● Swallowing dysfunction is a common disease in AD patients and can lead to many serious adverse consequences.
● Age and concomitant medical illness are the important influencing factors in swallowing dysfunction in patients with AD.
● Understanding the factors that influence swallowing dysfunction in patients with AD can facilitate screening the symptom in early stage and management of dysphagia to avoid serious complications.
1 IntroductionAccording to the World Health Organization, the number of people with dementia is currently 55 million, and by 2030, it is expected to increase to 78 million. The WHO global action plan on the public health response to dementia targets at least 50% of countries to diagnose 50% of the estimated number of people with dementia by 2025 (1). As the disease progresses, the self-care ability of patients with AD decreases, increasing the burden of care for families in patients with AD. Dysphagia is a geriatric syndrome that affects 10% to 30% of older adults. Oropharyngeal dysphagia was reported more commonly in older adults diagnosed as having neurologic disease (80% of AD and 60% of Parkinson disease) (2). With cerebral cortical involvement and cognitive impairment, the incidence of dysphagia in patients with severe AD is as high as 70%. Dysphagia is a common complication in patients with AD, occurring in 13%-57% and is one of the main causes of dehydration, malnutrition, aspiration pneumonia, and even asphyxia and multiple organ failure. The results of the study by Patel et al.(2018) found that patients with dysphagia had a 34% higher total hospital cost, a 2.8 times higher likelihood of needing acute follow-up care services, and a 1.7 times higher chance of dying in the hospital than patients without dysphagia, indicating that patients with dysphagia have a greater economic and survival burden.
A range of complications due to swallowing dysfunction is a major source of care burden. Consequences of dysphagia could be profound including malnutrition, volume depletion, quality of life issues, and aspiration, which may ultimately be the pivotal factor that precipitates a decline in an outcome of patients (3). In fact, dysphagia affects 1 in 25 adults annually and had significant psychosocial burden. Approximately 41% of patients had reported anxiety during mealtime and 36% avoided eating with others because of their dysphagia (4).
Understanding the factors that influence swallowing dysfunction in patients with AD can facilitate screening the symptom in early stage and management of dysphagia to avoid serious complications. This could reduce the burden of care on the patient’s family and even society. Therefore, we urgently need to find effective interventions to intervene swallowing dysfunction of patients with AD. At present, the research on the influencing factors of swallowing dysfunction mainly focuses on the age, dental condition, eating condition and course of the patients (5, 6). Our pilot observation found nutritional status, daily living skills, lifestyle habits might influence the swallowing function in patients with AD. Therefore, in this study we supplemented the assessment of the nutritional level and function of daily living in AD patients. Increasing awareness of these issues may contribute to more timely and efficient identification of Alzheimer’s disease patients with dysphagia (7), and provide scientific evidence for the development of nursing and medical interventions.
2 Materials and methods2.1 SubjectsA consecutive sampling method was used to recruit 109 hospitalized patients with AD from February 2021 to March 2022. Inclusion criteria: 1) age≥60 years; 2) compliance with the DSM-5 criteria for Alzheimer’s disease; 3) be able to respond naturally and complete scale rating; 4) agree to sign informed consent. The World Health Organization’s classification of the elderly is as follows: 60–74 years old are considered elderly, the senile age ranges from 75 years to 90 years, and individuals aged 90 years and above are considered long-livers. Herein, the subjects were divided into three groups based on their age for analysis: 60–74 years old (group 1), 75-89 years old (group 2), and higher than 90 years old (group 3).
2.2 QuestionnaireGender, age, poor lifestyle habits (drinking and smoking), illness duration, route to admission, and concomitant medical illness were collected by using a self-designed questionnaire. The scales used for evaluation include: Mini-Nutritional Assessment- Shortform (MNA-SF), Functional Questionnaire (FAQ) and Standardized Swallowing Assessment (SSA).
2.2.1 Mini-nutritional assessment- shortformThe Mini-Nutritional Assessment- Shortform was simplified by Rubenstein LZ equal to 2001 based on MNA. MNA-SF contained 7 items with a total of 14 points, which was designed and validated to provide a single, rapid assessment of nutritional status in elderly patients in outpatient clinics, hospitals and nursing homes. The scale scored ≤7 as malnutrition, 8~11 as at risk of malnutrition, and ≥12 as normal nutritional status (8).
2.2.2 Functional questionnaireFunctional Activities Questionnaire was developed by scholar Pfeffer et al. in 1982. The scale consisted of 10 items that mainly assess Instrumental Activities of Daily Living (IADL), including bill payment, tax administration, cooking, hobbies, following current events, traveling, remembering appointments, and taking medication. The scale used the Likert-4 scoring system, with higher scores meaning more dysfunction (9).
2.2.3 Standardized swallowing assessmentStandardized Swallowing Assessment was first reported by Ellul et al. in 1996, which was scientifically designed to assess the swallowing function of patients. This scale can not only be used as an effective tool for early identification of swallowing functions, but also as a criterion for determining whether or not to remove a gastric tube. The scale was divided into 3 steps: clinical examination, water test, and normal diet test, with a total score of 18-46 (10).
2.3 Data collectionIn the inpatient ward of a brain hospital, questionnaires were regularly distributed by four nurses trained as investigators. Once the questionnaire was completed, the nurse collected the questionnaire on the spot and checked the scale for missing or non-conformance.
2.4 Statistical analysisSPSS 25.0 was applied to process data. Measurement data was presented with Mean ± SD, and counting data was expressed using frequency and percentage. The difference between the two categories was performed by independent sample t-test, one-way ANOVA for three or more categories, and non-parametric K independent sample test for rank variable; The number of influencing factors related to the number of physical diseases in AD was analyzed by linear regression analysis. Before doing multiple linear regression analysis, our study supplemented with univariate linear regression analysis to ensure the accuracy of the results. The level of significance was set at 0.05.
3 Results3.1 The general information of participantsThe majority of participants in the study were female, with the highest proportion of participants over 70 years of age. The proportion of participants with spouses is much greater than that without spouses. The majority of participants had no poor lifestyle habits. More patients were admitted to the hospital for the first time through outpatient clinics. 96.3% of participants scored < 11 in nutrition and 92.7% scored > 5 points in social functioning. (Table 1)
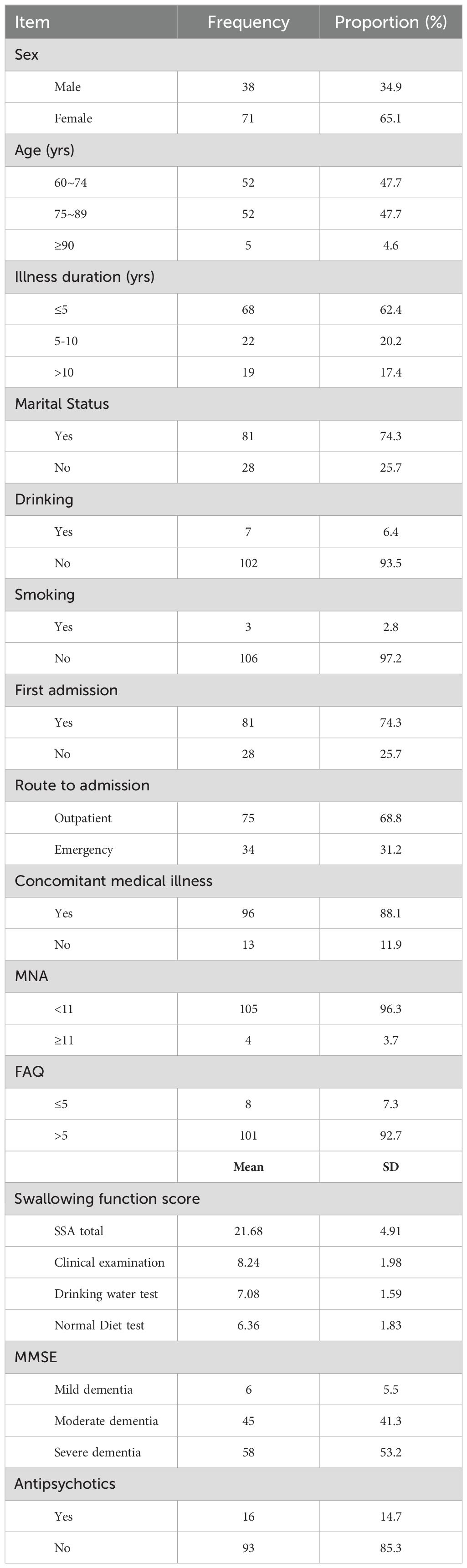
Table 1. General information.
The average score of the 109 participants on the swallowing function assessment was 21.68± 4.91. Among them, the average score of clinical examination was 8.24± 1.98, the average score of drinking water test was 7.08± 1.59, the average score of normal diet test was 6.36± 1.83. (Table 1)
3.2 Scores of SSA in patients with different characteristics of Alzheimer diseaseAccording to the grouping characteristics of different variables, the independent sample t-test or one-way ANOVA was carried out, and the results showed that the analysis results of age, route to admission, concomitant medical illness, MNA score and FAQ score (P<<0.05) (Table 2).
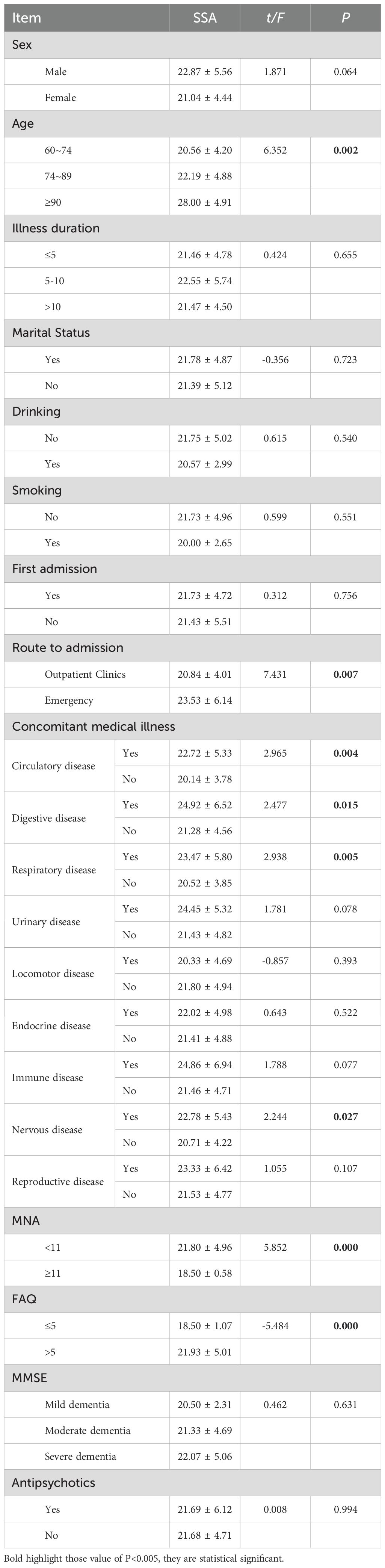
Table 2. Scores of SSA in patients with different characteristics of Alzheimer disease.
3.3 Uni-variate linear regressionThe above statistically significant results were analyzed by univariate linear regression, and the results showed that age (≥90 years old), route to admission (emergency), concomitant medical illness, and FAQ score (>5) (P< 0.05). (Table 3)
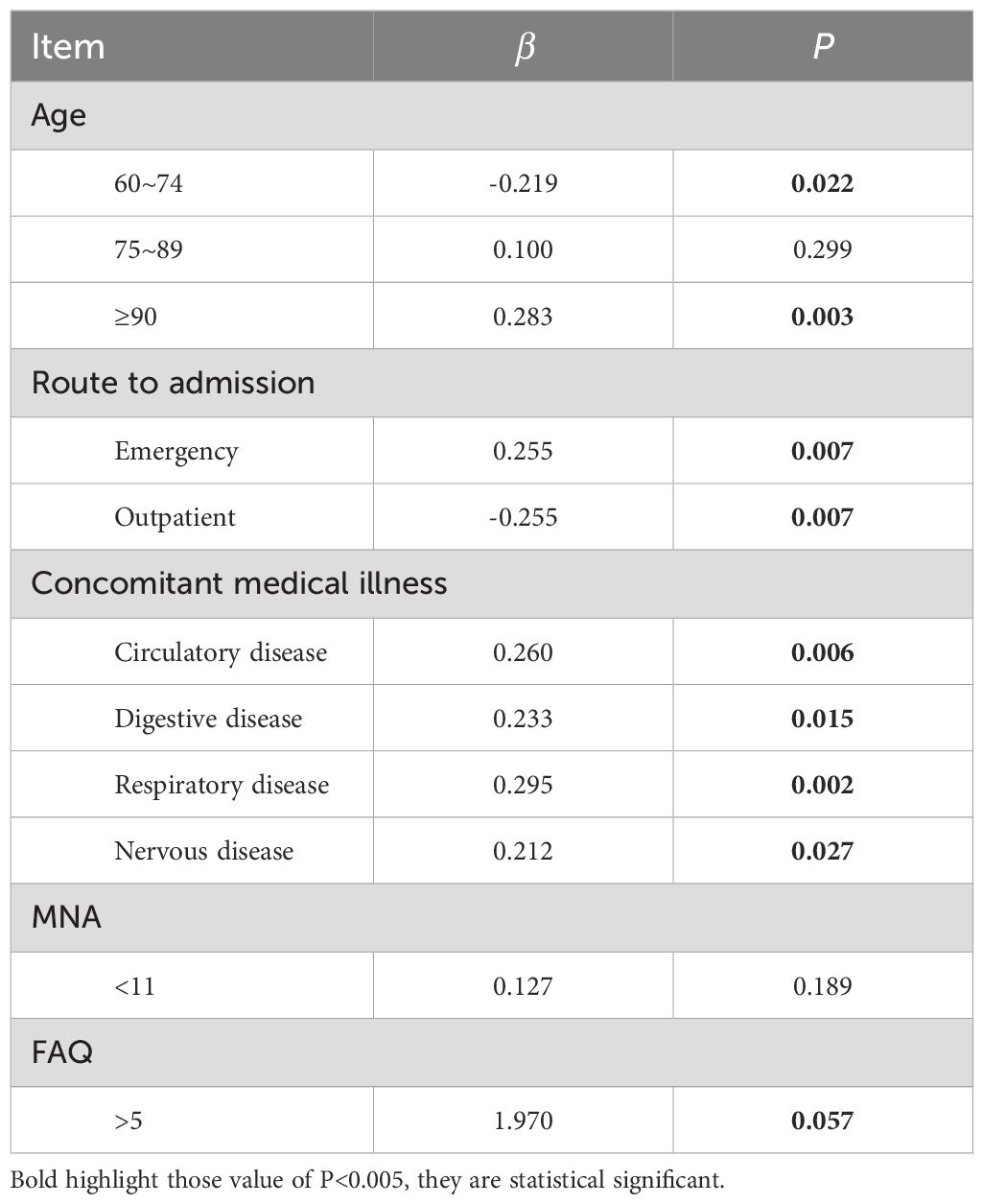
Table 3. Uni-variate linear regression.
3.4 Linear regression analysis of factors influencing Swallowing functionThe linear regression analysis model was established by taking swallowing function scores in patients with Alzheimer disease as the dependent variable, and the statistically significant variables of univariate linear regression as independent variables. The results showed that age (≥90 years old), concomitant medical illness (circulatory disease and respiratory disease)(P<0.05). (Table 4)
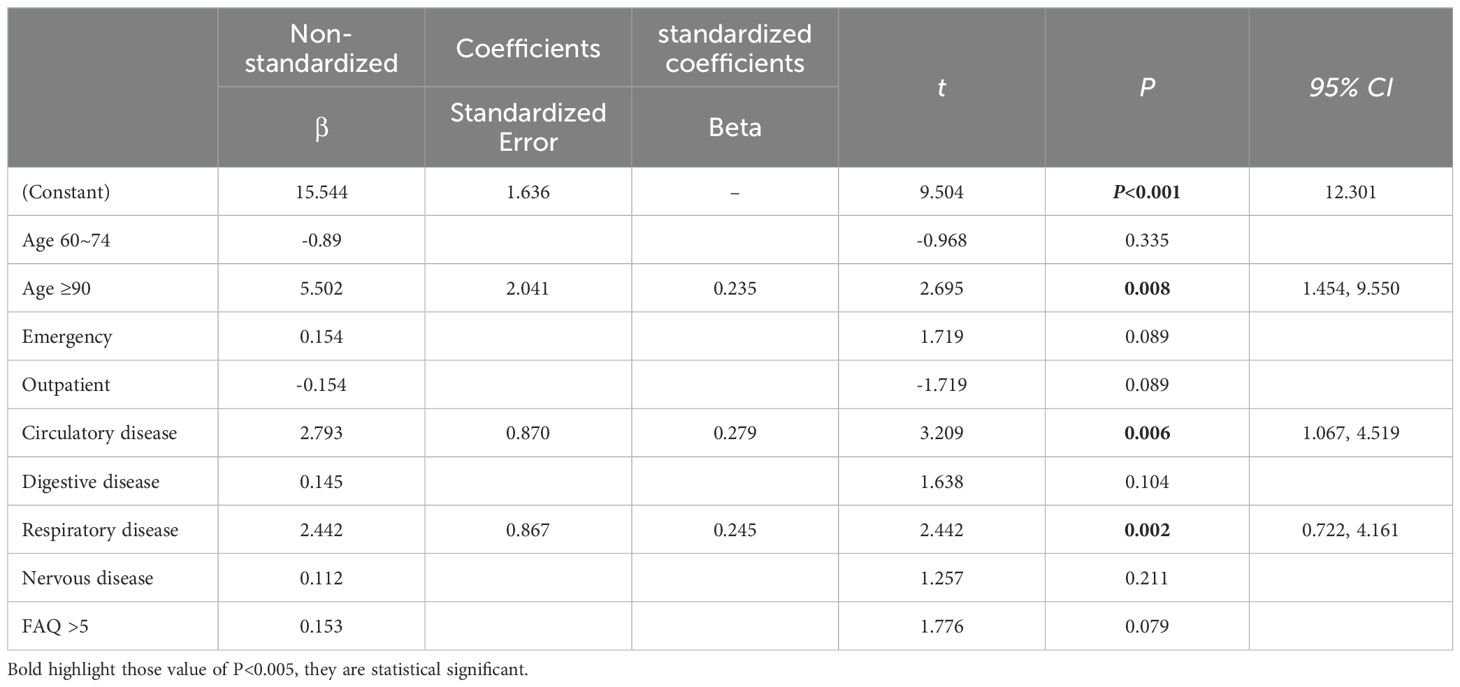
Table 4. Linear regression analysis of factors influencing Swallowing function.
4 DiscussionPrevious studies had found that patients with AD had a higher risk of dysphagia, but some clinical features and associated factors remained unclear (11). On the basis of empirical findings, this study investigated the swallowing function of patients with AD and reported that swallowing dysfunction was related to the age and its concomitant medical illness, including circulatory disease and respiratory disease.
Our study found that circulatory and respiratory diseases were important factors in swallowing dysfunction in patients with AD. Circulatory system dysfunction, such as reduced blood circulation or insufficient blood supply, might affect swallowing function. Intracranial circulation disorders could cause ischemia due to vascular wall lesions, blood composition or hemodynamic changes, including sensory dysfunction, autonomic nerve dysfunction, and motor nerve dysfunction, resulting in dysphagia (12). Insufficient blood supply to brain lead to impaired nerve function that regulates swallowing movement, resulting in dysphagia. Stroke is one of prevalent circulatory system diseases which influences the progression of dysphagia, because the lack of blood supply to the brain would affect function of nerves that control swallowing organ (13). In addition, studies found that long-term hypertension would affect blood circulation via hardening of brain vessels, abnormal brain circulation causes the injury of pharyngeal cortex center, leading to the descended cortical fibers, bulbar swallowing center and extrapyramidal system (14, 15). Consequently, prolonged oral transit time, delayed pharyngeal swallowing, and even aspiration during swallowing procedure, might occur after stroke (16).
This investigation suggested dysphagia was associated with respiratory system diseases, for instance chronic obstructive pulmonary disease (COPD), and pneumonia. Patients with respiratory diseases experience general respiratory symptoms that disrupt eating behavior, leading to choking episodes. This might due to the abnormal cough reflex cause food or liquids to stray into the airway and trigger dysphagia (17). Respiration and swallowing movement share reflective neuroanatomical mechanisms and pathways, individuals with tachypnea demonstrates shortened duration of apnea, disrupted swallowing, and even oropharyngeal dysphagia (18). In addition, long-term breathing difficulties and throat discomfort might induce dysphagia because of pharyngeal muscles fatigue (19). Thus, additional attention should be paid to medical comorbidities when screening swallowing function in patients with AD.
Aging was a vital factor influencing swallowing function in patients with AD: degenerative brain nerve function and abnormal swallowing reflex function weakened transport capacity and coordination of oral muscle groups, therefore older adults had greater risk of dysphagia (20). Research by Mayla Izumi et al. (2018) highlighted damaged tongue pressure had impacts on insufficient saliva secretion, weakened immunity, and impaired tongue motor function, resulting in dysphagia in aged adults (21). Given that aged patients with AD had clinical risk of dysphagia, interventions targeting on swallowing function should be conducted in early stage.
Previous study by Park, Y.H., et al. (2013) showed patients with moderate to severe dementia were at risk of dysphagia (22). In our study, the SSA scores (mean ± s.d.) of patients with mild (5.5%), moderate (41.3%), and severe (53.2%) AD were 20.50 ± 2.31, 21.33 ± 4.69, 22.07 ± 5.06, respectively. We had noticed an incline of SSA score with the increasing severity of AD, but further analysis had not clarified statistical different among three groups. These might due to proportions of participants with different dysphagia level varied significantly. To note, clinical evidence indicated that long-term use of antipsychotic medication lead to tardive dyskinesia, a movement disorder that disrupts effective swallowing (23, 24). In our study, we recorded the application of antipsychotics, but did not indicate significant difference in SSA score between participants underwent antipsychotics therapy or not. These might due to that clinically only very low does of antipsychotics was administrated to alleviated behavioral and psychological symptoms of dementia (BPSD), particularly in patients of first admission (74.3% in our study). Furthermore, it is worthy to mention the finding by Marta Miarons (2018) that the role of decreased physical condition caused by aging process and AD had greater impact on directing dysphagia than that of antipsychotics treatment (25). Therefore, future study unveiling how and to what extent antipsychotics influence dysphagia is demanding.
In summary, dysphagia of patients with AD is affected by age and medical comorbidities, despite the number of patients with AD is growing annually, understanding the caring demand benefits raising public awareness and developing targeted intervention (26). Limitation in this study should be mentioned: participants were recruited from a public psychiatric hospital in Guangzhou, China, this might affect the generality of our findings; besides, further study should focus on other factors encompassing AD combined with dysphagia, particularly gender balance, edentulism and frailty.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statementThe studies involving humans were approved by Guangzhou Medical University Human Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsJY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YP: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. TZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Writing – review & editing. JC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Writing – review & editing. WW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Writing – review & editing. XZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Writing – review & editing. DL: Data curation, Formal analysis, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing. SW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Writing – review & editing. ZL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. JG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. AX: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by Research Project of Guangzhou Municipal Health Commission (2023A031002), Research Project of Guangzhou Municipal Health Commission (SL2022A03J01476), Research Project of Department of Education of Guangdong Province (2021JD119) and Guangzhou Research oriented Hospital Project.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References3. Patel DA, Krishnaswami S, Steger E, Conover E, Vaezi MF, Ciucci MR, et al. Economic and survival burden of dysphagia among inpatients in the United States. Dis Esophagus. (2018) 31:1–7. doi: 10.1093/dote/dox131
PubMed Abstract | Crossref Full Text | Google Scholar
4. Ekberg O, Hamdy S, Woisard V, Wuttge-Hannig A, Ortega P. Social and psychological burden of dysphagia: its impact on diagnosis and treatment. Dysphagia. (2002) 17:139–46. doi: 10.1007/s00455-001-0113-5
PubMed Abstract | Crossref Full Text | Google Scholar
5. Lili C HL, Hong L, Rong L, Qiuhua C QH, Jing T. Prevalence and associated factors of dysphgia in elderly patients with dementia. J Nurs Sci. (2014) 29:24–6.
6. Ling Y GS, Qi C. Influencing factors and nursing progress of dysphgia in senile dementia patients. Chin Nurs Res. (2017) 31:3783–5.
7. Constantino EA, Julie R, Claire D. Physiological mechanisms and associated pathophysiology of dysphagia in older adults. J Gerontology Geriatric Med. (2022) 8:23337214221142949. doi: 10.1177/23337214221142949
Crossref Full Text | Google Scholar
9. Pfeffer RI, Kurosaki TT, Harrah CH Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. (1982) 37:323–9. doi: 10.1093/geronj/37.3.323
PubMed Abstract | Crossref Full Text | Google Scholar
10. Ellul J, Barer D. Interobserver reliability of a standardised swallowing assessment (SSA). Cerebrovascular Dis. (1996) 6:152–3.
12. Wang S, Zhao Q, Hong J. Pay attention to the clinical study of intracranial venous circulation disorder. Chin J Neurosurgical Dis. (2016) 15:481–4.
13. Londhe C, Agrawal A, Pednekar S, Pandey D, Khan MF. Swallowing dysfunction after acute stroke: the incidence, predictors and outcome. J Assoc Physicians India. (2023) 71:11–2. doi: 10.59556/japi.71.0301
PubMed Abstract | Crossref Full Text | Google Scholar
14. Dziewas R, Michou E, Trapl-Grundschober M, Lal A, Arsava EM, Bath PM, et al. European Stroke Organisation and European Society for Swallowing Disorders guideline for the diagnosis and treatment of post-stroke dysphagia. Eur Stroke J. (2021) 6:LXXXIX-CXV. doi: 10.1177/23969873211039721
PubMed Abstract | Crossref Full Text | Google Scholar
15. Sherman V, Greco E, Martino R. The benefit of dysphagia screening in adult patients with stroke: A meta-analysis. J Am Heart Assoc. (2021) 10:e018753. doi: 10.1161/JAHA.120.018753
PubMed Abstract | Crossref Full Text | Google Scholar
16. Yang C, Pan Y. Risk factors of dysphagia in patients with ischemic stroke: A meta-analysis and systematic review. PloS One. (2022) 17:e0270096. doi: 10.1371/journal.pone.0270096
PubMed Abstract | Crossref Full Text | Google Scholar
17. Allen JE. Deglutition, swallowing, and airway protection: physiology and pathophysiology. In: Meyer KC, Raghu G, editors. Gastroesophageal reflux and the lung. Springer New York, New York, NY (2013). p. 1–22.
18. Li W, Gao M, Liu J, Zhang F, Yuan R, Su Q, et al. The prevalence of oropharyngeal dysphagia in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Expert Rev Respir Med. (2022) 16:567–74. doi: 10.1080/17476348.2022.2086123
PubMed Abstract | Crossref Full Text | Google Scholar
19. Nativ-Zeltzer N, Nachalon Y, Kaufman MW, Seeni I, Bastea S, Aulakh SS, et al. Predictors of aspiration pneumonia and mortality in patients with dysphagia. Laryngoscope. (2022) 132:1172–6. doi: 10.1002/lary.v132.6
PubMed Abstract | Crossref Full Text | Google Scholar
20. Dallal York J, Leonard K, Anderson A, DiBiase L, Jeng EI, Plowman EK. Discriminant ability of the 3-ounce water swallow test to detect aspiration in acute postoperative cardiac surgical patients. Dysphagia. (2022) 37:831–8. doi: 10.1007/s00455-021-10333-0
PubMed Abstract | Crossref Full Text | Google Scholar
21. Izumi M, Akifusa S. Tongue cleaning in the elderly and its role in the respiratory and swallowing functions: Benefits and medical perspectives. J Oral Rehabil. (2021) 48:1395–403. doi: 10.1111/joor.v48.12
PubMed Abstract | Crossref Full Text | Google Scholar
22. Park YH, Han HR, Oh BM, Lee J, Park JA, Yu SJ, et al. Prevalence and associated factors of dysphagia in nursing home residents. Geriatr Nurs. (2013) 34:212–7. doi: 10.1016/j.gerinurse.2013.02.014
PubMed Abstract | Crossref Full Text | Google Scholar
23. Cicala G, Barbieri MA, Spina E, de Leon J. A comprehensive review of swallowing difficulties and dysphagia associated with antipsychotics in adults. Expert Rev Clin Pharmacol. (2019) 12:219–34. doi: 10.1080/17512433.2019.1577134
PubMed Abstract | Crossref Full Text | Google Scholar
24. Shunli R, Allescher HD. Drug-induced dysphagia. Chin J Gerontology. (2019) 39:2829–31.
25. Miarons M, Clavé P, Wijngaard R, Ortega O, Arreola V, Nascimento W, et al. Pathophysiology of oropharyngeal dysphagia assessed by videofluoroscopy in patients with dementia taking antipsychotics. J Am Med Dir Assoc. (2018) 19:812.e1–812.e10. doi: 10.1016/j.jamda.2018.04.016
PubMed Abstract | Crossref Full Text | Google Scholar
26. Fuhou C. (2019). Early prevention of Alzheimer’s disease, in: National Conference on Alzheimer’s and Cognitive Impairment-related Disease, Guizhou, China.
留言 (0)