Although our understanding of sleep’s functions is still limited, its role in memory consolidation is clear (1). Research demonstrates that both total sleep deprivation and partial sleep loss significantly impair memory tasks performance and the ability to form new memories (2, 3). Neuroimaging studies further reveal that sleep deprivation after learning crucially affects the long-term restructuring of memories in the brain (4). This review will examine the evidence of memory impairments induced by sleep deprivation and explore potential interventions to mitigate these effects. The inclusion of the gut-brain axis in the discussion of sleep deprivation and memory impairment advances the field by offering a novel perspective on how systemic health, particularly gut microbiota, can influence cognitive functions. This insight not only expands our understanding of the neurobiological mechanisms involved but also opens up potential new avenues for therapeutic interventions that could address both cognitive and systemic health issues.
The earliest studies highlighting the positive impact of sleep on memory date back to the 1920s (5). Researchers discovered that participants recalled syllables better if they slept after learning them, compared to staying awake. Presently, the concept of active systems consolidation during sleep lists among the most widely acknowledged theory explaining sleep-dependent memory consolidation (6). During learning or encoding new experiences, different aspects are processed and stored in various brain regions. The hippocampus plays a crucial role in integrating these components into a single episodic memory (7, 8). Sleep triggers the repeated reactivation of hippocampal neurons, which strengthens the memory representations in the neocortex through synaptic consolidation processes, leading to long-term memory formation (9).
Neuronal replay, essential for active systems consolidation, is predominantly observed during slow-wave sleep (SWS) and sometimes in wakefulness (10–12). This replay facilitates the formation of stable representations in extra-hippocampal networks, underlining the importance of sleep for memory consolidation. Advances in neuroimaging technologies like fMRI and EEG have provided more indirect evidence of memory replay during sleep (13, 14). Intracranial recordings in epilepsy patients have shown that gamma-band patterns, specific to the encoding of pictures, are crucial (15). Neuronal replay not only aids in system consolidation (the redistribution of hippocampus-dependent memories to neocortical sites for long-term storage) but may also contribute to synaptic consolidation (the enduring synaptic changes that stabilize memories).
Different sleep stages and specific wave patterns require special consideration. As mentioned, neuronal reactivation of spatio-temporal patterns during encoding primarily occurs in SWS. Neocortical slow oscillations (<1 Hz), thalamo-cortical spindles, and hippocampal sharp-wave ripples play a pivotal role in memory consolidation during SWS (9). During Rapid-eye-movement (REM) sleep, synaptic consolidation in the cortex may benefit from increased plasticity-related immediate-early gene activity. This stage is characterized by high-frequency brain waves, significant cholinergic, and theta activity. The slow oscillations’ depolarizing up-states initiate the formation of spindles and ripples, crucial for reactivating hippocampal memories and facilitating their efficient transfer to the neocortex (16). The alternating up- and down-states in neuronal activity induced by the neocortex’s slow oscillations create a higher-level temporal framework essential for redistributing memories for long-term storage (17, 18). Thalamo-cortical spindles prime cortical networks for long-term memory storage, with repeated spindle-associated discharges inducing long-term potentiation (19). This often occurs at synapses strengthened during memory encoding. Additionally, hippocampal sharp wave-ripples linked to sleep are associated with the reactivation of neuronal groups active during previous wakefulness (20, 21) (Figure 1).
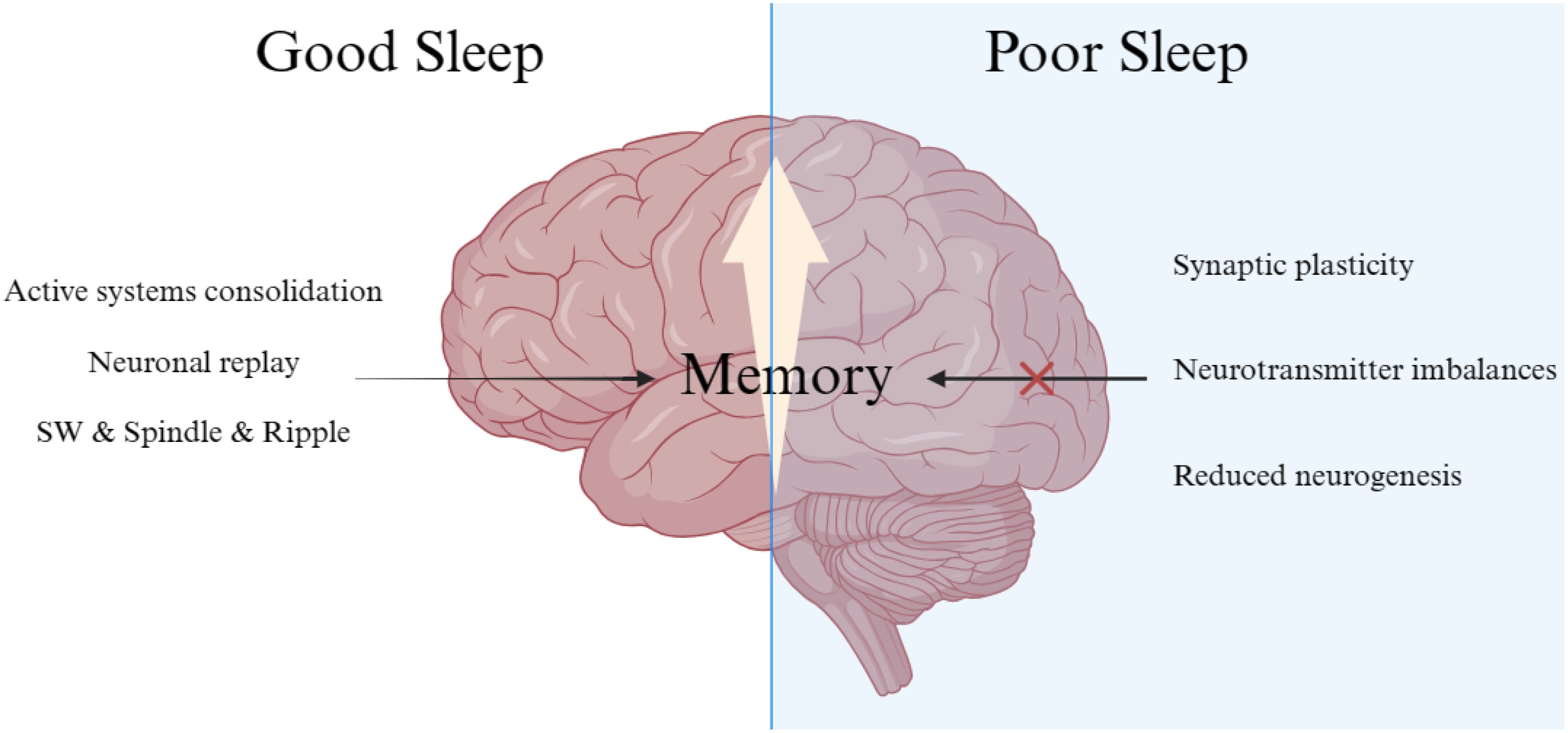
Figure 1. Neurobiological impact of sleep deprivation on memory.
Lifestyle factors, sleep health, and cognitive functionRecent studies have highlighted the intricate relationship between lifestyle factors, sleep health, and cognitive function. A large cross-sectional study in China explored the impact of various healthy lifestyle habits, including diet, exercise, and weight management, on sleep quality and duration, revealing that individuals adhering to healthier lifestyles experienced better sleep outcomes, including a lower incidence of insomnia and obstructive sleep apnea (22). In contrast, a meta-analysis investigating the health implications of habitual daytime napping found that longer naps (30 minutes or more) were associated with an increased risk of adverse health outcomes, such as cardiovascular disease and metabolic disorders (23). Interestingly, shorter naps did not pose significant risks, suggesting that while a healthy lifestyle can improve sleep health, the benefits of daytime napping are nuanced and dependent on nap duration. These findings underscore the importance of balanced lifestyle choices in promoting overall health and cognitive well-being.
The intricate relationship between sleep and memory has been a subject of extensive research, revealing that sleep plays a crucial role in both consolidating memories and preparing the brain for new memory formation. Sleep deprivation, therefore, has significant adverse effects on memory, which is a growing concern in today’s society where sleep curtailment is increasingly common due to various lifestyle factors (24). Studies have consistently shown that sleep deprivation impairs memory performance (25, 26). For example, participants who are deprived of sleep after learning new information tend to perform worse in memory tests. This finding supports theories that emphasize the importance of sleep in memory consolidation – the process by which short-term memories are converted into long-term ones. Additionally, sleep-deprived individuals have a reduced capacity to encode new memories when compared to well-rested controls, indicating that sleep is essential not just for consolidating existing memories but also for forming new ones.
Potential specific interventionsIn our modern society, sleep deprivation is increasingly widespread, driven by factors ranging from the pervasive use of smartphones and other electronic devices to academic pressures and stress, particularly among adolescents (27–29). This widespread reduction in sleep quality and quantity necessitates finding effective methods to counteract the negative impact of sleep deprivation on memory. Among the countermeasures to combat the effects of sleep deprivation, substances like caffeine, amphetamines, and modafinil are known for their ability to enhance alertness and vigilance (30). However, more specific interventions have been explored in recent research. For instance, donepezil, a cholinesterase inhibitor typically used in the treatment of Alzheimer’s disease, has been investigated for its potential to mitigate memory deficits associated with sleep deprivation (31). Studies have found that while donepezil does not offer significant benefits to well-rested individuals, it can improve performance in tasks related to memory among those who are sleep-deprived. This improvement is particularly noticeable in individuals who experience a significant decline in performance due to lack of sleep. These findings are supported by neuroimaging studies showing changes in brain activity related to the task in areas affected by donepezil, suggesting that it may enhance delayed recognition in sleep-deprived individuals by improving both attention and memory encoding.
Another compound, tolcapone – a selective inhibitor of catechol-O-methyltransferase (COMT) – improves cognition and cortical information processing in rested individuals (32). However, its effectiveness against sleep deprivation-induced memory impairment appears to be influenced by the individual’s COMT genotype, highlighting the complexity of neurochemical interactions in sleep and memory processes (33). The use of donepezil and memantine, both approved for treating Alzheimer’s disease, has also shown promise in preventing memory impairment induced by sleep deprivation (34). This is significant as it not only supports the potential use of these drugs in a wider context but also underscores the similarity in the underlying neurobiological mechanisms involved in sleep deprivation and neurodegenerative diseases like Alzheimer’s.
Gut-brain axisRecent research has turned its focus to the gut-brain axis, particularly the role of gut microbiota in sleep deprivation-induced memory impairment. Insomnia and other sleep disturbances can lead to significant changes in the gut microbiome, which in turn can impact cognitive functions. Studies have demonstrated that sleep-deprived mice exhibit disturbed intestinal microflora and intestinal barrier dysfunction (35). Interestingly, healthy mice that received gut microbiota transplants from insomnia-affected donors exhibited cognitive impairments, suggesting a direct link between gut health and brain function (36). Researchers have found that the administration of melatonin, a hormone known to regulate sleep, can ameliorate cognitive impairments induced by sleep deprivation. This effect is mediated through alterations in gut microbiota and their metabolites, which in turn modulate inflammatory responses and neuronal apoptosis in the hippocampus, a key brain region involved in memory (37). The gut-brain axis operates through several mechanistic pathways, including the modulation of the vagus nerve by gut microbiota, which in turn influences brain function. Recent studies suggest that disruptions in gut microbiota caused by sleep deprivation can lead to systemic inflammation, which exacerbates cognitive decline. Understanding these pathways is crucial for developing interventions that not only target cognitive symptoms but also address underlying systemic issues, potentially leading to more effective and holistic treatments.
Physical therapyExploring the enhancement of memory following sleep deprivation, physical therapy emerges as a promising method (38). This approach leverages physical factors—namely sound, light, electricity, and mechanical force—to modulate brain functions. Various forms of physical therapy, including transcranial magnetic stimulation (TMS), deep brain stimulation (DBS), and transcranial direct current stimulation (tDCS), have undergone investigation for their potential to mitigate memory impairments induced by sleep deprivation (39). Recent studies have demonstrated the efficacy of 1 Hz repetitive TMS (rTMS) in ameliorating spatial learning and memory deficits in sleep-deprived rats, attributed to the enhancement of synaptic structure and quantity in the hippocampus (39). Additionally, tDCS has shown promise in improving cognitive function following sleep deprivation without adversely affecting subsequent recovery sleep or post-recovery cognitive performance (40).
ConclusionThese findings highlight the complexity of the mechanisms underlying sleep deprivation-induced memory impairment and point towards a multifaceted approach to mitigating its effects. The exploration of chemical interventions like donepezil, memantine, compounds targeting the gut-brain axis, and physical therapy like rTMS and tDCS opens new avenues for research and potential treatments. However, these interventions should be considered alongside lifestyle changes that promote healthy sleep patterns, as the benefits of good sleep hygiene cannot be overstated. As research continues to unravel the intricate connections between sleep, the gut microbiome, and memory, a more holistic understanding of these processes will emerge, paving the way for more effective treatments and preventative strategies.
Author contributionsYF: Conceptualization, Writing – original draft, Writing – review & editing. JL: Writing – review & editing. SQ: Funding acquisition, Supervision, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsThe authors use the ChatGPT 4 version to help polish the language.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References8. McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. (1995) 102:419–57. doi: 10.1037/0033-295x.102.3.419
PubMed Abstract | Crossref Full Text | Google Scholar
12. Atherton LA, Dupret D, Mellor JR. Memory trace replay: the shaping of memory consolidation by neuromodulation. Trends Neurosci. (2015) 38:560–70. doi: 10.1016/j.tins.2015.07.004
PubMed Abstract | Crossref Full Text | Google Scholar
13. Piantoni G, van der Werf YD, Jensen O, Van Someren EJ. Memory traces of long-range coordinated oscillations in the sleeping human brain. Hum Brain Mapp. (2015) 36:67–84. doi: 10.1002/hbm.22613
PubMed Abstract | Crossref Full Text | Google Scholar
14. Schönauer M, Alizadeh S, Jamalabadi H, Abraham A, Pawlizki A, Gais S. Decoding material-specific memory reprocessing during sleep in humans. Nat Commun. (2017) 8:15404. doi: 10.1038/ncomms15404
PubMed Abstract | Crossref Full Text | Google Scholar
16. Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cognit Sci. (2007) 11:442–50. doi: 10.1016/j.tics.2007.09.001
Crossref Full Text | Google Scholar
17. Sirota A, Csicsvari J, Buhl D, Buzsáki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci USA. (2003) 100:2065–9. doi: 10.1073/pnas.0437938100
Crossref Full Text | Google Scholar
19. Werk CM, Harbour VL, Chapman CA. Induction of long-term potentiation leads to increased reliability of evoked neocortical spindles in vivo. Neuroscience. (2005) 131:793–800. doi: 10.1016/j.neuroscience.2004.12.020
PubMed Abstract | Crossref Full Text | Google Scholar
21. Nádasdy Z, Hirase H, Czurkó A, Csicsvari J, Buzsáki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. (1999) 19:9497–507. doi: 10.1523/jneurosci.19-21-09497.1999
PubMed Abstract | Crossref Full Text | Google Scholar
22. Zheng YB, Huang YT, Gong YM, Li MZ, Zeng N, Wu SL, et al. Association of lifestyle with sleep health in general population in China: a cross-sectional study. Transl Psychiatry. (2024) 14:320. doi: 10.1038/s41398-024-03002-x
PubMed Abstract | Crossref Full Text | Google Scholar
23. Yang YB, Zheng YB, Sun J, Yang LL, Li J, Gong YM, et al. To nap or not? Evidence from a meta-analysis of cohort studies of habitual daytime napping and health outcomes. Sleep Med Rev. (2024) 78:101989. doi: 10.1016/j.smrv.2024.101989
PubMed Abstract | Crossref Full Text | Google Scholar
24. Cousins JN, Teo TB, Tan ZY, Wong KF, Chee MWL. Sleep after learning aids the consolidation of factual knowledge, but not relearning. Sleep. (2021) 44(3):zsaa210. doi: 10.1093/sleep/zsaa210
PubMed Abstract | Crossref Full Text | Google Scholar
25. Aleman-Zapata A, Morris RGM, Genzel L. Sleep deprivation and hippocampal ripple disruption after one-session learning eliminate memory expression the next day. Proc Natl Acad Sci USA. (2022) 119:e2123424119. doi: 10.1073/pnas.2123424119
Crossref Full Text | Google Scholar
26. Newbury CR, Crowley R, Rastle K, Tamminen J. Sleep deprivation and memory: Meta-analytic reviews of studies on sleep deprivation before and after learning. Psychol Bull. (2021) 147:1215–40. doi: 10.1037/bul0000348
PubMed Abstract | Crossref Full Text | Google Scholar
28. Gillen-O'Neel C, Huynh VW, Fuligni AJ. To study or to sleep? The academic costs of extra studying at the expense of sleep. Child Dev. (2013) 84:133–42. doi: 10.1111/j.1467-8624.2012.01834.x
PubMed Abstract | Crossref Full Text | Google Scholar
29. Massar SAA, Liu JCJ, Mohammad NB, Chee MWL. Poor habitual sleep efficiency is associated with increased cardiovascular and cortisol stress reactivity in men. Psychoneuroendocrinology. (2017) 81:151–6. doi: 10.1016/j.psyneuen.2017.04.013
PubMed Abstract | Crossref Full Text | Google Scholar
30. Bonnet MH, Balkin TJ, Dinges DF, Roehrs T, Rogers NL, Wesensten NJ. The use of stimulants to modify performance during sleep loss: a review by the sleep deprivation and Stimulant Task Force of the American Academy of Sleep Medicine. Sleep. (2005) 28:1163–87. doi: 10.1093/sleep/28.9.1163
PubMed Abstract | Crossref Full Text | Google Scholar
31. Chuah LY, Chong DL, Chen AK, Rekshan WR, Tan JC, Zheng H, et al. Donepezil improves episodic memory in young individuals vulnerable to the effects of sleep deprivation. Sleep. (2009) 32:999–1010. doi: 10.1093/sleep/32.8.999.
PubMed Abstract | Crossref Full Text | Google Scholar
33. Valomon A, Holst SC, Borrello A, Weigend S, Müller T, Berger W, et al. Effects of COMT genotype and tolcapone on lapses of sustained attention after sleep deprivation in healthy young men. Neuropsychopharmacology. (2018) 43:1599–607. doi: 10.1038/s41386-018-0018-8.
PubMed Abstract | Crossref Full Text | Google Scholar
34. Rahman A, Lamberty Y, Schenker E, Cella M, Languille S, Bordet R, et al. Effects of acute administration of donepezil or memantine on sleep-deprivation-induced spatial memory deficit in young and aged non-human primate grey mouse lemurs (Microcebus murinus). PLoS One. (2017) 12:e0184822. doi: 10.1371/journal.pone.0184822
PubMed Abstract | Crossref Full Text | Google Scholar
35. Gao T, Wang Z, Dong Y, Cao J, Lin R, Wang X, et al. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J Pineal Res. (2019) 67:e12574. doi: 10.1111/jpi.12574.
PubMed Abstract | Crossref Full Text | Google Scholar
36. Wang Z, Chen WH, Li SX, He ZM, Zhu WL, Ji YB, et al. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol Psychiatry. (2021) 26:6277–92. doi: 10.1038/s41380-021-01113-1
PubMed Abstract | Crossref Full Text | Google Scholar
37. Wang X, Wang Z, Cao J, Dong Y, Chen Y. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome. (2023) 11:17. doi: 10.1186/s40168-022-01452-3
PubMed Abstract | Crossref Full Text | Google Scholar
38. Gomes-Osman J, Indahlastari A, Fried PJ, Cabral DLF, Rice J, Nissim NR, et al. Non-invasive brain stimulation: probing intracortical circuits and improving cognition in the aging brain. Front Aging Neurosci. (2018) 10:177. doi: 10.3389/fnagi.2018.00177
PubMed Abstract | Crossref Full Text | Google Scholar
39. Zheng X, Wang R, Ma B, Zhang J, Qian X, Fang Q, et al. rTMS reduces spatial learning and memory deficits induced by sleep deprivation possibly via suppressing the expression of kynurenine 3-monooxygenase in rats. Behav Brain Res. (2024) 456:114704. doi: 10.1016/j.bbr.2023.114704
PubMed Abstract | Crossref Full Text | Google Scholar
40. Cheng JX, Zhao X, Qiu J, Jiang Y, Ren J, Sun S, et al. Effects of transcranial direct current stimulation on performance and recovery sleep during acute sleep deprivation: a pilot study. Sleep Med. (2021) 79:124–33. doi: 10.1016/j.sleep.2021.01.014
留言 (0)