Sea buckthorn (Elaeagnus rhamnoides L.) is a nitrogen-fixing thorny deciduous shrub that is naturally distributed in Asia and Europe. It can grow in difficult conditions such as frost, drought, and polluted air. This botanical drug has been widely used for its nutritional and medicinal purposes (Patel et al., 2012; Jaśniewska and Diowksz, 2021). Raw fruits, various products made from them (e.g., juices, jams, tinctures), alcoholic extracts from different parts of this botanical drug and oil from the seeds have shown to possess antioxidant, antitumor, anti-inflammatory, immunomodulatory, and regenerative properties, which are related to a unique composition of bioactive metabolites, rich in phenolic metabolites (mainly phenolic acids and flavonoids), essential fatty acids (palmitic acid, palmitoleic acid, stearic acids), vitamins (A, B, C, E, K), phytosterols (cycloartenol, campesterol, sitosterol), and carotenoids (lycopene, lutein, zeaxantin) (Olas et al., 2018; Dudau et al., 2021; Stochmal et al., 2022). In general, more than 60 flavonoids and 10 phenolic acids have been identified in sea buckthorn. The most abundant flavonoids in fruits, leaves, and seeds are isorhamnetin and quercetin, followed by kaempferol, luteolin, myricetin, syringetin, naringenin, and epicatechin (Ren et al., 2020; He et al., 2023). Considering phenolic acids, gallic acid, caffeic acid, and ferulic acid are present in leaves and fruits (Jaśniewska and Diowksz, 2021; Danielski and Shahidi, 2024). This broad spectrum of bioactive metabolites can help prevent or treat a variety of conditions, such as cardiovascular diseases, diabetes mellitus, liver damage, gastrointestinal disorders, neuronal damage, skin lesions, retina damage, osteoporosis, and tumors (Ren et al., 2020).
In this review, we summarize the current knowledge from in vitro and in vivo studies regarding the effect of sea buckthorn, as well as its most widespread flavonoids isorhamnetin, quercetin, and kaempferol, on bone and breast tissue health. In individual studies, where it was relevant, we assessed the fulfillment of the requirements for the phytochemical characterization of the analysed extracts according to the ConPhyMP (Heinrich et al., 2022). Selected flavonoids exhibit both protective effects on bone tissue and antitumor impacts on breast tissue, potentially ameliorating bone loss in osteoporosis and inhibiting breast cancer progression. Therefore, we hypothesized that all of these bioactive metabolites should simultaneously attenuate bone damage and breast malignancy. These profitable effects of aforementioned bioactive metabolites on the health status of bone and breast tissues are still not sufficiently presented, as well as the interplay between both tissues.
2 The link between bone tissue and breast tissueThe relationships between bone and breast tissues are generally based on a constant dependence on the same regulatory and signaling molecules, as well as on mutual interactions through tissue-specific molecules that are mainly exposed at specific periods of life. Both bone tissue and breast tissue are dependent on estrogen, a key hormone that regulates bone mineral density (BMD), thereby maintaining the balance between bone formation and bone resorption. Estrogen is also an important mediator of mammary gland morphogenesis (Stingl, 2011). Furthermore, receptor activator of nuclear factor kappa β (RANK) and its ligand RANKL were discovered as key regulators of osteoclast development and activation. RANK and RANKL, however, also play an important role in the development of a functional lactating mammary gland during pregnancy (Sigl and Penninger, 2014). The mammary gland and bones are closely linked during lactation, when increased calcium requirements for milk production change bone and mineral metabolism (Athonvarangkul and Wysolmerski, 2023). In addition, with increasing age, cells and tissues, including breast and bone, become more susceptible to oxidative stress, which could modify the activity of key proteins and pathways needed to protect against bone and breast tissue damage (Figure 1). Disruption of the aforementioned important regulatory mechanisms and age-related changes in the organism, including oxidative stress, inflammation, and lipid accumulation contribute to simultaneous occurrence of osteoporosis and breast cancer in postmenopausal women (Cho et al., 2015; Muhammad et al., 2018; Martiniakova et al., 2023a). Overall, osteoporosis is the most common type of bone disease and its prevalence was reported at 18.3%. By 2050, more than 30 million people in Europe are expected to be affected by osteoporosis (Salari et al., 2021; Martiniakova et al., 2024). Globally, breast cancer is the second most common cause of cancer death in women, representing 12.5% of all new annual cancer cases worldwide (Muhammad et al., 2018; World Health Organization International Agency for Research on Cancer, 2024). There is a close clinically significant relationship between osteoporosis and breast cancer. Estrogen deficiency is considered the main cause of postmenopausal osteoporosis. Conversely, elevated estrogen levels during life (e.g., late menopause, obesity, estrogen replacement therapy) are associated with increased incidence of breast malignancy. Deregulation of the RANK/RANKL system also contributes to the development of postmenopausal osteoporosis. The RANK/RANKL pathway has also been found to be involved in hormone-induced breast cancer development and metastatic spread to bone (Muhammad et al., 2018; Martiniakova et al., 2023a).
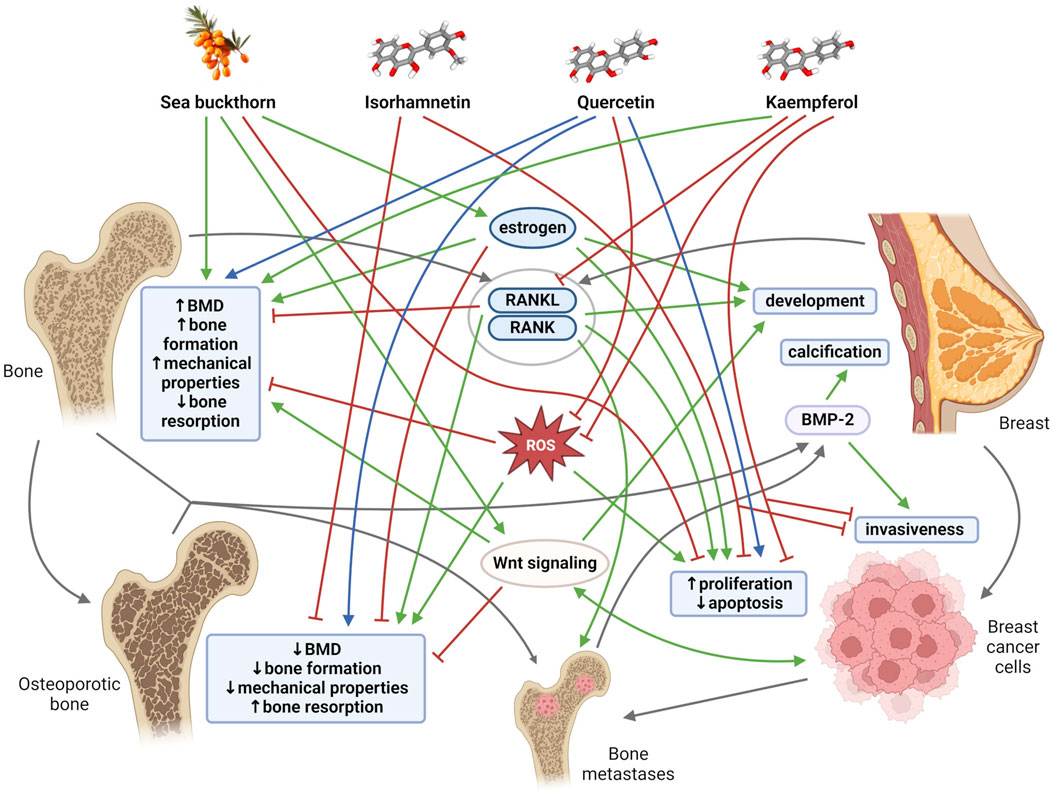
Figure 1. General mechanisms and links among bone tissue, breast tissue, sea buckthorn, its flavonoids (isorhamnetin, quercetin, kaempferol). Blunt red arrows indicate an inhibitory effect, sharp green arrows designate a stimulatory effect. Blue arrows show a dose-different effect. Gray arrows indicate the connection of individual mechanisms, molecules and structures. BMD, bone mineral density; BMP-2, bone morphogenetic protein-2; RANK, receptor activator of nuclear factor kappa β; RANKL, receptor activator of nuclear factor kappa β ligand; ROS, reactive oxygen species. Created with BioRender.com using chemical structures from PubChem (Kim et al., 2023); CIDs: 5281654, 5280343, 5280863, 122228.
Another clinical interrelationship between bone tissue and breast tissue is demonstrated by breast microcalcifications and breast cancer bone metastases (Antonacci et al., 2018). The presence of breast microcalcifications and localized deposits of hydroxyapatite in the breast tissue is actually considered an early mammographic sign of breast cancer (Johnson et al., 1999). Approximately 70%–80% of patients with advanced breast cancer experienced bone metastases (Chen et al., 2010), which seriously affect their quality of life and can lead to death. Moreover, osteoblasts and osteoclasts represent an important switch in tumor cell dormancy during bone metastasis (Haider et al., 2020; Dai et al., 2022). Several researchers have also demonstrated the expression of typical bone markers in breast cells, including predominantly bone morphogenetic proteins (BMPs) and Wnt signaling (Liu et al., 2008; Bramwell et al., 2014). In general, BMPs are cytokines belonging to the transforming growth factor (TGF)-β superfamily that play multiple functions during development and tissue homeostasis, including the regulation of bone homeostasis (Sánchez-Duffhues et al., 2015). Current studies have shown that BMPs may also be involved in breast tissue. They can support oncogenic behavior by affecting apoptosis, migration, invasion, and angiogenesis (Alarmo and Kallioniemi, 2010), initiate microcalcification (Liu et al., 2008), and promote the phenomenon of epithelial-mesenchymal transition (EMT) (Gonzalez and Medici, 2014). Canonical Wnt signaling activity supports bone formation (Turashvili et al., 2006), and it is also involved in several stages of mammary gland growth and differentiation and human breast cancer development (Howe and Brown, 2004). Additionally, it is also important to mention that the implication of other bone-derived factors (e.g., osteocalcin, fibroblast growth factor 23, sclerostin, lipocalin 2) in cancer biology has attracted research interest in recent years (Mansinho et al., 2019; Santiago-Sánchez et al., 2020; Martiniakova et al., 2023b), as they may be used as promising tumor biomarkers. Conversely, breast cancer cells are able to secrete various cytokines, including parathyroid hormone (PTH)-related protein, vascular endothelial growth factor (VEGF), RANKL, various interleukins (ILs) that serve as crucial bone modulators (Shemanko et al., 2016). These facts also contribute to a profound interplay between bone tissue and breast tissue.
Current pharmacological treatments for both osteoporosis and breast cancer often cause undesirable side effects; therefore, various natural metabolites, including those found in sea buckthorn, are being intensively researched to discover an alternative and effective treatment method with less harmful impacts (Li Y. et al., 2017; Martiniakova et al., 2020).
3 The impact of sea buckthorn and its flavonoids isorhamnetin, quercetin, and kaempferol on bone tissue healthSeveral studies have shown that sea buckthorn fatty acids significantly elevated levels of serum estrogen, TGF, insulin-like growth factor (IGF), BMD, cortical bone thickness, trabecular number, and bone mechanical properties in aged female rats (Liu et al., 2006b; Liu et al., 2006a). In this context, Yuan et al. (2022) found that sea buckthorn was able not only to increase BMD and estrogen levels, but also to raise levels of bone turnover markers (e.g., procollagen type 1N propeptide: P1NP, C-terminal telopeptide: CTX) and improve trabecular bone microstructure (relative bone volume, trabecular thickness) in ovariectomized (OVX) rats. Furthermore, it enhanced the efficacy of a traditional Chinese medicine QiangGuYin (used to treat osteoporosis) by inhibiting Casein kinase 2-interacting protein-1 (CKIP-1) and Notum expression through the Wnt/β-catenin pathway. Molecular docking analysis revealed that seven active components, including isorhamnetin, quercetin, and kaempferol, were able to potentially influence CKIP-1 and Notum (Yuan et al., 2022). However, this study did not provide important details about the material used (Supplementary Table 1). Moreover, the discovery of the ‘active’ metabolites of sea buckthorn was based only on the calculated results of molecular docking and was not followed by experimental studies. According to Park et al. (2022), an alcoholic extracts of sea buckthorn fruits and their fractions increased BMD and exerted the protective effects against cartilage damage and disturbed trabecular bone microarchitecture. Moreover, the extracts and their fractions stimulated the differentiation of murine mesenchymal stem cells into osteoblasts and elevated gene expression of osteogenic factors and markers, e.g., alkaline phosphatase (ALP), runt-related transcription factor 2 (RUNX2), osteopontin (OPN), osterix (OSX). Lee et al. (2023) pointed out the anti-osteoporotic impacts of triterpenoids from sea buckthorn fruit by promoting osteoblast differentiation from mesenchymal stem cells. However, the extracts used in the studies mentioned above would require better characterization. Relevant information related to this issue is presented in Supplementary Table 1; Figure 1.
There is a limited number of studies examining the effect of isorhamnetin in relation to bone damage. According to Yamaguchi et al. (2007), isorhamnetin inhibited PTH-stimulated osteoclastogenesis in mouse bone marrow cells and elevated PTH-decreased calcium content in femoral-diaphyseal tissue cultures. Moreover, isorhamnetin reduced osteoclast formation in bone marrow macrophages through inhibition of mitogen-activated protein kinase (MAPK), nuclear factor-kappa B (NF-κB), and protein kinase B (AKT) signaling (Zhou et al., 2019).
In contrast to isorhamnetin, more in vitro studies have shown the ability of quercetin to reduce osteoblast apoptosis, osteoclastogenesis, and oxidative stress (Woo et al., 2004; Yamaguchi et al., 2007; Tsuji et al., 2009; Yamaguchi and Weitzmann, 2011; Tripathi et al., 2015; Niu et al., 2020; Wong et al., 2020). On the contrary, quercetin significantly increased osteoblastogenesis, ALP activity, calcium content, expression of bone formation-associated proteins in mouse preosteoblastic MC3T3-E1 cells (Tripathi et al., 2015), rat osteoblast-like ROS 17/2.8 cells (Kim et al., 2007), human osteoblast-like MG-63 cells (Prouillet et al., 2004), and bone marrow mesenchymal stem cells (BMSCs) (Yuan et al., 2018; Feng et al., 2023). In some researches mentioned above, the effect of quercetin on NF-κB and Wnt/β-catenin signaling was also reported. Notably, higher doses of quercetin had either suppressive or decreased activity on osteoblast-specific gene expression, osteoblast growth and mineralization in several studies (Yamaguchi and Weitzmann, 2011; Wong et al., 2020). Furthermore, quercetin has an ability to bind to the estrogen receptor (ER) (Ross and Kasum, 2002) and influence osteoblast and osteoclast activity, as well as the expression and activity of various inflammatory cytokines, participating in bone remodeling (Wang et al., 2017). Numerous in vivo studies have established the protective effects of quercetin against bone loss through increased BMD, improved bone microarchitecture and bone strength, elevated bone growth, decreased bone resorption markers, and increased bone formation markers (Tsuji et al., 2009; Oršolić et al., 2018; Yuan et al., 2018; Niu et al., 2020; Sun et al., 2022; Feng et al., 2023). Yurteri et al. (2023) revealed that quercetin was also able to strengthen bone in both the early and late stages of fracture healing.
Several in vitro studies have shown that inhibitory effects of kaempferol on osteoclastogenesis may be linked to the downregulation of osteoclastogenic factors, such as RANKL, nuclear factor of activated T-cells cytoplasmic 1 (NFAT-c1), tumor necrosis factor receptor-associated factor 6 (TRAF6), c-Fos proto-oncogene, NF-κB signaling (Pang et al., 2006; Lee et al., 2014; Kim et al., 2018; Wong et al., 2019). On the other hand, conducted experiments have reported that kaempferol increased ALP activity and promoted osteogenic differentiation and mineralization in rat bone marrow mesenchymal stem cells (rBMSCs) via mediation of SOX2/miR-124-3p/PI3K/AKT/mTOR axis as well as in osteoblastic MC3T3-E1 cells by inducing autophagy and activating osteoblast differentiation markers such as RUNX2, OSX, collagen 1 (Kim I. R. et al., 2016; Xie et al., 2021; Gan et al., 2022). In addition, kaempferol could affect bone through the regulation of AKT serine/threonine kinase 1 (AKT1) and matrix metalloproteinase (MMP)-9 gene expressions, which are closely related to the pathogenesis of bone loss (Dong et al., 2024). Furthermore, kaempferol can modulate bone metabolism also through the ER (Jia et al., 2012). Tang et al. (2008) revealed that kaempferol activated ERβ-mediated estrogen response element (ERE) transcription in MG-63 cell line. In vivo studies have demonstrated that kaempferol can increase BMD and ALP activity, reduce bone turnover, and improve trabecular bone microarchitecture and bone strength in OVX rats (Trivedi et al., 2008; Nowak et al., 2017; Liu et al., 2021). Moreover, kaempferol was found to be able to ameliorate osteoporosis by raising C-X-C motif ligand 12 (CXCL12) expression and decreasing miR-10a-3p (Liu et al., 2021).
4 The impact of sea buckthorn and its flavonoids isorhamnetin, quercetin, and kaempferol on breast tissue healthSeveral in vitro studies have demonstrated the antitumor activity of sea buckthorn. Zhang et al. (2005) examined changes in apoptosis-related gene expression profiles in human breast carcinoma Bcap-37 cells induced by flavonoids from sea buckthorn seeds. These authors found that the expression of 32 apoptosis-related genes (e.g., IGFBP4, CTNNB1, CASP3, GADD34) was affected by flavonoid treatment. According to Wang et al. (2014), sea buckthorn procyanidins isolated from the seeds could induce apoptosis of human breast cancer MDA-MB-231 cells through fatty acid synthase inhibition. Indeed, high levels of this enzyme have been identified in cancer cells. Olsson et al. (2004) reported that sea buckthorn fruit extracts reduced the proliferation of breast cancer MCF-7 cells in a concentration-dependent manner. Similarly, Boivin et al. (2007) determined the inhibition of breast cancer (MCF-7 and MDA-MB-231) cell proliferation by sea buckthorn berry juice, as well as the suppression of tumor necrosis factor (TNF)-induced activation of the nuclear transcription factor NF-κB. A limitation of the studies mentioned above is the lack of characterization of the experimental material. In addition, many studies have determined a positive role of sea buckthorn oil in cancer treatment, including chemotherapy and radiotherapy (Olas, 2018; Olas et al., 2018). Table 1; Figure 1 summarize relevant information related to this issue.
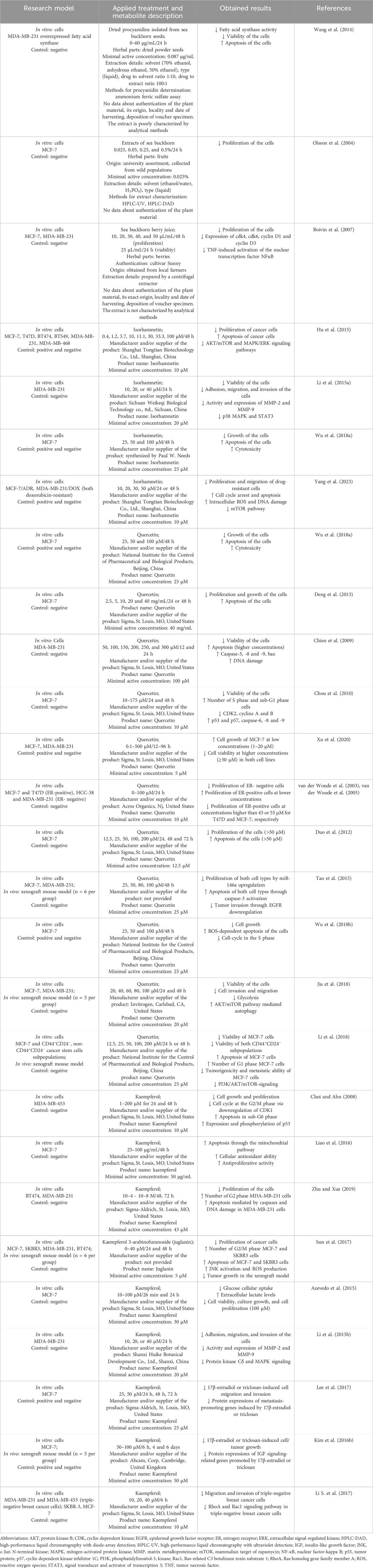
Table 1. In vitro and in vivo studies reflecting the potential of sea buckthorn and its flavonoids isorhamnetin, quercetin, and kaempferol against breast cancer.
Hu et al. (2015) revealed the anti-proliferative and pro-apoptotic effects of isorhamnetin in breast cancer mediated through inhibition of AKT/mTOR and MEK/ERK signaling pathways. Li et al. (2015a) discovered an inhibitory impact of isorhamnetin on the invasion of human breast carcinoma MDA-MB-231 cells by reducing the expression and activity of MMP-2 and MMP-9. This inhibition can be potentially linked to p38 MAPK and STAT3 suppression. Wu et al. (2018a) found that isorhamnetin dose-dependently inhibited the growth of human breast cancer MCF-7 cells, and exerted a strong cytotoxic effect through the reactive oxygen species (ROS)-dependent apoptosis pathway. According to Yang et al. (2023), isorhamnetin significantly reduced cell proliferation and migration and enhanced antitumor competence of doxorubicin (DOX) against resistant breast cancer cells both in vitro and in vivo, indicating its anti-breast tumor action as a DOX sensitizer.
Numerous in vitro studies have shown that quercetin at high concentrations exerts anti-proliferative impacts on breast cancer cells by arresting the cell cycle and inducing apoptosis (Chien et al., 2009; Chou et al., 2010; Deng et al., 2013). Conversely, lower doses of quercetin resulted in strong pro-proliferative effects (van der Woude et al., 2003; Xu et al., 2020). van der Woude et al. (2005) found that quercetin-induced stimulation of breast cancer cell proliferation was mediated by the ER. In ER+ (e.g., MCF-7) cells, lower concentrations of quercetin led to proliferative effects, while higher concentrations decreased cell viability. In ER− (e.g., MDA-MB-231) cells, reduced cell proliferation was observed even at low doses of quercetin. According to Duo et al. (2012), quercetin at higher concentrations was able to induce apoptosis through induction of BAX with concomitant inhibition of BCL-2 in human breast cancer MCF-7 cells and also through mitochondria- and caspase-3-dependent pathways in human breast carcinoma MDA-MB-231 cells (Chien et al., 2009). Tao et al. (2015) found that quercetin strongly inhibited cell proliferation in human breast cancer cells in a time- and dose-dependent fashion, which was associated with upregulation of miR-146a expression and induction of apoptosis through activation of caspase-3 and mitochondrial-dependent pathways. Wu et al. (2018b) pointed out the antitumor effects of quercetin through the induction of ROS-dependent apoptosis in MCF-7 cells. Jia et al. (2018) revealed that quercetin suppressed breast cancer progression by inhibiting cell motility and glycolysis via the induction of autophagy mediated by the AKT/mTOR pathway. Animal studies using tumor xenografts revealed that quercetin administration led to a reduction in tumor volume and decreased the markers associated with tumor growth and metastatic properties (Tao et al., 2015; Jia et al., 2018; Li et al., 2018).
In breast cancer, kaempferol can inhibit cell growth by destroying the cell cycle and induce apoptosis through p53 phosphorylation (Choi and Ahn, 2008), mitochondria-dependent pathway (Liao et al., 2016; Zhu and Xue, 2019), ROS/c-Jun N-terminal kinase (JNK) signaling pathway (Sun et al., 2017). The primary intracellular antioxidant mechanism of kaempferol involves scavenging the ROS accumulation and maintaining the activity of antioxidant enzymes at a physiological level. According to Azevedo et al. (2015), kaempferol at high concentrations strongly inhibited glucose uptake by breast carcinoma MCF-7 cells, leading to a significant decline in cell viability and proliferative capability. Li et al. (2015b) reported that kaempferol suppressed the invasion of human breast cancer MDA-MB-231 cells by downregulating the activity and expression of MMP-9. Kaempferol was also able to inhibit triclosan-induced EMT and metastatic behavior in breast cancer MCF-7 cells (Lee et al., 2017). Zhu and Xue (2019) demonstrated that the inhibitory effects of kaempferol on cell proliferation are greater in ER− (MDA-MB-231) cells compared to ER+ breast carcinoma (BT474) cells. According to Kim S. H. et al. (2016), kaempferol exerted anti-proliferative activity against breast cancer by suppressing triclosan- and estrogen-induced cancer progression by acting as an antagonist of ER and IGF-1R signaling in both cellular and xenograft breast cancer models. Li S. et al. (2017) revealed that a low dose of kaempferol inhibited the migration and invasion of triple-negative breast cancer (TNBC) cells by targeting the Rac1 or RhoA signaling pathway. In a mouse xenograft model, kaempferol inhibited the growth of breast cancer in vivo (Sun et al., 2017).
5 Methodological aspects and limitations of the reviewed studiesFrom the point of view of the interpretation and validity of the studies listed in Supplementary Table 1; Table 1, it is necessary to focus attention on several methodological aspects that should be fulfilled during pharmacological research (Heinrich et al., 2020). An important limitation of the studies analysing sea buckthorn extracts is the lack of characterization of the experimental material. Moreover, although relevant animal and cell models, negative and in several cases positive controls were used in those researches, no comparable healthy controls were available for cultured tumor cells to monitor metabolite selectivity. On the contrary, it is positive that studies showing antioxidant effects of metabolites used cell-based antioxidant assays, which, unlike chemical ones, are pharmacologically relevant. Other potential methodological risks arise from the structure and nature of flavonoids investigated. In general, polyphenols are categorized as Pan-Assay INterference compounds (PAINs or promiscuous inhibitors) and can interfere with the results of various assays. They also bind broadly to the protein targets of the assays themselves (Sheridan and Spelman, 2022). It has been revealed that polyphenols can self-associate to form colloids, which can affect their affinity for proteins. Flavonoids are more prone to aggregation than other phenolic metabolites under the conditions of the biochemical assays, with quercetin confirmed as promiscuous inhibitor (Pohjala and Tammela, 2012). Consequently, flavonoids interfere with colorimetric protein assays in a concentration- and structure-dependent manner (Singh et al., 2020) and affect other commonly used assays, such as MTT, by altering succinate dehydrogenase activity or directly interacting with MTT (Wang et al., 2010). In addition, such properties of flavonoids can be a potential source of misleading results in molecular docking analysis, therefore its findings should be verified in experimental studies. Thus, all circumstances, specifics, and risks of using individual methods should always be considered when analysing flavonoids and the failure to provide details on dealing with these methodological aspects can be considered as another limitation.
6 ConclusionBotanical drugs have recently achieved remarkable success in promoting the treatment of various diseases. Sea buckthorn shows great medicinal and therapeutic potential due to its high content of bioactive metabolites with anti-proliferative, antioxidant, and anti-inflammatory activities. This review described the contemporary knowledge from in vitro and in vivo studies on the effect of sea buckthorn and its flavonoids isorhamnetin, quercetin, and kaempferol on bone and breast tissue health with an emphasis on osteoporosis and breast cancer, given their raising incidence in postmenopausal women. Conducted studies related to bone damage have demonstrated favorable impacts of all aforementioned bioactive metabolites on bone remodeling and mineralization, oxidative stress, bone microarchitecture and strength. In relation to breast cancer, sea buckthorn and its flavonoids inhibited cancer cell proliferation while inducing apoptosis, reduced tumor expansion and metastatic properties. In any case, it should be noted that several studies using extracts did not provide a sufficiently detailed definition of the study material or reports on the phytochemical analysis of the extracts as recommended by the best practice guidelines, indicating limitations and lower reliability of these studies. In addition, the known interference of flavonoids with commonly used assays (such as protein or MTT assays) should be always considered and may be another source of limitation. On the contrary, all these investigations used standard research models or cell lines and were published in peer-reviewed journals. By evaluating the available studies that analysed extracts and flavonoids mentioned in our manuscript, we can state that our hypothesis was confirmed, as all bioactive metabolites improved the impaired health status of both bone and breast tissues. In addition, some research has investigated the role of sea buckthorn extracts in reducing chemotherapy- and radiotherapy-related side effects, suggesting their potential benefits to improve overall treatment outcomes. Further in vitro studies and animal model studies that provide enough detailed information on the investigated material are needed, as well as clinical trials involving osteoporotic/non-osteoporotic and breast cancer/non-breast cancer patients, which may provide the key findings for identifying more effective therapies against bone and breast tissue damage. In this regard, the appropriate selection of the optimal dose and type of bioactive agent for inducing protective effects on bone and breast tissues in humans requires careful consideration and further validation in clinical trials.
Author contributionsMM: Conceptualization, Funding acquisition, Methodology, Supervision, Writing–original draft, Writing–review and editing. NP: Formal Analysis, Visualization, Writing–original draft. RB: Formal Analysis, Visualization, Writing–review and editing. AS: Formal Analysis, Visualization, Writing–review and editing. VK: Formal Analysis, Visualization, Writing–review and editing. VM: Formal Analysis, Visualization, Writing–review and editing. SC: Supervision, Writing–review and editing. RO: Funding acquisition, Supervision, Visualization, Writing–original draft, Writing–review and editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Ministry of Education, Research, Development and Youth of the Slovak Republic, grant numbers VEGA 1/0416/22 and VEGA 1/0328/24.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1462823/full#supplementary-material
ReferencesAntonacci, C., Scimeca, M., Bonfiglio, R., Fazi, S., and Bonanno, E. (2018). Breast and bone: a story of hidden similarities. Chronicles Oncol. 1. doi:10.33582/2638-4248/1001
CrossRef Full Text | Google Scholar
Athonvarangkul, D., and Wysolmerski, J. J. (2023). Crosstalk within a brain-breast-bone axis regulates mineral and skeletal metabolism during lactation. Front. Physiol. 14, 1121579. doi:10.3389/fphys.2023.1121579
PubMed Abstract | CrossRef Full Text | Google Scholar
Azevedo, C., Correia-Branco, A., Araújo, J. R., Guimarães, J. T., Keating, E., and Martel, F. (2015). The chemopreventive effect of the dietary compound kaempferol on the MCF-7 human breast cancer cell line is dependent on inhibition of glucose cellular uptake. Nutr. Cancer 67, 504–513. doi:10.1080/01635581.2015.1002625
PubMed Abstract | CrossRef Full Text | Google Scholar
Boivin, D., Blanchette, M., Barrette, S., Moghrabi, A., and Béliveau, R. (2007). Inhibition of cancer cell proliferation and suppression of TNF-induced activation of NFkappaB by edible berry juice. Anticancer Res. 27, 937–948.
PubMed Abstract | Google Scholar
Bramwell, V. H., Tuck, A. B., Chapman, J.-A. W., Anborgh, P. H., Postenka, C. O., Al-Katib, W., et al. (2014). Assessment of osteopontin in early breast cancer: correlative study in a randomised clinical trial. Breast Cancer Res. 16, R8. doi:10.1186/bcr3600
PubMed Abstract | CrossRef Full Text | Google Scholar
Chien, S.-Y., Wu, Y.-C., Chung, J.-G., Yang, J.-S., Lu, H.-F., Tsou, M.-F., et al. (2009). Quercetin-induced apoptosis acts through mitochondrial- and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Hum. Exp. Toxicol. 28, 493–503. doi:10.1177/0960327109107002
PubMed Abstract | CrossRef Full Text | Google Scholar
Cho, J.-H., Kim, J.-H., and Lee, H.-K. (2015). The relationship between breast density and bone mineral density after menopause. J. Phys. Ther. Sci. 27, 1243–1246. doi:10.1589/jpts.27.1243
PubMed Abstract | CrossRef Full Text | Google Scholar
Choi, E. J., and Ahn, W. S. (2008). Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells. Nutr. Res. Pract. 2, 322–325. doi:10.4162/nrp.2008.2.4.322
PubMed Abstract | CrossRef Full Text | Google Scholar
Chou, C.-C., Yang, J.-S., Lu, H.-F., Ip, S.-W., Lo, C., Wu, C.-C., et al. (2010). Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch. Pharm. Res. 33, 1181–1191. doi:10.1007/s12272-010-0808-y
PubMed Abstract | CrossRef Full Text | Google Scholar
Dai, R., Liu, M., Xiang, X., Xi, Z., and Xu, H. (2022). Osteoblasts and osteoclasts: an important switch of tumour cell dormancy during bone metastasis. J. Exp. Clin. Cancer Res. 41, 316. doi:10.1186/s13046-022-02520-0
PubMed Abstract | CrossRef Full Text | Google Scholar
Danielski, R., and Shahidi, F. (2024). Phenolic composition and bioactivities of sea buckthorn (Hippophae rhamnoides L.) fruit and seeds: an unconventional source of natural antioxidants in North America. J. Sci. Food Agric. 104, 5553–5564. doi:10.1002/jsfa.13386
PubMed Abstract | CrossRef Full Text | Google Scholar
Deng, X.-H., Song, H.-Y., Zhou, Y.-F., Yuan, G.-Y., and Zheng, F.-J. (2013). Effects of quercetin on the proliferation of breast cancer cells and expression of survivin in vitro. Exp. Ther. Med. 6, 1155–1158. doi:10.3892/etm.2013.1285
PubMed Abstract | CrossRef Full Text | Google Scholar
Dong, Q., Ren, G., Li, Y., and Hao, D. (2024). Network pharmacology analysis and experimental validation to explore the mechanism of kaempferol in the treatment of osteoporosis. Sci. Rep. 14, 7088. doi:10.1038/s41598-024-57796-3
PubMed Abstract | CrossRef Full Text | Google Scholar
Dudau, M., Vilceanu, A. C., Codrici, E., Mihai, S., Popescu, I. D., Albulescu, L., et al. (2021). Sea-buckthorn seed oil induces proliferation of both normal and dysplastic keratinocytes in basal conditions and under UVA irradiation. J. Pers. Med. 11, 278. doi:10.3390/jpm11040278
PubMed Abstract | CrossRef Full Text | Google Scholar
Duo, J., Ying, G.-G., Wang, G.-W., and Zhang, L. (2012). Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol. Med. Rep. 5, 1453–1456. doi:10.3892/mmr.2012.845
PubMed Abstract | CrossRef Full Text | Google Scholar
Feng, L., Yang, Z., Hou, N., Wang, M., Lu, X., Li, Y., et al. (2023). Long non-coding RNA Malat1 increases the rescuing effect of quercetin on tnfα-impaired bone marrow stem cell osteogenesis and ovariectomy-induced osteoporosis. Int. J. Mol. Sci. 24, 5965. doi:10.3390/ijms24065965
PubMed Abstract | CrossRef Full Text | Google Scholar
Gan, L., Leng, Y., Min, J., Luo, X.-M., Wang, F., and Zhao, J. (2022). Kaempferol promotes the osteogenesis in rBMSCs via mediation of SOX2/miR-124-3p/PI3K/Akt/mTOR axis. Eur. J. Pharmacol. 927, 174954. doi:10.1016/j.ejphar.2022.174954
PubMed Abstract | CrossRef Full Text | Google Scholar
He, N., Wang, Q., Huang, H., Chen, J., Wu, G., Zhu, M., et al. (2023). A comprehensive review on extraction, structure, detection, bioactivity, and metabolism of flavonoids from sea buckthorn (hippophae rhamnoides L.). J. Food Biochem. 2023, 1–27. doi:10.1155/2023/4839124
CrossRef Full Text | Google Scholar
Heinrich, M., Appendino, G., Efferth, T., Fürst, R., Izzo, A. A., Kayser, O., et al. (2020). Best practice in research - overcoming common challenges in phytopharmacological research. J. Ethnopharmacol. 246, 112230. doi:10.1016/j.jep.2019.112230
PubMed Abstract | CrossRef Full Text | Google Scholar
Heinrich, M., Jalil, B., Abdel-Tawab, M., Echeverria, J., Kulić, Ž., McGaw, L. J., et al. (2022). Best Practice in the chemical characterisation of extracts used in pharmacological and toxicological research-The ConPhyMP-Guidelines. Front. Pharmacol. 13, 953205. doi:10.3389/fphar.2022.953205
PubMed Abstract | CrossRef Full Text | Google Scholar
Hu, S., Huang, L., Meng, L., Sun, H., Zhang, W., and Xu, Y. (2015). Isorhamnetin inhibits cell proliferation and induces apoptosis in breast cancer via Akt and mitogen-activated protein kinase kinase signaling pathways. Mol. Med. Rep. 12, 6745–6751. doi:10.3892/mmr.2015.4269
PubMed Abstract | CrossRef Full Text | Google Scholar
Jaśniewska, A., and Diowksz, A. (2021). Wide spectrum of active compounds in sea buckthorn (hippophae rhamnoides) for disease prevention and food production. Antioxidants (Basel) 10, 1279. doi:10.3390/antiox10081279
PubMed Abstract | CrossRef Full Text | Google Scholar
Jia, L., Huang, S., Yin, X., Zan, Y., Guo, Y., and Han, L. (2018). Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy induction. Life Sci. 208, 123–130. doi:10.1016/j.lfs.2018.07.027
PubMed Abstract | CrossRef Full Text | Google Scholar
Jia, M., Nie, Y., Cao, D.-P., Xue, Y.-Y., Wang, J.-S., Zhao, L., et al. (2012). Potential antiosteoporotic agents from plants: a comprehensive review. Evid. Based Complement. Altern. Med. 2012, 364604. doi:10.1155/2012/364604
PubMed Abstract | CrossRef Full Text | Google Scholar
Johnson, J. M., Dalton, R. R., Wester, S. M., Landercasper, J., and Lambert, P. J. (1999). Histological correlation of microcalcifications in breast biopsy specimens. Arch. Surg. 134, 712–715. doi:10.1001/archsurg.134.7.712
PubMed Abstract | CrossRef Full Text | Google Scholar
Kim, C.-J., Shin, S.-H., Kim, B.-J., Kim, C.-H., Kim, J.-H., Kang, H.-M., et al. (2018). The effects of kaempferol-inhibited autophagy on osteoclast formation. Int. J. Mol. Sci. 19, 125. doi:10.3390/ijms19010125
PubMed Abstract | CrossRef Full Text | Google Scholar
Kim, D.-S., Takai, H., Arai, M., Araki, S., Mezawa, M., Kawai, Y., et al. (2007). Effects of quercetin and quercetin 3-glucuronide on the expression of bone sialoprotein gene. J. Cell Biochem. 101, 790–800. doi:10.1002/jcb.21233
PubMed Abstract | CrossRef Full Text | Google Scholar
Kim, I. R., Kim, S.-E., Baek, H.-S., Kim, B.-J., Kim, C.-H., Chung, I.-K., et al. (2016). The role of kaempferol-induced autophagy on differentiation and mineralization of osteoblastic MC3T3-E1 cells. BMC Complementary Altern. Med. 16, 333. doi:10.1186/s12906-016-1320-9
PubMed Abstract | CrossRef Full Text | Google Scholar
Kim, S., Chen, J., Cheng, T., Gindulyte, A., He, J., He, S., et al. (2023). PubChem 2023 update. Nucleic Acids Res. 51, D1373–D1380. doi:10.1093/nar/gkac956
PubMed Abstract | CrossRef Full Text | Google Scholar
Kim, S. H., Hwang, K.-A., and Choi, K.-C. (2016). Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. J. Nutr. Biochem. 28, 70–82. doi:10.1016/j.jnutbio.2015.09.027
PubMed Abstract | CrossRef Full Text | Google Scholar
Lee, D., Park, K., Hong, J.-H., Kim, S., Park, K.-M., and Kim, K. H. (2023). Anti-osteoporosis effects of triterpenoids from the fruit of sea buckthorn (Hippophae rhamnoides) through the promotion of osteoblast differentiation in mesenchymal stem cells, C3H10T1/2. Archives Pharmacal Res. 46, 771–781. doi:10.1007/s12272-023-01468-9
CrossRef Full Text | Google Scholar
Lee, G.-A., Choi, K.-C., and Hwang, K.-A. (2017). Kaempferol, a phytoestrogen, suppressed triclosan-induced epithelial-mesenchymal transition and metastatic-related behaviors of MCF-7 breast cancer cells. Environ. Toxicol. Pharmacol. 49, 48–57. doi:10.1016/j.etap.2016.11.016
PubMed Abstract | CrossRef Full Text | Google Scholar
Lee, W.-S., Lee, E.-G., Sung, M.-S., and Yoo, W.-H. (2014). Kaempferol inhibits IL-1β-stimulated, RANKL-mediated osteoclastogenesis via downregulation of MAPKs, c-Fos, and NFATc1. Inflammation 37, 1221–1230. doi:10.1007/s10753-014-9849-6
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, C., Yang, D., Zhao, Y., Qiu, Y., Cao, X., Yu, Y., et al. (2015a). Inhibitory effects of isorhamnetin on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-2/9. Nutr. Cancer 67, 1191–1200. doi:10.1080/01635581.2015.1073763
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, C., Zhao, Y., Yang, D., Yu, Y., Guo, H., Zhao, Z., et al. (2015b). Inhibitory effects of kaempferol on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-9. Biochem. Cell Biol. 93, 16–27. doi:10.1139/bcb-2014-0067
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, S., Yan, T., Deng, R., Jiang, X., Xiong, H., Wang, Y., et al. (2017). Low dose of kaempferol suppresses the migration and invasion of triple-negative breast cancer cells by downregulating the activities of RhoA and Rac1. OncoTargets Ther. 10, 4809–4819. doi:10.2147/OTT.S140886
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, X., Zhou, N., Wang, J., Liu, Z., Wang, X., Zhang, Q., et al. (2018). Quercetin suppresses breast cancer stem cells (CD44+/CD24−) by inhibiting the PI3K/Akt/mTOR-signaling pathway. Life Sci. 196, 56–62. doi:10.1016/j.lfs.2018.01.014
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, Y., Li, S., Meng, X., Gan, R.-Y., Zhang, J.-J., and Li, H.-B. (2017). Dietary natural products for prevention and treatment of breast cancer. Nutrients 9, 728. doi:10.3390/nu9070728
PubMed Abstract | CrossRef Full Text | Google Scholar
Liao, W., Chen, L., Ma, X., Jiao, R., Li, X., and Wang, Y. (2016). Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur. J. Med. Chem. 114, 24–32. doi:10.1016/j.ejmech.2016.02.045
留言 (0)