B), and periaqueductal mesencephalon tegmental region (arrrowheads in C). Lower row (D through F same acquisition planes as above) presents patient’s 13-year follow-up MRI. T2-FLAIR weighted images show occurrence of severe lateral ventricular (arrows) and aqueductal enlargement (arrowheads), related to parenchymal volume loss.
In the years following the accident, the patient experienced a partial improvement in her neurological symptoms but continued to suffer from severe psychiatric issues. These issues included impulsivity that lead to destructive behavior and self-harm, delusions, mood instability with depressive episodes, emotional suppression, and obsessive-compulsive behaviors.
The severity of these symptoms often required emergency psychiatric interventions, including compulsory hospital admissions. The treatment involved a combination of antipsychotic medications (clotiapine, quetiapine, and clozapine) and mood stabilizers (valproic acid, oxcarbazepine, and lithium).
At the last clinical visit (June 2023), the patient’s neurological and psychiatric conditions remained stable, with no significant improvement. A follow-up brain MRI in February 2021 (T2-FLAIR; Scanner: 3 Tesla Skyra [Siemens, Erlangen, Germany]; TR = 8,000 ms, TE = 85 ms, FA = 150 deg., TI = 2,372 ms, FOV = 220*220 mm, matrix = 256*179, slice thickness = 3 mm, interslice gap = 0.3 mm; acquisition time = 3′42″) revealed minimal changes compared to the previous MRI (T2* gradient echo; scanner: 1.5 Tesla Skyra [Siemens, Erlangen, Germany]; TR = 800, TE = 31, FA = 35 deg., FOV = 230*200, matrix = 256*168, slice thickness = 5 mm, interslice gap = 0.8 mm, acquisition time = 2′13″). The MRI revealed slight further enlargement of the ventricles and aqueduct, indicating parenchymal volume loss (Figure 1; lower row).
At the time, the patient was on psychopharmacological treatment consisting of lithium (1,350 mg/day) and quetiapine (450 mg/day) in titration. Given her partial response to previous treatments and the persistence of disabling behavioral symptoms, rTMS treatment was proposed.
2.2 Neurological, psychiatric, and neurocognitive assessmentThe study timeline is illustrated in Figure 2.
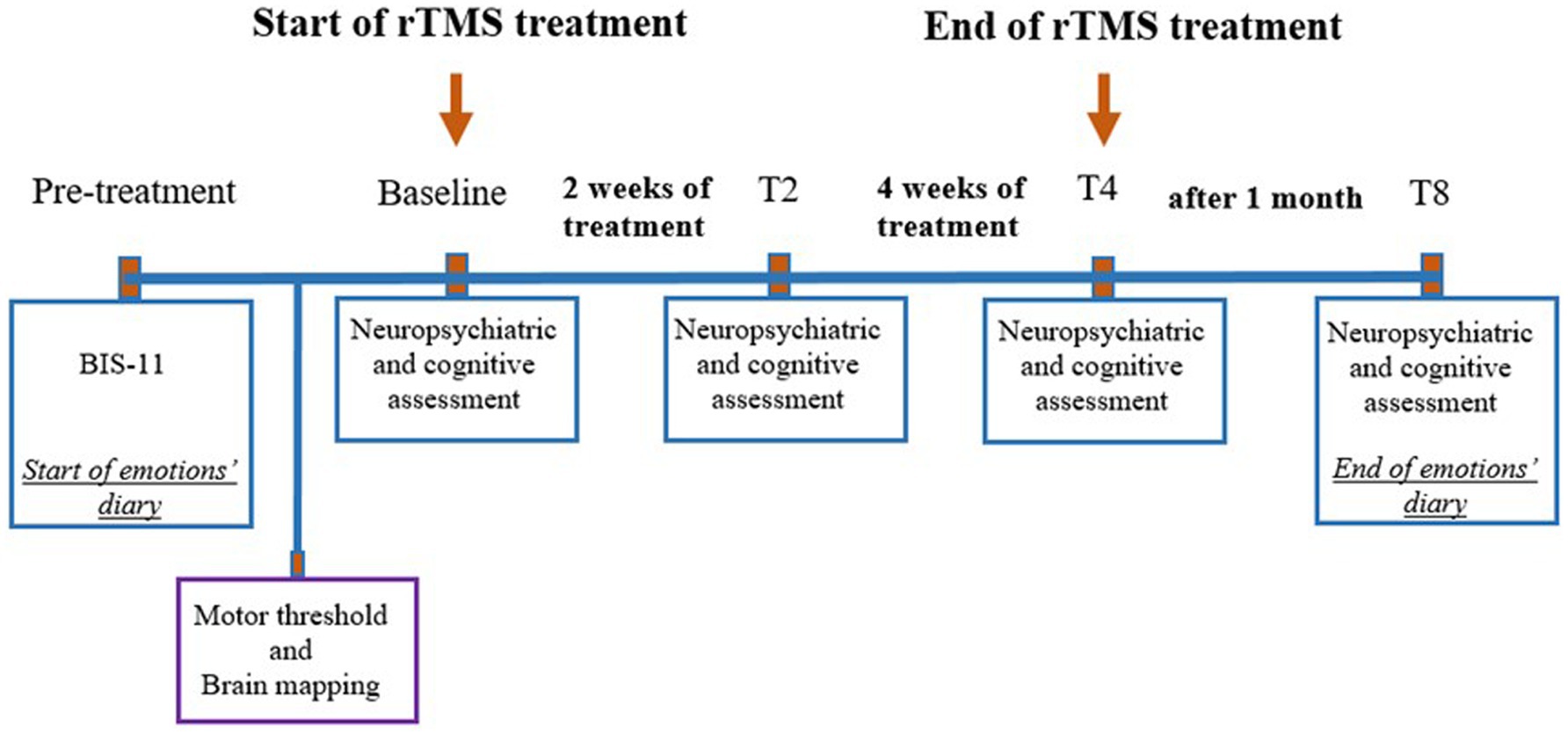
Figure 2. Timeline of events related to treatment. rTMS, Repetitive transcranial magnetic stimulation; BIS11, Barratt Impulsiveness Scale, T2, 2 weeks after the beginning of the treatment; T4, 4 weeks after the beginning of the treatment; T8, 8 weeks after the beginning of the treatment (follow-up).
The neurological examination included an assessment of movement disorders, possible side effects of medical therapy (iatrogenic), and psychiatric evaluation using the Clinical Global Impression Scale (CGI) (21). This scale was used to evaluate global illness severity (CGI-S), overall improvement from the start of treatment (CGI-I), and therapeutic response (CGI-E).
To assess impulsivity and obsessive-compulsive symptoms, the Barratt Impulsiveness Scale (BIS-11) (22) and the Yale-Brown Obsessive-Compulsive Scale (YBOCS) (23) were administered, respectively.
The neurocognitive evaluation tested sustained attention, cognitive flexibility, and processing speed using the Color Trails Test (24), and working memory and interference control using the Night and Day Test (25). The results from the neuropsychological tests were scored using a standardized method (26).
All assessments were conducted at four time points:
1. before rTMS treatment (baseline),
2. two weeks after the start of rTMS treatment,
3. four weeks after the start of rTMS treatment, and
4. eight weeks after the start of rTMS treatment (follow-up).
To control for social desirability bias, two pre-treatment BIS-11 measures were compared (one taken a week before treatment and one just before the treatment began).
In addition to the psychiatric and neurocognitive assessment, starting 1 week before the rTMS treatment, the patient was asked to maintain a diary to track daily mood fluctuations. The diary recorded both positive emotions (e.g., happiness, enjoyment, and satisfaction) and negative emotions (e.g., sadness, anger, fear, surprise, melancholy, loneliness, and annoyance), rated on a scale from 0 (“absent”) to 10 (“very high”).
2.3 Neuromodulation treatmentThe rTMS protocol was delivered using a 70-mm cooled coil connected to a Magstim Rapid 2 stimulator (Magstim Co., Whitland, United Kingdom). On a separate day prior to the first treatment session, the resting motor threshold (RMT) for the right abductor pollicis brevis muscle was determined using an amplaid electromyograph (Fa. Micromed, Freiburg, Germany) according to the method of limits (27). The stimulation intensity for the experiment was set at 100% of the RMT.
A T1-weighted MRI scan (TR = 1,900 ms; TE = 2.1 ms; TI = 900 ms; FOV = 240 mm2; matrix = 256 × 256; voxel size = 0.9 × 0.9 × 0.9 mm3) of the patient was used as an anatomical reference. The target points, expressed in Talairach space, were automatically registered to the patient’s native space using SoftTaxic software.
To further enhance the accuracy of stimulation, we utilized an open-source software tool (SimNIBS version 3.2) (28) to position the TMS coil precisely where the electric field strength was optimal.
Briefly, the patient’s T1-weighted MRI data were processed in SimNIBS to create a personalized head model and simulate the electric field distribution from the TMS coil. The resulting 3D map of the electric field was exported and aligned with SofTaxic’s coordinate system, which then guided the TMS coil in real time, optimizing the stimulation by targeting the areas of maximum electric field strength. For additional information, please refer to the Supplementary materials (29, 30).
Within the course of 1 month, the patient underwent 20 daily rTMS sessions, with one session per day, 5 days a week. The treatment included both inhibitory and excitatory rTMS stimulation.
Inhibitory rTMS (1 Hz) was applied to the right DLPFC in a continuous 15-min train, delivering one pulse per second for a total of 900 pulses per session. Excitatory rTMS (10 Hz) was applied to the left DLPFC in 30 trains of 10 s each, with a 10-s interval between trains, for a total of 1,500 pulses per session. Each session began with stimulation of the right DLPFC, followed by the left DLPFC. The patient had no prior experience with rTMS before the study.
2.4 Statistical analysisGiven the exploratory nature of the study, clinical, neuropsychiatric, and cognitive changes were assessed using univariate descriptive statistics.
3 ResultsThe results of the psychiatric and neurocognitive assessments are reported in Table 1.
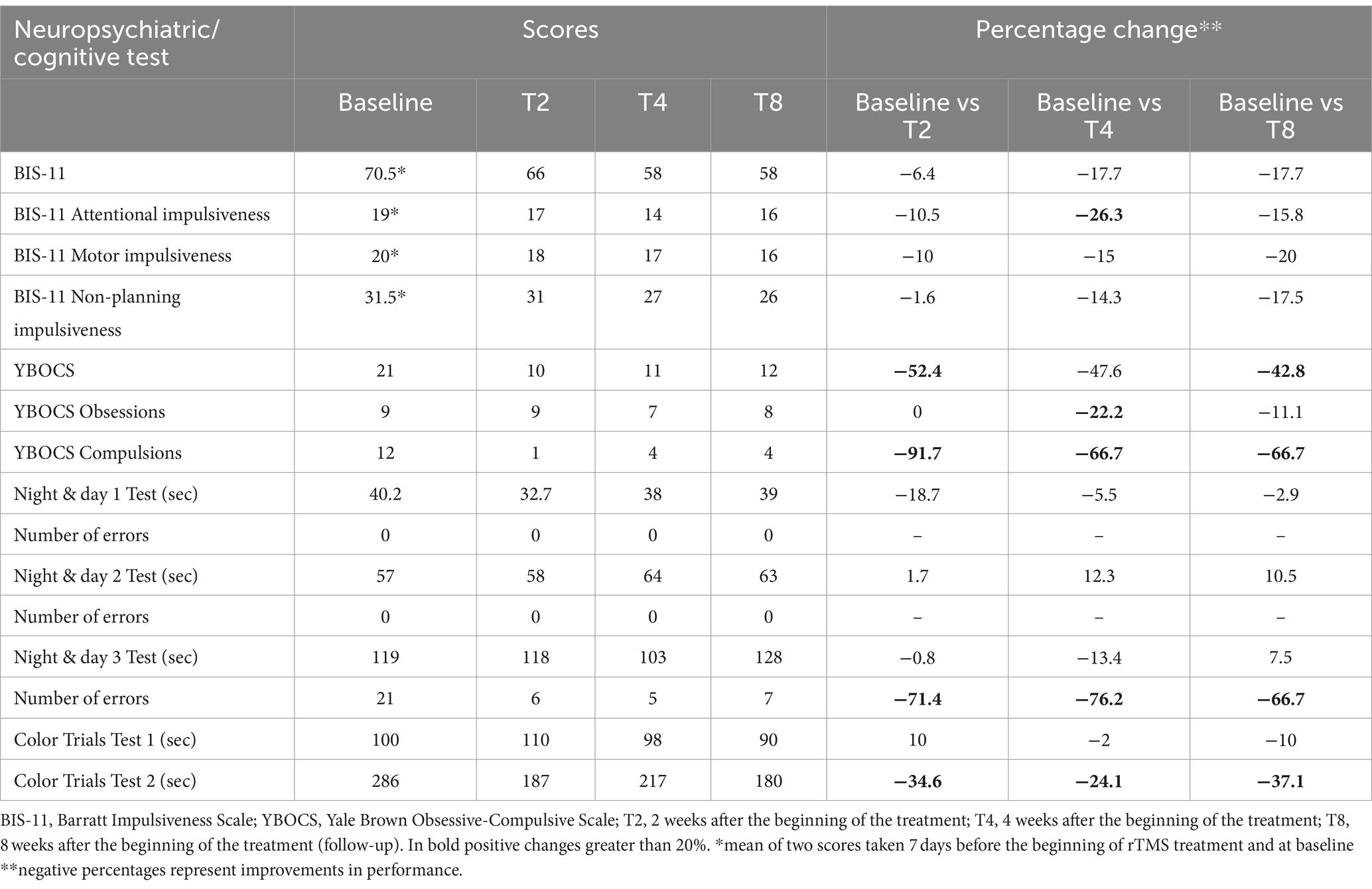
Table 1. Neuropsychiatric and cognitive assessment results over time.
At baseline, the extent of the patient’s illness was classified as severe (CGI = 6). Personality and behavior exhibited moderate disturbances (Barratt Impulsiveness Scale = 70.5; Yale-Brown Obsessive-Compulsive Scale = 21), while attentive and executive functions were impaired, displaying a significant number of errors due to diminished inhibition control.
After 2 weeks of treatment, notable improvements were observed in physical control, including reduced tremors, better posture, and improved fluidity in oral and gestural communication. Concurrently, there was a slight decrease in impulsivity (6.4%) and a significant reduction in obsessive-compulsive behavior (52.4%), with a substantial improvement in compulsive attitude (91.7%). Improvements were also observed in attentive-executive functions, including processing speed (18.7%), self-shifting (0.8%), and inhibitory control (34.6%), which led to a 71.4% increase in answer accuracy.
After 4 weeks, the patient experienced a reduction in illness severity to a moderate level (CGI = 4). Impulsivity control improved further, with a 17.7% reduction, and attentive impulsiveness showed a notable improvement of 26.3%. Although obsessive-compulsive tendencies increased slightly by 4.8%, the overall reduction remained significant at 47.6%. Cognitive performance in areas such as processing speed and sustained attention remained stable, while improvements in set-shifting and inhibitory control led to a further increase in answer accuracy, reaching 76.2%.
At the follow-up assessment, the neuropsychiatric and cognitive improvements observed earlier were still present, although there was a slight decrease in inhibitory control (13%) and response accuracy (10%).
A qualitative increase in positive daily emotions was observed over time, with baseline mean = 3.9, rising to 5.9 after 2 weeks, 5.4 after 4 weeks, and 6.5 after 8 weeks. Simultaneously, there was a consistent decrease in negative emotions, from a baseline mean of 3.9 to 2.8 after 2 weeks, 2.2 after 4 weeks, and 1.9 after 8 weeks (Figure 3). These trends suggests a sustained improvement in emotional regulation over the course of the study.
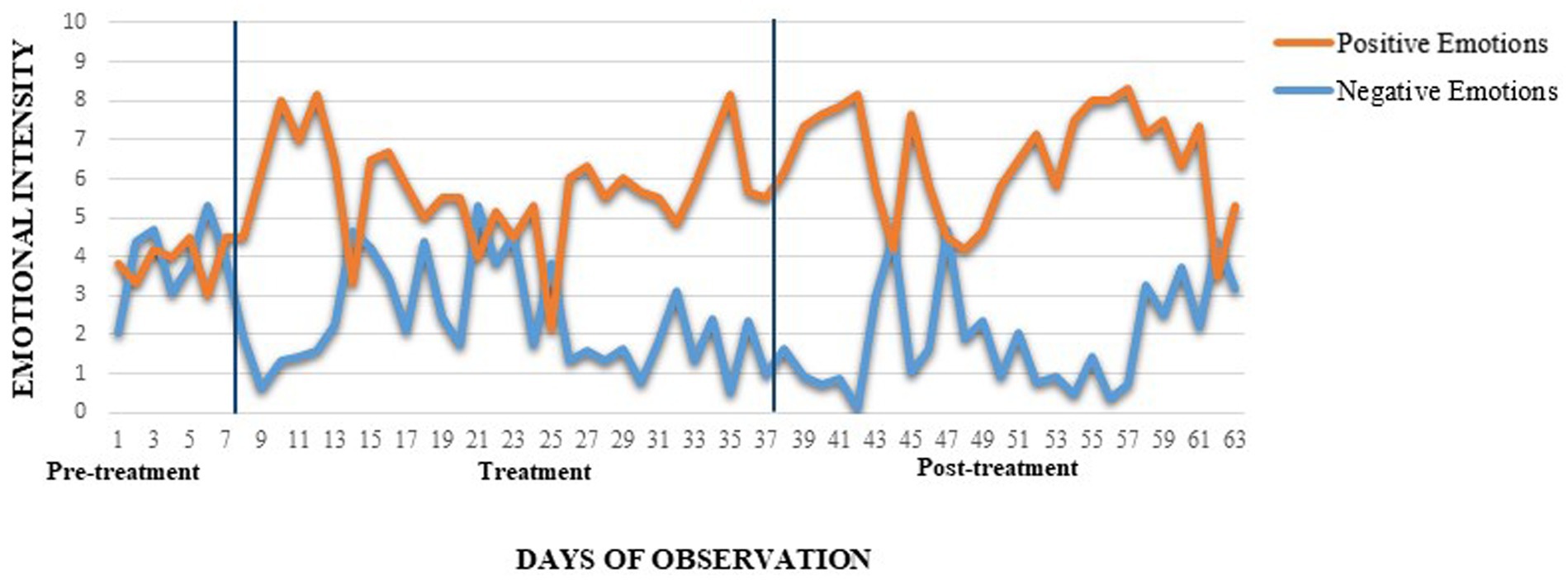
Figure 3. Daily self-report of positive and negative emotions intensity. BIS-11, Barratt Impulsiveness Scale; YBOCS, Yale Brown Obsessive-Compulsive Scale; T2, 2 weeks after the beginning of the treatment; T4, 4 weeks after the beginning of the treatment; T8, 8 weeks after the beginning of the treatment (follow-up). The two lines delimit treatment administration.
4 DiscussionTo the best of our knowledge, this is the first case to examine the effects of dual-site sequential focal coil rTMS, using 1 Hz (inhibitory) stimulation on the right DLFFC and 10 Hz (excitatory) stimulation on the left DLPFC, in a TBI patient with severe personality and emotional disorders.
Daily, sequential inhibitory and excitatory monitored stimulation was conducted to (1) increase the neuronal activity in the right subcortical prefrontal circuit to manage negative emotions (31) and obsessive-compulsive behavior (32); (2) stimulate the inhibitor control of the left DLPFC over the ipsilateral amygdala to regulate positive emotions (33, 34); and (3) increase interhemispheric connectivity to intensify the synaptic response of the basolateral amygdalae, thereby regulating amygdala-dependent behaviors (35).
The results indicate significant and lasting improvements in neuropsychiatric symptoms, with marked enhancements in clinical stability and social interactions.
Within 2 weeks, rTMS treatment led to improvements in physical balance, control, and posture, along with a reduction in obsessive-compulsive symptoms, executive dysfunction, and emotional instability.
Interestingly, previous findings have shown that inhibitory rTMS applied to the right DLPFC has a medium-term effect in reducing obsessive-compulsive symptoms and anxiety (5), suggesting that it rebalances prefrontal cortex activity. This rebalancing likely enhances executive function and control over impulsive, obsessive, and compulsive behaviors by modulating the activity of the cortico-striato-thalamo-cortical circuit (36).
After 4 weeks, rTMS treatment had a notable effect on impulsivity, particularly in reducing attentive impulsiveness, resulting in a substantial improvement in answer accuracy during cognitive tasks.
The treatment’s delayed impact on impulsivity dysregulation may be attributed to the complex nature of impulsivity, as its behavioral effects are often delayed due to learning processes that foster adaptive behaviors (37).
In line with previous findings on various psychiatric and personality disorders (10, 38, 39), we observed a qualitative increase in positive emotions (upregulation) and a decrease in negative emotions (downregulation) during treatment, with these effects persisting after 8 weeks. These emotional regulation improvements could be due to the sequential right and left rTMS stimulation, which contributes to emotional balance (40).
Finally, the sustained benefits of rTMS may be indicative of induced changes in cortical and subcortical synaptic efficacy and connectivity within the network responsible for controlling impulsivity, emotional instability, and emotional regulation.
The main limitation of this exploratory study is the absence of a control group. However, we are confident that the rigorous application of the rTMS intervention and the longitudinal assessment of within-subject changes provide valuable preliminary findings, serving as a reference point for future randomized control trials.
Additionally, our study did not assess motor threshold (MT) during TMS treatment, despite recent studies suggesting its potential to predict changes in symptomatology. Moreover, the application of neuroimaging techniques such as functional MRI to assess brain activity within the targeted network and quantitative electroencephalograms (EEG) for detailed analysis of electrical patterns could significantly enhance our understanding of the underlying mechanisms and the efficacy of rTMS in treating neuropsychiatric disturbances in TBI patients who are unresponsive to conventional medical treatments.
The current study provides preliminary evidence supporting the effectiveness of a sequential, alternated-frequency rTMS protocol in reducing impulsivity, OCD symptoms, and executive dysfunction in TBI patients. This preliminary evidence suggests that this rTMS protocol may have potential applications in the treatment of other conditions with similar symptom profiles, such as attention-deficit hyperactivity disorders (ADHD), Tourette’s syndrome, BPD, bipolar disorder, autism spectrum disorder, and OCD.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statementThe studies involving humans were approved by Comitato etico cantonale c/o Ufficio di sanità. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsGR: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. RB: Investigation, Methodology, Writing – original draft. MV: Data curation, Investigation, Project administration, Writing – original draft. EP: Resources, Visualization, Writing – review & editing. AK-L: Funding acquisition, Methodology, Supervision, Validation, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsThe authors would like to express their sincere gratitude to the patient in this study, whose strength and dedication were instrumental in making these findings possible.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fneur.2024.1412304/full#supplementary-material
References1. Torregrossa, W, Raciti, L, Rifici, C, Rizzo, G, Raciti, G, Casella, C, et al. Behavioral and psychiatric symptoms in patients with severe traumatic brain injury: a comprehensive overview. Biomedicines. (2023) 11:1449. doi: 10.3390/biomedicines11051449
PubMed Abstract | Crossref Full Text | Google Scholar
4. Kan, RLD, Padberg, F, Giron, CG, Lin, TTZ, Zhang, BBB, Brunoni, AR, et al. Effects of repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex on symptom domains in neuropsychiatric disorders: a systematic review and cross-diagnostic meta-analysis. Lancet Psychiatry. (2023) 10:252–9. doi: 10.1016/S2215-0366(23)00026-3
PubMed Abstract | Crossref Full Text | Google Scholar
5. Elbeh, KAM, Elserogy, YMB, Khalifa, HE, Ahmed, MA, Hafez, MH, and Khedr, EM. Repetitive transcranial magnetic stimulation in the treatment of obsessive-compulsive disorders: double blind randomized clinical trial. Psychiatry Res. (2016) 238:264–9. doi: 10.1016/j.psychres.2016.02.031
PubMed Abstract | Crossref Full Text | Google Scholar
6. Jahanbakhsh, G, alireza Haji seyed javadi, S, Majidi, M, khademi, M, and Karimi, R. Effectiveness of adjunctive low-frequency repetitive transcranial magnetic stimulation therapy over the left dorsolateral prefrontal cortex in patients with obsessive-compulsive disorder refractory to medical treatment: a double-blind, randomized clinical trial. Asian J Psychiatr. (2023) 80:103384. doi: 10.1016/j.ajp.2022.103384
PubMed Abstract | Crossref Full Text | Google Scholar
7. Rao, V, Bechtold, K, McCann, U, Roy, D, Peters, M, Vaishnavi, S, et al. Low-frequency right repetitive transcranial magnetic stimulation for the treatment of depression after traumatic brain injury: a randomized sham-controlled pilot study. J Neuropsychiatry Clin Neurosci. (2019) 31:306–18. doi: 10.1176/appi.neuropsych.17110338
PubMed Abstract | Crossref Full Text | Google Scholar
8. Galimberti, A, Tik, M, Pellegrino, G, and Schuler, AL. Effectiveness of rTMS and tDCS treatment for chronic TBI symptoms: a systematic review and meta-analysis. Prog Neuro-Psychopharmacol Biol Psychiatry. (2024) 128:110863. doi: 10.1016/j.pnpbp.2023.110863
PubMed Abstract | Crossref Full Text | Google Scholar
9. Asgharian Asl, F, and Vaghef, L. The effectiveness of high-frequency left DLPFC-rTMS on depression, response inhibition, and cognitive flexibility in female subjects with major depressive disorder. J Psychiatr Res. (2022) 149:287–92. doi: 10.1016/j.jpsychires.2022.01.025
PubMed Abstract | Crossref Full Text | Google Scholar
10. van Kleef, RS, Marsman, JC, van Valen, E, Bockting, CLH, Aleman, A, and van Tol, MJ. Neural basis of positive and negative emotion regulation in remitted depression. NeuroImage Clin. (2022) 34:102988. doi: 10.1016/j.nicl.2022.102988
PubMed Abstract | Crossref Full Text | Google Scholar
11. Rodriguez Delgado, A, Morelos Santana, E, Torres Marcial, A, Arango de Montis, I, Miranda Terres, E, and Gonzalez, OJ. Effect of repetitive transcraneal magnetic stimulation on aggressive impulsive behavior in subjects with bpd in a of social exclusion paradigm. Brain Stimul. (2019) 12:512. doi: 10.1016/j.brs.2018.12.681
Crossref Full Text | Google Scholar
12. Boggio, PS, Rocha, M, Oliveira, MO, Fecteau, S, Cohen, RB, Campanhã, C, et al. Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. J Clin Psychiatry. (2010) 71:992–9. doi: 10.4088/JCP.08m04638blu
PubMed Abstract | Crossref Full Text | Google Scholar
13. Reyes-López, J, Ricardo-Garcell, J, Armas-Castañeda, G, García-Anaya, M, Arango-De Montis, I, González-Olvera, JJ, et al. Clinical improvement in patients with borderline personality disorder after treatment with repetitive transcranial magnetic stimulation: preliminary results. Braz J Psychiatry. (2018) 40:97–104. doi: 10.1590/1516-4446-2016-2112
Crossref Full Text | Google Scholar
14. Dixon, ML, Thiruchselvam, R, Todd, R, and Christoff, K. Emotion and the prefrontal cortex: an integrative review. Psychol Bull. (2017) 143:1033–81. doi: 10.1037/bul0000096
Crossref Full Text | Google Scholar
16. Glahn, DC, Lovallo, WR, and Fox, PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol Psychiatry. (2007) 61:1306–9. doi: 10.1016/j.biopsych.2006.09.041
PubMed Abstract | Crossref Full Text | Google Scholar
17. Hariri, AR, Drabant, EM, Munoz, KE, Kolachana, BS, Mattay, VS, Egan, MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. (2005) 62:146–52. doi: 10.1001/archpsyc.62.2.146
Crossref Full Text | Google Scholar
18. Graham, BM, and Milad, MR. Prefrontal cortex regulation of emotion and anxiety In: KJ Ressler, DS Charney, JD Buxbaum, P Sklar, and EJ Nestler, editors. Neurobiology of mental illness. Oxford: Oxford University Press (2013). 580–92.
19. Forbes, EE, and Dahl, RE. Neural systems of positive affect: relevance to understanding child and adolescent depression? Dev Psychopathol. (2005) 17:827–50. doi: 10.1017/S095457940505039X
PubMed Abstract | Crossref Full Text | Google Scholar
20. Van Kleef, GA, and Lelieveld, GJ. Moving the self and others to do good: the emotional underpinnings of prosocial behavior. Curr Opin Psychol. (2022) 44:80–8. doi: 10.1016/j.copsyc.2021.08.029
PubMed Abstract | Crossref Full Text | Google Scholar
21. Busner, J, and Targum, SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont (PA: Township)). (2007) 4:28–37.
PubMed Abstract | Google Scholar
22. Fossati, A, Di Ceglie, A, Acquarini, E, and Barratt, ES. Psychometric properties of an Italian version of the Barratt impulsiveness Scale-11 (BIS-11) in nonclinical subjects. J Clin Psychol. (2001) 57:815–28. doi: 10.1002/jclp.1051
PubMed Abstract | Crossref Full Text | Google Scholar
23. Melli, G, Avallone, E, Moulding, R, Pinto, A, Micheli, E, and Carraresi, C. Validation of the Italian version of the Yale-Brown obsessive compulsive scale-second edition (Y-BOCS-II) in a clinical sample. Compr Psychiatry. (2015) 60:86–92. doi: 10.1016/j.comppsych.2015.03.005
PubMed Abstract | Crossref Full Text | Google Scholar
24. Tyburski, E, Karabanowicz, E, Mak, M, Lebiecka, Z, Samochowiec, A, Pełka-Wysiecka, J, et al. Color trails test: a new set of data on cognitive flexibility and processing speed in schizophrenia. Front Psych. (2020) 11:521. doi: 10.3389/fpsyt.2020.00521
PubMed Abstract | Crossref Full Text | Google Scholar
25. Carlock, RH , editor. Defining and measuring executive functions in adults: applications for practice and policy; (2011).
26. Spinnler, H, and Tognoni, G. Standardizzazione e taratura italiana di test neuropsicologici: gruppo italiano per lo studio neuropsicologico dell'invecchiamento. Milano: Masson Italia Periodici Milano (1987).
27. Pascual-Leone, A, Rubio, B, Pallardó, F, and Catalá, MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet (London, England). (1996) 348:233–7. doi: 10.1016/S0140-6736(96)01219-6
PubMed Abstract | Crossref Full Text | Google Scholar
28. Weise, K, Numssen, O, Thielscher, A, Hartwigsen, G, and Knösche, TR. A novel approach to localize cortical TMS effects. NeuroImage. (2020) 209:116486. doi: 10.1016/j.neuroimage.2019.116486
PubMed Abstract | Crossref Full Text | Google Scholar
29. Ziegler, E, Chellappa, SL, Gaggioni, G, Ly, JQM, Vandewalle, G, André, E, et al. A finite-element reciprocity solution for EEG forward modeling with realistic individual head models. NeuroImage. (2014) 103:542–51. doi: 10.1016/j.neuroimage.2014.08.056
PubMed Abstract | Crossref Full Text | Google Scholar
30. Gomez, LJ, Dannhauer, M, Koponen, LM, and Peterchev, AV. Conditions for numerically accurate TMS electric field simulation. Brain Stimul. (2020) 13:157–66. doi: 10.1016/j.brs.2019.09.015
PubMed Abstract | Crossref Full Text | Google Scholar
31. Pripfl, J, and Lamm, C. Focused transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex modulates specific domains of self-regulation. Neurosci Res. (2015) 91:41–7. doi: 10.1016/j.neures.2014.09.007
PubMed Abstract | Crossref Full Text | Google Scholar
32. Liang, K, Li, H, Bu, X, Li, X, Cao, L, Liu, J, et al. Efficacy and tolerability of repetitive transcranial magnetic stimulation for the treatment of obsessive-compulsive disorder in adults: a systematic review and network meta-analysis. Transl Psychiatry. (2021) 11:332. doi: 10.1038/s41398-021-01453-0
PubMed Abstract | Crossref Full Text | Google Scholar
33. Zotev, V, Phillips, R, Young, KD, Drevets, WC, and Bodurka, J. Prefrontal control of the amygdala during real-time fMRI neurofeedback training of emotion regulation. PLoS One. (2013) 8:e79184. doi: 10.1371/journal.pone.0079184
PubMed Abstract | Crossref Full Text | Google Scholar
34. Boggio, PS, Zaghi, S, and Fregni, F. Modulation of emotions associated with images of human pain using anodal transcranial direct current stimulation (tDCS). Neuropsychologia. (2009) 47:212–7. doi: 10.1016/j.neuropsychologia.2008.07.022
PubMed Abstract | Crossref Full Text | Google Scholar
35. Huang, TN, Hsu, TT, Lin, MH, Chuang, HC, Hu, HT, Sun, CP, et al. Interhemispheric connectivity potentiates the basolateral amygdalae and regulates social interaction and memory. Cell Rep. (2019) 29:34–48.e4. doi: 10.1016/j.celrep.2019.08.082
PubMed Abstract | Crossref Full Text | Google Scholar
37. Bari, A, and Robbins, TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. (2013) 108:44–79. doi: 10.1016/j.pneurobio.2013.06.005
Crossref Full Text | Google Scholar
38. Diefenbach, GJ, Bragdon, LB, Zertuche, L, Hyatt, CJ, Hallion, LS, Tolin, DF, et al. Repetitive transcranial magnetic stimulation for generalised anxiety disorder: a pilot randomised, double-blind, sham-controlled trial. Br J Psychiatry. (2016) 209:222–8. doi: 10.1192/bjp.bp.115.168203
PubMed Abstract | Crossref Full Text | Google Scholar
39. Molavi, P, Aziziaram, S, Basharpoor, S, Atadokht, A, Nitsche, MA, and Salehinejad, MA. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. (2020) 274:93–102. doi: 10.1016/j.jad.2020.05.007
PubMed Abstract | Crossref Full Text | Google Scholar
40. Qiu, X, He, Z, Cao, X, and Zhang, D. Transcranial magnetic stimulation and transcranial direct current stimulation affect explicit but not implicit emotion regulation: a meta-analysis. Behav Brain Funct. (2023) 19:15. doi: 10.1186/s12993-023-00217-8
留言 (0)