Air pollution is a globally recognized environmental health hazard (1). With rapid global socioeconomic development, there is growing concern about the adverse health effects of air pollutants (2). Environmental air pollution and indoor air pollution are considered major risk factors leading to premature mortality and an increased incidence of diseases. The burden of diseases and deaths caused by these factors has become a global public health challenge, imposing significant direct and indirect costs on society (3). In 2021, particulate matter air pollution was the leading contributor to the global disease burden in 2021 (4). Air pollutants can be categorized into two main types based on their state of existence: gaseous pollutants and particulate pollutants. Gaseous pollutants include nitrogen dioxide (NO2), carbon monoxide (CO), ozone (O3), sulfur dioxide (SO2), and others. Atmospheric particulate matter (PM) comprises total suspended particles, inhalable particles (PM10), fine particulate matter (PM2.5), and ultrafine particles. Among these, PM2.5 refers to particles in the atmosphere with a diameter equal to or less than 2.5μm. PM2.5 represents the majority of PM in the atmosphere (5, 6).
Thyroid disorders have been recognized as some of the most prevalent diseases worldwide (7). Common biomarkers for assessing thyroid homeostasis include free thyroxine (FT4), free triiodothyronine (FT3), and thyroid-stimulating hormone (TSH) (8). Thyroid hormones (THs) play a pivotal role in maintaining metabolic balance, cardiovascular health, and neurological development, exhibiting pleiotropic effects on multiple organ systems (9). Abnormal TH levels, whether elevated or decreased, are closely associated with various thyroid disorders (10). While iodine nutrition status is significantly linked to thyroid disease, research indicates that the prevalence of thyroid disorders continues to rise in iodine-sufficient populations, such as in China (11). Furthermore, the global incidence of thyroid disease is also on the rise (12–14). Animal studies and extensive epidemiological research suggest that air pollutants can disrupt thyroid hormone levels, impair metabolic homeostasis, and ultimately contribute to thyroid dysfunction (15, 16). Epidemiological studies often utilize large population samples, enabling researchers to observe the effects of air pollutants in real-world settings. Additionally, long-term epidemiological investigations help establish temporal associations between exposure and disease onset. However, establishing a causal relationship between exposure and disease requires further experimental evidence.
This review aims to retrieve and synthesize published epidemiological studies on the relationship between air pollutant exposure and thyroid diseases, as well as thyroid hormones, across various populations, including children, adults, and pregnant women. The findings are interpreted from an epidemiological perspective, offering theoretical insights and directions for future systematic research, and providing new perspectives on the prevention of thyroid diseases.
MethodsWe conducted a scoping review to facilitate the mapping of the literature on emerging topics and provide avenues for future research. Our aim was to gain a comprehensive understanding of the literature regarding the relationship between exposure to air pollutants and thyroid disorders and thyroid hormone levels. Our findings were reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) (17). The scoping review protocol was registered with the Open Science Framework (https://doi.org/10.17605/OSF.IO/V8ERP).
Data sources and search strategyThe preliminary search was conducted on PubMed to identify relevant MeSH terms and keywords. Subsequently, a comprehensive systematic search strategy was developed for the PubMed, Embase, and Web of Science databases using the identified keywords and indexing terms (Tables 1, 2). The final literature retrieval was carried out on August 1, 2023.
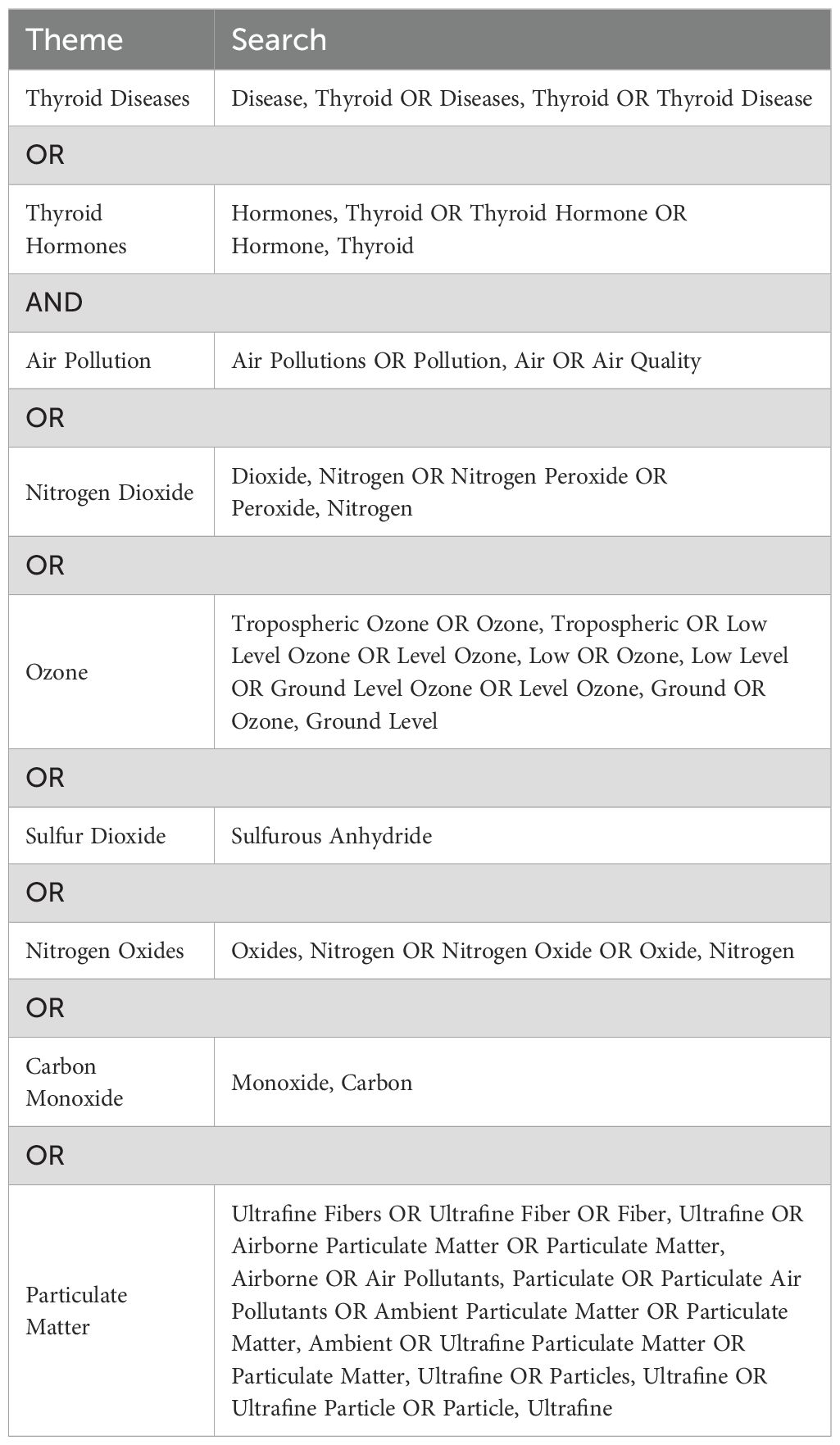
Table 1. Index terms and keywords used for the literature search.
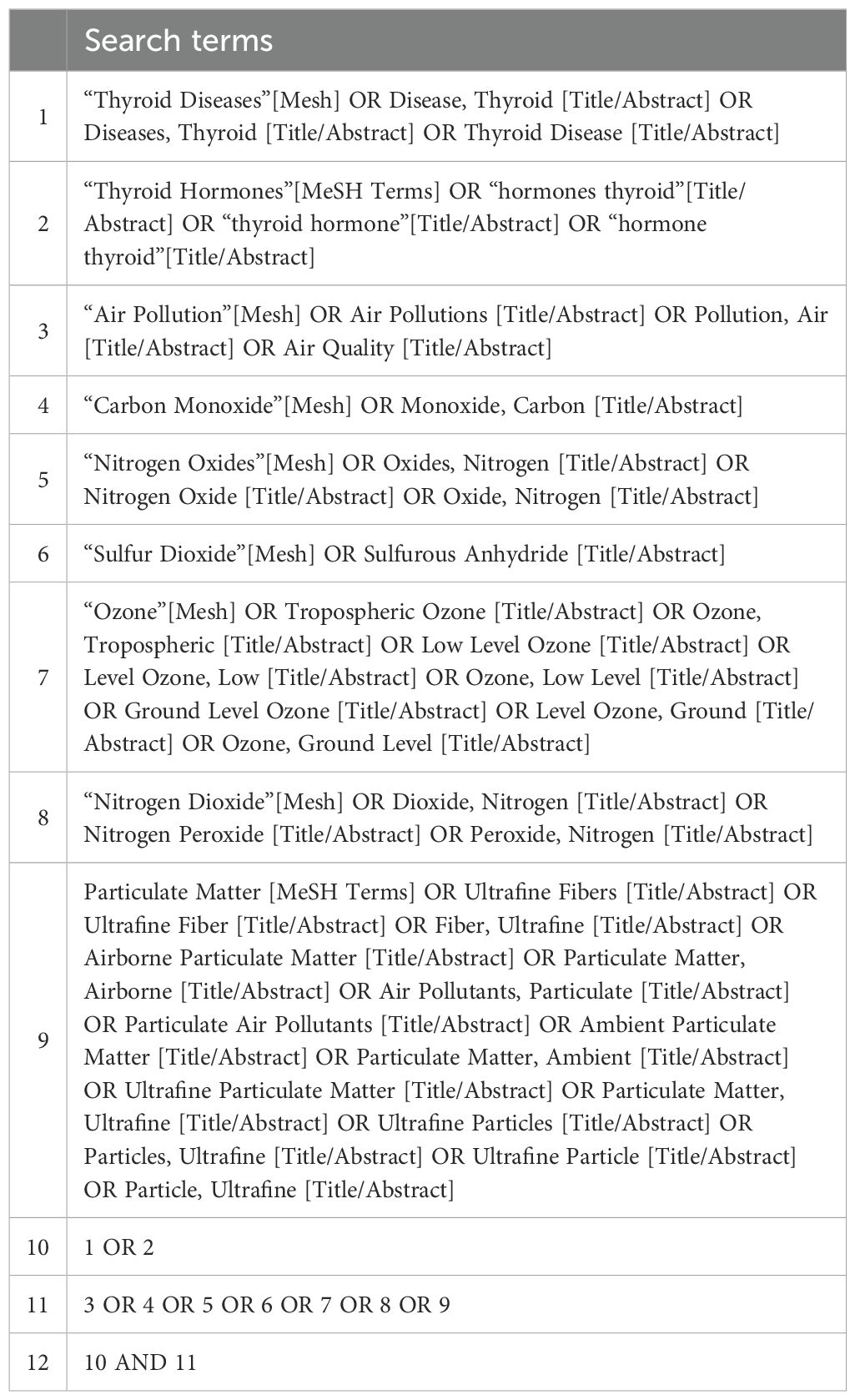
Table 2. Example of full search strategy in PUBMED.
Inclusion and exclusion criteriaThe inclusion criteria for the literature were as follows: (1) the exposure factor studied was air pollutants as the primary focus; (2) the literature examined outcomes related to thyroid diseases and thyroid hormone levels; and (3) the literature included results from epidemiological studies.
Exclusion criteria for the literature were as follows: (1) literature that did not meet the inclusion criteria; (2) literature investigating exposure factors such as organic pollutants or chemical substances, among others; (3) duplicate literature, reviews, meta-analyses, letters, replies, comments, or meeting abstracts; and (4) literature for which full text was unavailable, and data extraction was not possible.
Data extractionTwo researchers conducted a full-text screening, independently reviewed the literature included in the final selection, and extracted the data into tables for the purpose of data visualization, data synthesis, and result reporting. Discrepancies arising during this process were resolved through discussions involving all the authors. For studies meeting the inclusion criteria, we extracted information on the author, year of study, study type, country, study period, study population, sample size, pollutants, pollutants exposure evaluation, pollutants exposure time, thyroid related outcomes. Due to heterogeneity across studies and insufficient support for aggregation of the results, a meta-analysis was not conducted.
ResultsA total of 3,373 articles related to air pollutants and thyroid diseases were identified in the search. After removing duplicates (313 articles) and performing the initial screening of titles and abstracts, 68 articles remained. Upon further full-text examination and the exclusion of articles not meeting the criteria, 25 articles were considered relevant. Figure 1 illustrates the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart for the selection of included studies (Figure 1).

Figure 1. PRISMA flow diagram of study selection.
Study characteristicsThe included literature consists of population-based epidemiological studies. Most studies employed a cohort study design (12 studies), followed by cross-sectional studies (8 studies), case−control studies (4 studies), and one Mendelian randomization study (Table 3). All the included articles were published within the past ten years, with nearly 70% published after 2020. Among the 25 studies, more than half were conducted in Asia, including 12 from China. This study examines eight different air pollutants (PM2.5, PM10, PM10-2.5, O3, NO2, NOx, SO2, CO) and twelve thyroid-related outcomes, including TSH (n=12), FT4 (n=10), FT3 (n=7), hypothyroidism [n=7, of which 3 are congenital hypothyroidism (CH)], and thyroid cancer (n=3), among others. The 25 studies primarily focus on pregnant women and newborns, with 10 studies involving pregnant women, 7 studies involving newborns, and 6 studies involving the general adult population.

Table 3. Association between air pollutants and thyroid disorders.
Cohort studiesOut of the 25 studies included, 12 were cohort studies. Of these, 11 addressed thyroid hormone (THs) levels. Research found that, during late pregnancy, exposure to PM2.5 was negatively correlated with both umbilical cord blood TSH levels and the FT4/FT3 ratio (18). Higher PM2.5 exposure during pregnancy was associated with decreased maternal FT4 levels (19) and a reduced FT4/FT3 ratio (20). However, some studies have found no statistically significant associations between maternal PM2.5 exposure and neonatal TSH concentrations (19). Regarding thyroid disease, one large-scale cohort study spanning a decade among the Chinese population evaluated the relationship between O3 and thyroid diseases (TNs). The study indicates that long-term exposure to high levels of O3 in Hunan Province may be associated with an increased detection rate of TNs in general adults, potentially mediated by TSH (21). The other three studies focused on hypothyroidism, with one addressing full-term newborns with CH. Ghassabian et al. conducted a cohort study using data from five birth cohorts, comprising a total of 9,931 pregnant women. The study found that exposure to PM2.5 in early pregnancy was linked to mild thyroid dysfunction persisting throughout pregnancy. However, exposures to NOx and NO2 were not associated with hypothyroxinemia or high TSH during pregnancy (22). Another cohort study conducted in Shanghai, China, reached similar conclusions, reporting that early pregnancy (0-12 weeks) and mid-pregnancy (13-26 weeks) exposure to PM2.5 was associated with an increased risk of hypothyroidism in pregnant women. However, there was no significant association between hypothyroidism and NO2 exposure (23). An Israeli cohort study demonstrated a positive correlation between late-pregnancy exposure to nitrogen oxide (NOx) and the likelihood of newborns developing CH (OR 1.23 [95% CI 1.08 to 1.41]). However, there was no association between early and mid-pregnancy exposure to NOx and NO2 and the risk of CH (24). In a cohort study conducted at the University Clinical Centre in Tuzla focusing on autoimmune thyroid disease (AITD), five major air pollutants (PM2.5, NO2, SO2, CO, and O3) were analyzed. However, the findings indicated that the average concentrations of these pollutants were not statistically associated with an increased risk of AITD in the population of the region (25).
Cross-sectional studyMore than half of these cross-sectional studies were based on national databases from China, and seven studies utilized monitoring stations to assess pollutant exposure levels. Thyroid hormone levels were the primary outcomes in five of these studies. A study from Korea found a positive association between PM10 exposure and TSH levels in adults, while annual mean exposure to NO2 and CO was significantly associated with elevated TSH levels and reduced FT4 concentrations (26). In Chinese adults, increased PM2.5 levels were significantly negatively correlated with FT4 and the FT4/FT3 ratio, but positively correlated with FT3 levels (2). Higher exposure to PM2.5-bound metals was associated with lower FT4 and higher FT3 levels in pregnant women (27). Additionally, studies in pregnant women in Greece and adults in Spain found that PM2.5 exposure was linked to increased TSH levels, but no associations were observed between NO2 exposure and thyroid hormone levels (28, 29).
Three cross-sectional studies on air pollution and thyroid diseases have been conducted in China. Two of these studies utilized environmental air pollution data from the Chinese Air Quality Online Monitoring and Analysis Platform (https://www.aqistudy.cn/) (30, 31). They examined the relationship between maternal exposure to air pollutants and the likelihood of CH in offspring. Among these pollutants, O3 (OR 1.06 [95% CI 1.01 to 1.10]), NO2 (OR 1.10 [95% CI 1.02 to 1.18]), and PM2.5 (OR 1.02 [95% CI 1.00 to 1.03]) were significantly positively associated with the risk of CH in offspring (30, 31). However, there were no significant associations of exposure to SO2, CO, or PM10 with the risk of CH. Another study, which included a cohort of 4.9 million Chinese adults, examined the associations between exposure to PM2.5, PM10, NO2, SO2, CO, and O3 and the risk of TNs. The findings revealed significant linear associations between each of the six air pollutants and the risk of TNs (32).
Case−control studiesThere were four case−control studies, one of which was nested within a cohort (33–36). Out of the 13 included articles, three investigated thyroid cancer using case−control study designs. A nested case−control study in South Korea concerning thyroid cancer revealed a positive association between thyroid cancer incidence and NO2 exposure (OR 1.33 [95% CI 1.24 to 1.43]) and an inverse association between thyroid cancer incidence and PM10 exposure (OR 0.64 [95% CI 0.60 to 0.69]). These associations remained consistent in subgroup analyses (33). Two additional case-control studies on papillary thyroid cancer (PTC) conducted at the Johns Hopkins Medical Institution in the United States provided further evidence. They demonstrated a significant association between long-term exposure to PM2.5 and an increased diagnosis rate of PTC (34). Prolonged exposure to PM2.5 over 2 years (OR 1.18 [95% CI 1.00 to 1.40]) and 3 years (OR 1.23 [95% CI 1.05 to 1.44]) was significantly correlated with an increased incidence of PTC (36). Two studies included 1,990 PTC patients as the experimental group. The main difference between the two was that Crepeau et al. had a larger sample size in their control group, but their final conclusions were consistent with the other study. Furthermore, Crepeau et al. found that this association was most significant in populations with a higher median household income (34). Another case−control study in China investigated the correlation between preconception and early pregnancy exposure to environmental particulate matter and hypothyroidism during pregnancy. The study revealed that exposure to PM2.5 and PM10 during various intervals before the last menstrual period month (LMPM), including LMPM-60 days, LMPM-30 days, and all other distances before LMPM, was associated with an increased risk of hypothyroidism. Notably, the most significant associations with hypothyroidism risk were observed for PM2.5 (OR 1.14 [95% CI 1.10 to 1.18]) and PM10 (OR 1.10 [95% CI 1.07 to 1.13]) within a 250-metre buffer zone during the LMPM period (35).
Mendelian randomization studyIn Europe, a causal relationship between PM2.5 exposure and hypothyroidism was investigated through a two-sample Mendelian randomization study (37). That study revealed an association between exposure to increased PM2.5 concentrations and an increased risk of developing hypothyroidism.
DiscussionThe objective of this scoping review is to explore the relationship between exposure to air pollutants and thyroid diseases, as well as thyroid hormones, from an epidemiological perspective. A review of published studies indicates that different types of air pollution may have varying health effects. Exposure to particulate matter, particularly PM2.5, has been shown to impair thyroid function in pregnant women and negatively affect their offspring. However, the findings of Shang et al. suggest no association between PM10 exposure and the risk of CH in offspring (38). This discrepancy may be attributed to differences in study design and exposure assessment methods.
Among the 25 studies included, cohort studies were the most common, with 12 studies, followed by cross-sectional and case-control studies. In situations where randomized trials are not feasible, cohort studies are often considered one of the most reliable forms of observational epidemiological research. Cohort studies are particularly effective in establishing causal relationships by tracking health outcomes after exposure to factors such as air pollution, helping to understand how these exposures affect thyroid function or disease progression. However, they may be prone to selection bias. For example, individuals in susceptible populations may already have the disease at the start of the study, which can skew the associations being investigated. Cross-sectional studies can quickly assess the correlation between air pollution and thyroid function, but they are limited in their ability to infer causality. The limitations of case-control studies primarily arise from the selection of control groups. If there are geographical differences between the control and case groups, the study may fail to draw clear conclusions. In cases where associations exist, bias could lead to inaccurate results. Nevertheless, no single study design is flawless. A comprehensive understanding of the multifaceted relationship between air pollutants and thyroid diseases can be best achieved by integrating studies that offer complementary strengths and limitations. In the future, large-scale cohort studies with extensive exposure levels and long-term follow-up may provide the most powerful means to elucidate these associations.
The genetic background of the study subjects, as well as the potential interactions between air pollutants and genetic factors, may increase susceptibility to diseases. Therefore, these factors should be considered as confounders in research design. Thyroid hormones play a crucial role in the development of fetuses and neonates (39). In early pregnancy, since the fetal thyroid is not yet functional, the fetus relies on maternal thyroid hormones to maintain normal growth and development (40, 41). Consequently, maternal thyroid dysfunction is recognized as a known risk factor for restricted fetal development (42). This explains why researchers often focus on thyroid diseases and related hormone levels in pregnant women and neonates (41, 43, 44).
Differences exist in the methods used to assess exposure to environmental air pollutants in various studies. Qi et al.’s research, for instance, directly employed data disseminated by air quality monitoring stations for analysis (31). However, the majority of monitors involved in direct measurements are situated in urban or polluted areas (such as power plants), and not all study subjects reside in areas where monitoring stations are present. Furthermore, the data scope can be constrained by the specific years for which information is available, rendering the obtained data suitable only for coarse approximations. The land use regression (LUR) model utilized by Zhao et al. can be employed to forecast outdoor pollutant levels at the residential addresses of each participant. Building upon data derived from satellite systems at monitoring stations and further accounting for meteorological and spatial factors, the model incorporates adjustments based on geographic information such as population density, sea level, and meteorological data (45). Consequently, this approach enables the assessment of exposure levels in areas lacking air pollution monitoring, thus significantly enhancing the accuracy of exposure evaluations. This model has been validated in several studies (38, 46). Furthermore, research has estimated the average air pollutant exposure concentrations for study participants within circular buffer zones with diameters of 250 meters, 500 meters, and 750 meters based on the location of each residence, allowing for a more comprehensive analysis of the relationships (35). Exposure duration represents a common concern, necessitating assessment based on disease susceptibility and the target population. In the context of research related to thyroid cancer, the temporal scope of exposure is notably extended compared to other thyroid conditions. In the case of specific demographic groups, such as pregnant women, some researchers have structured exposure periods to encompass distinct gestational stages (22, 23).
Among the 25 studies we included, PM2.5 was mentioned 21 times, while PM10 was noted 10 times. This highlights the widespread concern about particulate matter pollution. In particular, China, where the prevalence of thyroid diseases is high and pollution is becoming increasingly severe, may prompt local researchers to investigate the potential link between air pollution exposure and hypothyroidism (47). Additionally, nitrogen oxides, such as nitrogen dioxide (NO2), were the second most frequently discussed pollutants, cited 13 times, followed by SO2, CO, and O3. A substantial body of epidemiological and toxicological research has shown that PM2.5 can enter the respiratory system through the lungs, triggering a series of pathophysiological responses, including systemic inflammation, oxidative stress, and vascular dysfunction, which can severely impact multiple bodily systems (11). Moreover, PM2.5 has been shown to impair thyroid function by disrupting thyroid hormone levels, potentially leading to various thyroid diseases (48–51). Animal experiments have demonstrated that PM2.5 can disrupt thyroid homeostasis by affecting the synthesis of thyroid hormones, but further basic research is needed to explore these mechanisms in greater detail (16).
In summary, large-scale, long-term cohort studies are needed to better understand the prolonged effects of air pollution on thyroid hormone levels and related diseases. Interdisciplinary collaboration is essential to further elucidate the complex interactions between air pollution and the endocrine system. For vulnerable populations, such as pregnant women and newborns, enhanced health protection measures should be prioritized. In high-risk areas, stricter air quality management and control of major pollutant emissions are necessary to reduce the burden of thyroid diseases. Future research should focus on elucidating how pollutants affect thyroid function through endocrine-disrupting mechanisms and quantify these effects across various exposure scenarios. A deeper exploration of these mechanisms could lead to more effective clinical and public health interventions.
Author contributionsKY: Data curation, Formal Analysis, Investigation, Validation, Writing – original draft, Writing – review & editing. GZ: Conceptualization, Project administration, Resources, Supervision, Validation, Writing – review & editing. YL: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work is supported by the National Natural Science Foundation of China (Grant No. 82000753), and the China Postdoctoral Science Foundation (Grant No. 2021MD703910 and 2023T160724). The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or the writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. WHO Guidelines Approved by the Guidelines Review Committee. WHO global air quality guidelines: Particulate matter (PM(25) and PM(10)), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. Geneva: World Health Organization (2021). © World Health Organization 2021.
2. Zeng Y, He H, Wang X, Zhang M, An Z. Climate and air pollution exposure are associated with thyroid function parameters: a retrospective cross-sectional study. J Endocrinol Invest. (2021) 44:1515–23. doi: 10.1007/s40618-020-01461-9
PubMed Abstract | Crossref Full Text | Google Scholar
3. Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. (2017) 389:1907–18. doi: 10.1016/S0140-6736(17)30505-6
PubMed Abstract | Crossref Full Text | Google Scholar
4. GBD 2021 Risk Factors Collaborators. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. (2024) 403:2162–203.
PubMed Abstract | Google Scholar
5. Yang J, Zhou M, Li M, Yin P, Hu J, Zhang C, et al. Fine particulate matter constituents and cause-specific mortality in China: A nationwide modelling study. Environ Int. (2020) 143:105927. doi: 10.1016/j.envint.2020.105927
PubMed Abstract | Crossref Full Text | Google Scholar
6. Pope CA 3rd, Coleman N, Pond ZA, Burnett RT. Fine particulate air pollution and human mortality: 25+ years of cohort studies. Environ Res. (2020) 183:108924. doi: 10.1016/j.envres.2019.108924
PubMed Abstract | Crossref Full Text | Google Scholar
7. Garmendia Madariaga A, Santos Palacios S, Guillén-Grima F, Galofré JC. The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. J Clin Endocrinol Metab. (2014) 99:923–31. doi: 10.1210/jc.2013-2409
PubMed Abstract | Crossref Full Text | Google Scholar
8. Van Uytfanghe K, Ehrenkranz J, Halsall D, Hoff K, Loh TP, Spencer CA, et al. Thyroid stimulating hormone and thyroid hormones (Triiodothyronine and thyroxine): an American thyroid association-commissioned review of current clinical and laboratory status. Thyroid: Off J Am Thyroid Assoc. (2023) 33:1013–28. doi: 10.1089/thy.2023.0169
Crossref Full Text | Google Scholar
10. Ittermann T, Khattak RM, Nauck M, Cordova CM, Völzke H. Shift of the TSH reference range with improved iodine supply in Northeast Germany. Eur J endocrinology. (2015) 172:261–7. doi: 10.1530/EJE-14-0898
Crossref Full Text | Google Scholar
11. Li Y, Xu L, Shan Z, Teng W, Han C. Association between air pollution and type 2 diabetes: an updated review of the literature. Ther Adv Endocrinol Metab. (2019) 10:2042018819897046. doi: 10.1177/2042018819897046
PubMed Abstract | Crossref Full Text | Google Scholar
13. Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. (2015) 47:127–41. doi: 10.4143/crt.2015.060
PubMed Abstract | Crossref Full Text | Google Scholar
14. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. (2002) 87:489–99. doi: 10.1210/jcem.87.2.8182
PubMed Abstract | Crossref Full Text | Google Scholar
15. Della Guardia L, Shin AC. The role of adipose tissue dysfunction in PM(2.5)-induced vascular pathology. Am J Physiol Heart Circ Physiol. (2022) 322:H971–h2. doi: 10.1152/ajpheart.00156.2022
PubMed Abstract | Crossref Full Text | Google Scholar
16. Tang S, Li D, Ding H, Jiang M, Zhao Y, Yu D, et al. GLIS3 mediated by the Rap1/PI3K/AKT signal pathway facilitates real-ambient PM(2.5) exposure disturbed thyroid hormone homeostasis regulation. Ecotoxicology Environ Saf. (2022) 232:113248. doi: 10.1016/j.ecoenv.2022.113248
Crossref Full Text | Google Scholar
17. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-scR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
PubMed Abstract | Crossref Full Text | Google Scholar
18. Janssen BG, Saenen ND, Roels HA, Madhloum N, Gyselaers W, Lefebvre W, et al. Fetal thyroid function, birth weight, and in utero exposure to fine particle air pollution: A birth cohort study. Environ Health Perspect. (2017) 125:699–705. doi: 10.1289/EHP508
PubMed Abstract | Crossref Full Text | Google Scholar
19. Wang X, Liu C, Zhang M, Han Y, Aase H, Villanger GD, et al. Evaluation of maternal exposure to PM(2.5) and its components on maternal and neonatal thyroid function and birth weight: A cohort study. Thyroid. (2019) 29:1147–57. doi: 10.1089/thy.2018.0780
PubMed Abstract | Crossref Full Text | Google Scholar
20. Zhang X, Huels A, Makuch R, Zhou A, Zheng T, Xia W, et al. Association of exposure to ambient particulate matter with maternal thyroid function in early pregnancy. Environ Res. (2022) 214:113942. doi: 10.1016/j.envres.2022.113942
PubMed Abstract | Crossref Full Text | Google Scholar
21. He Q, Wu M, Shi Q, Tan H, Wei B, Tang N, et al. Association of Ozone Exposures with the risk of thyroid nodules in Hunan Province: a population-based cohort study. Environ Health. (2022) 21:65. doi: 10.1186/s12940-022-00874-8
PubMed Abstract | Crossref Full Text | Google Scholar
22. Ghassabian A, Pierotti L, Basterrechea M, Chatzi L, Estarlich M, Fernández-Somoano A, et al. Association of exposure to ambient air pollution with thyroid function during pregnancy. JAMA Netw Open. (2019) 2:e1912902. doi: 10.1001/jamanetworkopen.2019.12902
PubMed Abstract | Crossref Full Text | Google Scholar
23. Zhao Y, Cao Z, Li H, Su X, Yang Y, Liu C, et al. Air pollution exposure in association with maternal thyroid function during early pregnancy. J Hazard Mater. (2019) 367:188–93. doi: 10.1016/j.jhazmat.2018.12.078
PubMed Abstract | Crossref Full Text | Google Scholar
24. Harari-Kremer R, Calderon-Margalit R, Korevaar TIM, Nevo D, Broday D, Kloog I, et al. Associations between prenatal exposure to air pollution and congenital hypothyroidism. Am J Epidemiol. (2021) 190:2630–8. doi: 10.1093/aje/kwab187
PubMed Abstract | Crossref Full Text | Google Scholar
25. Izic B, Husejnovic MS, Caluk S, Fejzic H, Kundalic BS, Custovic A. Urban air pollution associated with the incidence of autoimmune thyroid diseases. Med Arch. (2022) 76:115–21. doi: 10.5455/medarh.2022.76.115-121
PubMed Abstract | Crossref Full Text | Google Scholar
26. Kim HJ, Kwon H, Yun JM, Cho B, Park JH. Association between exposure to ambient air pollution and thyroid function in Korean adults. J Clin Endocrinol Metab. (2020) 105: e2912–20. doi: 10.1210/clinem/dgaa338
Crossref Full Text | Google Scholar
27. Qiu L, Shen W, Ye C, Wu J, Zheng S, Lou B, et al. Association of exposure to PM(2.5)-bound metals with maternal thyroid function in early pregnancy. Sci Total Environ. (2022) 810:151167. doi: 10.1016/j.scitotenv.2021.151167
PubMed Abstract | Crossref Full Text | Google Scholar
28. Ilias I, Kakoulidis I, Togias S, Stergiotis S, Michou A, Lekkou A, et al. Atmospheric pollution and thyroid function of pregnant women in Athens, Greece: A pilot study. Med Sci (Basel Switzerland). (2020) 8(2):19. doi: 10.3390/medsci8020019
Crossref Full Text | Google Scholar
29. Valdés S, Doulatram-Gamgaram V, Maldonado-Araque C, Lago-Sampedro A, García-Escobar E, García-Serrano S, et al. Ambient air pollution and thyroid function in Spanish adults. A nationwide population-based study (Di@bet.es study). Environ Health. (2022) 21:76. doi: 10.1186/s12940-022-00889-1
PubMed Abstract | Crossref Full Text | Google Scholar
30. Shang L, Huang L, Yang W, Qi C, Yang L, Xin J, et al. Maternal exposure to PM(2.5) may increase the risk of congenital hypothyroidism in the offspring: a national database based study in China. BMC Public Health. (2019) 19:1412. doi: 10.1186/s12889-019-7790-1
PubMed Abstract | Crossref Full Text | Google Scholar
31. Qi C, Shang L, Yang W, Huang L, Yang L, Xin J, et al. Maternal exposure to O(3) and NO(2) may increase the risk of newborn congenital hypothyroidism: a national data-based analysis in China. Environ Sci pollut Res Int. (2021) 28:34621–9. doi: 10.1007/s11356-021-13083-6
PubMed Abstract | Crossref Full Text | Google Scholar
32. Zhang Y, Wang K, Qin W, Jin C, Song Y, Jia P, et al. Six air pollutants associated with increased risk of thyroid nodules: A study of 4.9 Million Chinese Adults. Front Endocrinol (Lausanne). (2021) 12:753607. doi: 10.3389/fendo.2021.753607
PubMed Abstract | Crossref Full Text | Google Scholar
33. Park SJ, Min C, Yoo DM, Choi HG. National cohort and meteorological data based nested case-control study on the association between air pollution exposure and thyroid cancer. Sci Rep. (2021) 11:21562. doi: 10.1038/s41598-021-00882-7
PubMed Abstract | Crossref Full Text | Google Scholar
34. Crepeau P, Zhang Z, Udyavar R, Morris-Wiseman L, Biswal S, Ramanathan M Jr., et al. Socioeconomic disparity in the association between fine particulate matter exposure and papillary thyroid cancer. Environ Health. (2023) 22:20. doi: 10.1186/s12940-023-00972-1
PubMed Abstract | Crossref Full Text | Google Scholar
35. Sun Q, Chen Y, Ye F, Liu J, Liu D, Ao B, et al. Association of hypothyroidism during pregnancy with preconception and early pregnancy exposure to ambient particulate matter. Environ Sci pollut Res Int. (2023) 30:88084–94. doi: 10.1007/s11356-023-28683-7
PubMed Abstract | Crossref Full Text | Google Scholar
36. Karzai S, Zhang Z, Sutton W, Prescott J, Segev DL, McAdams-DeMarco M, et al. Ambient particulate matter air pollution is associated with increased risk of papillary thyroid cancer. Surgery. (2022) 171:212–9. doi: 10.1016/j.surg.2021.05.002
留言 (0)