Macrophages represent an ancient cell type in the phylogeny of metazoans (Wittamer et al., 2011; Buchmann, 2014). They are capable of engulfing foreign or endogenous materials and are found in many organisms with multicellular organization, where they have versatile roles in maintaining homeostasis and immunological defense (Mosser and Edwards, 2008; Wynn et al., 2013; Barreda et al., 2016; Mosser et al., 2021). They were named (“makros” = big, “phagein” = to eat, “big eaters”) after their most characteristic ability and main function, the active uptake of particles bigger than 0.5 µm, by a process called phagocytosis, firstly discovered by Metchnikoff in the beginning of 1880 (Underhill and Goodridge, 2012; Tauber et al., 2017).
1.1 Phagocytosis: a crucial cellular mechanism in macrophages for immune defense and tissue homeostasisPhagocytosis is an important cellular mechanism conserved in all multicellular organisms from protozoans to mammals, including humans (Boulais et al., 2010; Gordon, 2016). Macrophages phagocytose endogenous material, like apoptotic cells (Fadok et al., 1998; Liu et al., 2006; Erwig and Henson, 2008; Kono and Rock, 2008; Suzanne and Steller, 2013; Kourtzelis et al., 2020) and cell debris, or foreign objects, such as pathogens (Chen et al., 2007; Gluschko et al., 2018) and toxic substances, like asbestos or silica particles (Murray and Wynn, 2011b; Nakayama, 2018).
Macrophages play a vital role in engulfing and digesting particles through phagocytosis, a function that aids in identifying different subtypes within the macrophage classification based on how efficiently and extensively they perform this process (Ku et al., 1992; Kirkpatrick et al., 1997; Neve et al., 2014; Kresinsky et al., 2016).
For scanning their extracellular surroundings, macrophages express numerous surface and cytoplasmic receptors that detect irregular signals not typically found in the organism’s physiological milieu (Uribe-Querol and Rosales, 2020). These include scavenger receptors, which bind apoptotic and necrotic cells, opsonized pathogens, and cell debris; and pattern recognition receptors (PRRs) that detect ‘non-self’ or ‘damaged’ signals (Kawai and Akira, 2011), such as Toll-like receptors (TLRs), C-type lectin receptors (CLRs), retinoic acid-inducible gene 1 (RIG1)-like helicase receptors (RLRs), Fc‐receptors, and NOD-like receptors (NLRs) (Elinav et al., 2011; Osorio and Reis e Sousa, 2011; Freeman and Grinstein, 2014; Uribe-Querol and Rosales, 2020).
The interaction of these receptors with ligands recruits actin filaments to internalize the particle, which is then enclosed within a phagosome (Sarantis and Grinstein, 2012; Weiss and Schaible, 2015). Phagosomes fuse with lysosomes to form phago-lysosomes, leading to the degradation of the cargo (Jutras and Desjardins, 2005; Haas, 2007; Levin et al., 2016).
1.2 Efferocytosis: macrophages’ strategy for infection control and tissue maintenanceMacrophages can contain the spread of infection through a clearance of infected and dead host cells (Martin et al., 2012). This behavior is called efferocytosis and serves as a protective mechanism to clear dying or dead cells from tissues during growth and remodeling (Boada-Romero et al., 2020). This process is primarily carried out by tissue macrophages and, during the onset of inflammation, by monocyte-derived macrophages (Watanabe et al., 2019).
In instances of infection, a significant number of cells engaged in host defense undergo cell death. It becomes imperative to remove these cells to minimize tissue damage and inflammation (Labbe and Saleh, 2008). Additionally, since these cells are often harboring intracellular pathogens, which lose their habitats due to cell death, containing the infection becomes critical, rendering efferocytosis an indispensable process during the host’s response to intracellular bacteria (Weiss and Schaible, 2015; Karaji and Sattentau, 2017). In addition, efferocytosis promotes the transition of macrophages to the anti-inflammatory phenotype, leading to a reduction in pro-inflammatory cytokines and an increase in the release of anti-inflammatory mediators, such as interleukin (IL)-10 and transforming growth factor-beta (TGF-β) and pro-resolving molecules (resolvins and protectins) (Dalli and Serhan, 2012; Angsana et al., 2016; Ge et al., 2022), which aid in the reduction of inflammation during infection (Elliott et al., 2017; Chazaud, 2020).
Moreover, efferocytosis plays a crucial role in tissue restructuring during growth and development, as well as in the process of wound healing (Vaught et al., 2015; Mehrotra and Ravichandran, 2022). Throughout the development of complex organisms, cell death is a natural occurrence that aids in growth and tissue restructuring. Thus, eliminating senescent and deceased cells becomes vital for preserving tissue balance and structure, while also facilitating the healing process (Morioka et al., 2019; Gerlach et al., 2021).
1.3 Optimizing macrophage function: the crucial role of the extracellular matrix and fibroblast interactionsEnsuring a favorable environment for macrophages is important for their optimal function as phagocytes (Chen et al., 2023). The extracellular matrix (ECM) serves as a sophisticated system that offers a structural scaffold supporting immune cells. Particularly, collagen, predominantly synthesized by fibroblasts, plays a key role in facilitating various cellular functions, including the differentiation and adhesion of myeloid cells (Khalaji et al., 2017; Makdissi and Mass, 2021). In addition, the interplay of macrophages and fibroblasts is important for growth factor exchange and reducing abnormal proliferation (Zhou et al., 2018). Finally, modification of ECM can trigger a mechanosensitive response in macrophages which is critical for tissue regeneration and fibrosis (Meizlish et al., 2024).
As the origin of macrophages, whether isolated from animal or human samples, differentiated with growth factors from precursor cells, isolated as cancer cell lines exhibiting macrophage-like behavior, or generated as in vitro cell lines, understanding alterations in macrophage behavior solely based on its origin is futile. Moreover, characterizing macrophages by their phagocytic activity needs to be expanded, as macrophage function during processes such as body development, homeostasis, repair, and immune responses against invading pathogens is of paramount importance.
2 Macrophage development: diverse origins and functional adaptationsAs macrophages are pivotal components of the innate immune system, their development is tightly regulated in a series of stages within the hematopoietic system (Wynn et al., 2013). For many years, there was a prevailing belief that macrophages constituted a largely uniform group originating from hematopoietic stem cells (HSCs) in the bone marrow and underwent a defined developmental process known as monopoiesis (Mosser and Edwards, 2008). However, recent findings show that numerous macrophages residing in tissues of adult mice do not depend on HSCs, instead, they originate from yolk-sac progenitors in early embryonic stages (Gomez Perdiguero et al., 2015).
These precursor cells, termed pre-macrophages (pMacs), travel through the bloodstream and settle in various organs. Upon exiting the bloodstream and undergoing differentiation, they develop a unique genetic profile specific to the tissue they inhabit (Mass, 2023; Wang et al., 2023). This maturation process equips macrophages with the ability to adapt to particular tissue contexts (Gordon and Pluddemann, 2017). The resulting macrophages can assume diverse roles, ranging from resident tissue macrophages contributing to homeostasis or to activated macrophages responding dynamically to infections or inflammatory stimuli (Gomez Perdiguero et al., 2015). Tissue-resident macrophages possess the ability to renew themselves and typically do not rely on input from HSCs (Mass et al., 2023).
Furthermore, each organ contains monocyte-derived macrophages (MDMs) with varying lifespans, some persisting long-term while others have shorter lifespans and are continuously replaced by HSCs from the bone marrow. This leads to a complex situation where macrophages originating from fetal and HSC sources coexist in certain tissues (Jenkins and Allen, 2021; Dick et al., 2022).
At the site of inflammation, monocytes are attracted to the tissue and differentiate into macrophages, which cooperate with or replace resident cells for sustaining immunity or promoting resolution of inflammation and tissue regeneration (Italiani and Boraschi, 2015; Muller et al., 2020; Mass et al., 2023).
2.1 Tissue macrophagesTissue macrophages show remarkable functional diversity and plasticity, which are influenced by the surrounding tissue (Sreejit et al., 2020; Mass et al., 2023). Studies employing fate-mapping techniques involving conditional reporter genes have indicated that most resident tissue macrophage populations are established during embryonic development and sustain themselves in adulthood through self-renewal, with limited contribution from bone marrow progenitors or blood monocytes (van Furth and Cohn, 1968; Hume, 2023). Tissue macrophage progenitors (for skin, spleen, pancreas, liver, brain) are derived from yolk sac and fetal liver (Bertrand et al., 2005; Gomez Perdiguero et al., 2015) or have mixed origins from yolk sac and bone marrow (for lung, kidney) (Hoeffel et al., 2012; Epelman et al., 2014).
Some macrophage populations, that are characterized by low F4/80 receptor expression, can be replenished from bone marrow-derived progenitor cells under certain circumstances, such as tissue infection (Ginhoux et al., 2010; Hoeffel et al., 2012; Schulz et al., 2012). In their basal state, resident tissue macrophages exhibit considerable variation in function, requiring different morphologies, transcriptional responses, and locations (Davies and Taylor, 2015). This functional heterogeneity probably results from the dynamic crosstalk between resident tissue macrophages and client cells that they support (de Sousa et al., 2019).
The roles of tissue resident macrophages have been reviewed (Davies et al., 2013a; Epelman et al., 2014; Davies and Taylor, 2015; Varol et al., 2015; Lazarov et al., 2023). Macrophages are divided into subpopulations based on their anatomical location and functional phenotype (Figure 1). A remarkably large reservoir of tissue resident macrophages lies in the intestine (Gordon et al., 2014).
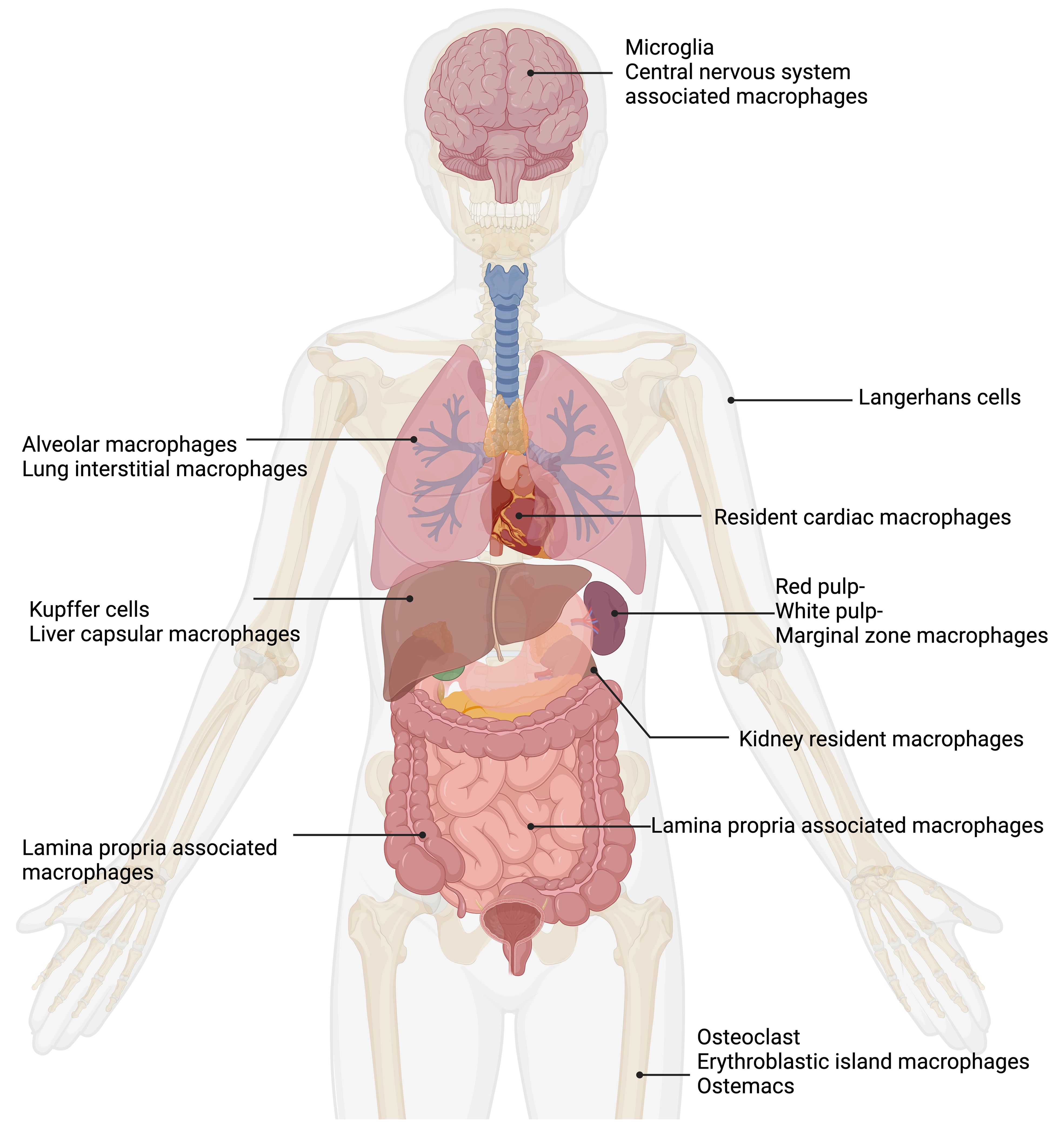
Figure 1. Tissue macrophages exhibit remarkable functional diversity depending on their tissue of residence. In the central nervous system (CNS), microglia (Pallares-Moratalla and Bergers, 2024) and CNS-associated macrophages (Prinz et al., 2021) reside. In the skin, tissue-resident macrophages are Langerhans cells (Merad et al., 2002). The lung contains two types of tissue-resident macrophages: alveolar macrophages (Joshi et al., 2018) and lung interstitial macrophages (Schyns et al., 2018). Tissue-resident macrophages of the spleen are divided into red pulp, white pulp, and marginal zone macrophages (Fujiyama et al., 2019). Resident cardiac macrophages critically contribute to heart tissue remodeling (Wong et al., 2021; Suku et al., 2022). Kidney resident macrophages support organ healing (Cheung et al., 2022). The liver comprises two main types of tissue-resident macrophages: Kupffer cells (Dixon et al., 2013) and liver capsular macrophages (Bleriot and Ginhoux, 2019). The gut contains lamina propria-associated macrophages (Nagashima et al., 1996). In the bone, three types of tissue-resident macrophages are present: osteoclasts (Drissi and Sanjay, 2016), erythroblastic island macrophages (Seu et al., 2017), and osteomacs (Batoon et al., 2017). Figure was adapted from the template “Human Internal Organs” by Eunice Huang. Retrieved from https://app.biorender.com/biorender-templates; Biorender.com.
Interestingly, it is believed that murine intestinal tissue-resident macrophages are regularly replenished by circulating monocytes via a phenomenon referred to as the “monocyte waterfall” (Bsat et al., 2020; Muller et al., 2020). Embryonic precursors colonize the intestinal mucosa and undergo significant proliferation in situ during the neonatal phase. However, they are replaced around weaning by Ly6Chigh monocytes. These monocytes then mature into anti-inflammatory macrophages locally. This process, primarily influenced by the microbiota, needed to persist throughout adulthood to uphold a healthy population of intestinal macrophages (Bain et al., 2014; Desalegn and Pabst, 2019).
Tissue macrophages contribute to the maintenance of healthy tissues by removing dead and dying cells, as well as toxic materials (Murray and Wynn, 2011b). Tissue macrophages also suppress inflammation mediated by inflammatory monocytes, thereby ensuring that tissue homeostasis is restored after infection or injury. Indeed, important homeostatic functions have been assigned to the mononuclear phagocytes in almost every tissue of the body (Murray and Wynn, 2011b; Wynn et al., 2013; Chovatiya and Medzhitov, 2014; Davies and Taylor, 2015; Mosser et al., 2021).
3 Macrophage plasticity and polarization: beyond the M1/M2 paradigmMacrophages display an incredible plasticity, where their functions can be significantly and specifically altered by cytokine signals (Murray and Wynn, 2011b; Wynn et al., 2013). Due to their ability to change and adapt to various exogenous and endogenous factors, macrophages can rapidly alter their functional profile through a process known as polarization (Mosser et al., 2021). Macrophage polarization is the process by which macrophages respond to incoming stimuli from the local microenvironment (Murray, 2017; Arora et al., 2018). After recognition of the phagocytosed material and/or additional factors, such as secreted pro- or anti-inflammatory cytokines from other cells, several signaling pathways are activated. These pathways ultimately lead to the development of a specific macrophage phenotype tailored to meet the functional requirements (Stout et al., 2005).
Currently, there is a partial consensus among researcher in the macrophage field concerning the classification of various macrophage activation phenotypes (Martinez and Gordon, 2014; Ginhoux et al., 2016; Murray, 2017). Initially, macrophage activation relied on a simplistic binary model, such as classic and alternative activation concepts, also known as M1 and M2. It is now widely acknowledged that this model is overly broad and misinterpreted, hindering the understanding of pathogenesis (Martinez and Gordon, 2014; Muraille et al., 2014).
Classically activated macrophages (or M1-like macrophages) release a plethora of pro-inflammatory cytokines, and produce antimicrobial factors, like reactive nitrogen species (RNS) or reactive oxygen species (ROS) (Nathan et al., 1983; Filipe-Santos et al., 2006; Mosser and Edwards, 2008; O’Shea and Murray, 2008; Orecchioni et al., 2019; Dalby et al., 2020; Herb and Schramm, 2021). Alternatively activated macrophages (or M2-like macrophages) have anti-inflammatory functions and regulate wound healing and tissue repair (Stein et al., 1992; Doyle et al., 1994; Loke et al., 2002; Kreider et al., 2007; Wynn et al., 2011; Sica and Mantovani, 2012; Corliss et al., 2016; Wynn and Vannella, 2016; de Groot and Pienta, 2018). There are in addition many subtypes of M2-like macrophages depending on the anti-inflammatory stimuli: M2a, M2b, M2c, M2d (Mantovani et al., 2004).
The currently prevailing perspective on macrophage activation involves adopting a multidimensional model that incorporates the diverse signals encountered by macrophages within their unique microenvironments i.e.: M(IL-4), M(IL-13), M(IFN-γ), M(LPS). This system sidesteps the intricacies of classifications, where different laboratories might define activation differently, enabling comparisons and contrasts of new activation conditions with these fundamental examples (Murray et al., 2014; Ginhoux et al., 2016).
Macrophages demonstrate remarkable phenotypic plasticity by rapidly modulating their functional capability in response to exogenous factors, such as infection or injury (Mosser and Edwards, 2008). These immune effectors are extremely dynamic, initially engaging in pro-inflammatory activities and subsequently transitioning to facilitate the resolution phase. This flexibility allows macrophages to regulate immune responses, from inflammation to restoring tissue balance (Mills, 2012; Gordon and Martinez-Pomares, 2017).
Taken together, there is not “the macrophage” per se, but a broad set of different macrophage types that constantly detect and respond to environmental stimuli as well as tissue physiology and change their functional and metabolic state accordingly (Nathan et al., 1983; Pace et al., 1983; Stein et al., 1992; Doyle et al., 1994; Mills and O’Neill, 2016; O’Neill and Pearce, 2016; Formentini et al., 2017). Having said this, it comes with no surprise that there cannot be a one fit all model for studying macrophage biology but that careful consideration is required upfront.
4 Macrophage colony-stimulating factor and its role in macrophage differentiation and cultivationMacrophage colony-stimulating factor (M-CSF) is a growth factor responsible for the proliferation and differentiation of myeloid progenitors into macrophages in the bodies of humans and mice (Lin et al., 2008; Hamilton and Achuthan, 2013; Roszer, 2018; Hamidzadeh et al., 2020).
In the plasma of mice, approximately 10 ng/mL M-CSF is constantly present, maintained by secretion from various cells in the body, thereby ensuring the recruitment and differentiation of circulating blood monocytes (Hume and MacDonald, 2012; Hamilton and Achuthan, 2013). Under physiological conditions, M-CSF levels are regulated by colony stimulating factor 1 receptor (CSF-1R)-mediated endocytosis, providing feedback control to regulate macrophage production based on the number of mature macrophages (Bartocci et al., 1987). A key characteristic of in vivo macrophages is their dependence on M-CSF receptor signaling for differentiation and survival (Ushach and Zlotnik, 2016).
M-CSF is frequently utilized in vitro to generate bone marrow-derived macrophages (BMDM) from myeloid progenitor cells in the bone marrow of mice and to differentiate macrophages from peripheral blood monocytes (PBMC) for human-related research (Ushach and Zlotnik, 2016). These cells need M-CSF for both, differentiation and survival in vitro (Erbel et al., 2013; Chen et al., 2021b). In contrast, ex vivo macrophages, such as peritoneal macrophages, are isolated as mature macrophages and therefore do not need M-CSF for further differentiation. However, they only survive 2-3 days during in vitro cultivation without M-CSF supplementation (Wang et al., 2013).
In sharp contrast to in vitro-differentiated or ex vivo-cultivated macrophages, the macrophage-like cell lines RAW264.7 and J774 do not need M-CSF or any other constantly applied growth factor for cultivation or survival in vitro (Ralph and Nakoinz, 1975; Raschke et al., 1978).
Islam et al. reported minimal production of M-CSF in RAW264.7 cells, which was significantly increased due to receptor activator of nuclear factor kappa B (NF-κB) ligand (RANKL) treatment (Islam et al., 2008). Another study on osteoclast formation from the macrophage-like cell line RAW264.7 stated that the immortalized cell line produces M-CSF (Collin-Osdoby and Osdoby, 2012). Moreover, M-CSF levels in the supernatant of RAW264.7 cells were detected via ELISA in another study (Yang et al., 2014).
Checking available high-throughput RNA-sequencing data, two publications showed the expression of M-CSF in RAW264.7 cells (Chen et al., 2021a; Liu et al., 2023a). The levels of M-CSF significantly increased due to stimulation with lipopolysaccharide (LPS) (Liu et al., 2023a) and decreased due to the infection with Mycobacterium tuberculosis (Mtb) (Chen et al., 2021a). One publication by Andreu et al. showed that J774 cells express higher levels of the gene for M-CSF compared to BMDM. The level of M-CSF increased over time and in response to infection with Mtb in J774 cells (Andreu et al., 2017). The published results of the RNA-sequencing data are summarized in Table 1.
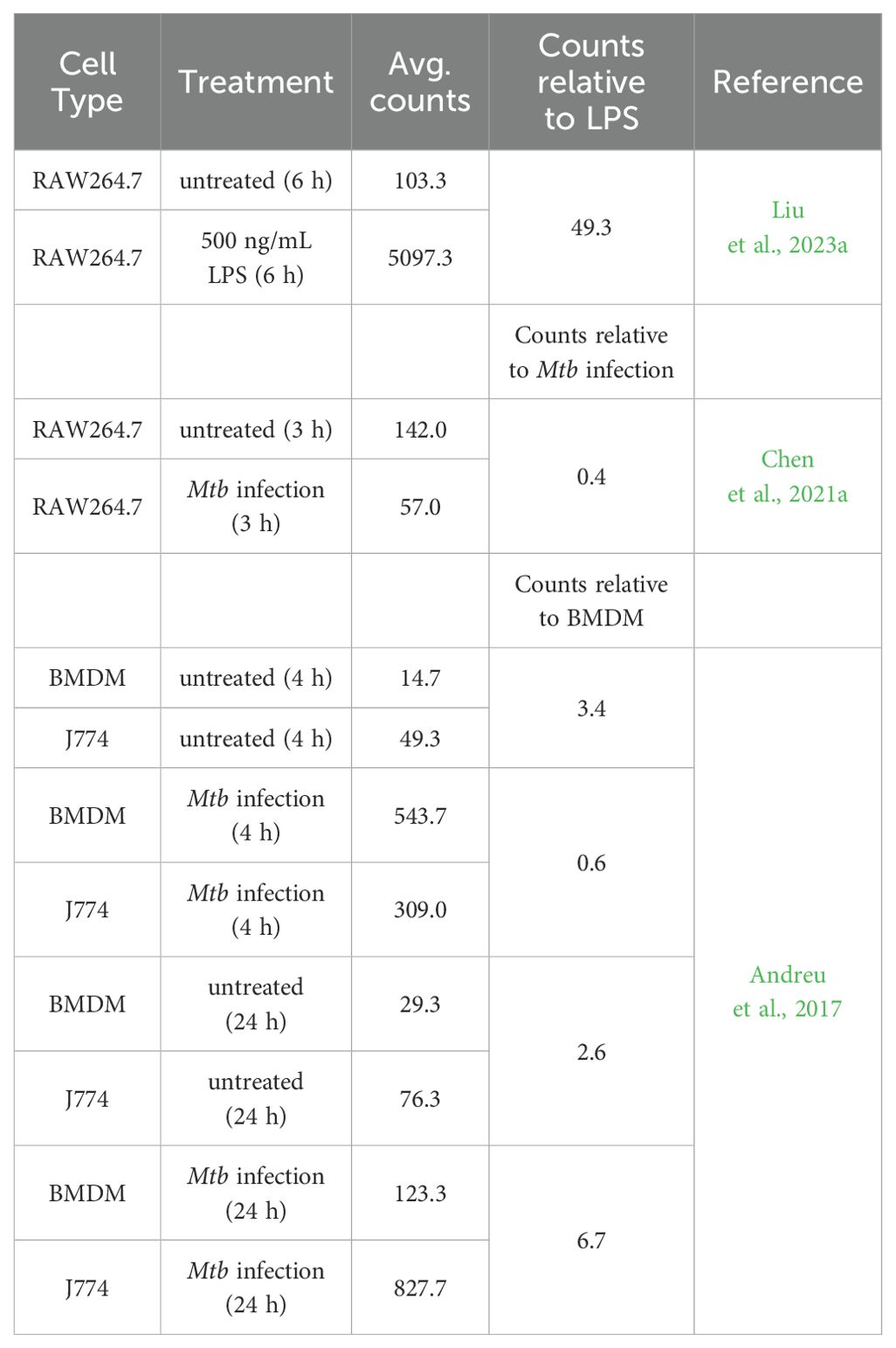
Table 1. M-CSF expression in macrophage-like cell lines and bone marrow-derived macrophages.
Table 1 shows the total number of aligned reads for the M-CSF gene (raw counts; not shown) for each biological replicate was taken from the published data and used to estimate M-CSF expression by averaging (Avg. counts). The relative M-CSF expression was determined by forming a ratio between J774 and BMDM, RAW264.7 untreated and LPS stimulated or with Mtb infected samples. Independent filtering of the DESeq2 R package confirmed that all values have passed the filter threshold for low expressed genes (not shown).
However, only RNA data has been published and no study has reported M-CSF secretion in the macrophage cell line J774. Therefore, it remains unclear whether J774 cells produce their own M-CSF and thus do not require supplementation, or if this cell line simply does not need M-CSF due to their inherent proliferative capabilities as tumor-derived cells.
5 Immortalized and primary mouse macrophage models for in vitro studiesIn general, immortalized macrophages derived from tumor cells, which exhibit continuous division or cells that have been deliberately modified to proliferate indefinitely, enable their cultivation over numerous generations (Taciak et al., 2018; Lucy et al., 2022; Chalak et al., 2024; Xie et al., 2024). Nonetheless, primary macrophages are the mainstay of study of macrophage function in vitro (Murray and Wynn, 2011a).
Each in vitro macrophage model has both positive and negative aspects that need to be carefully considered before choosing a model.
5.1 Immortalized mouse macrophage cell linesThe development of tissue culture techniques and establishment of macrophage cell lines has been indispensable for biological research for several decades (Freshney; Ralph et al., 1980; Sumiya et al., 2015; Taciak et al., 2018). Macrophage-like cell lines are important tools to unravel macrophage function, with RAW264.7 and J774 cells as the two most commonly used immortalized macrophage cell lines available from cell banks (Lam et al., 2009; Taciak et al., 2018). Immortalized macrophage cell lines have several advantages: (I) they are easy to handle and self-replicate, thereby representing an unlimited source of cells with various genetic conditions. (II) The cells can be cultivated in almost limitless quantities, (III) they can be stored frozen for a long time, and (IV) they are easily replaced if lost due to e. g. contamination (Kaur and Dufour, 2012; Voloshin et al., 2023; Weiskirchen et al., 2023).
However, there are a couple of limitations if immortalized macrophage cell lines are used. They are derived either from cancerous single cells/tumors or were generated by viral infection. That is why they are susceptible to genotypic and phenotypic drift during culture and passaging. Consequently, macrophage cell lines can lose macrophage-specific functions and acquire a molecular phenotype that is quite different from that of cells in vivo or primary isolated cells (Burdall et al., 2003; Pan et al., 2009; Frattini et al., 2015; Bosshart and Heinzelmann, 2016).
For example, RAW264.7 macrophages are lacking apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC) which serves as adaptor molecule for various inflammasome receptors (Pelegrin et al., 2008). This hinders RAW264.7 cells to produce and secrete mature IL-1β (while the production of pro-IL-1β is unaffected) after stimulation with nigericin for NLR family pyrin domain containing 3 (NLRP3), double-stranded DNA for absent in myeloma 2 (AIM), and Clostridioides difficile toxin b (TcdB) for pyrin inflammasome activation (Zheng et al., 2020). Transfection of RAW264.7 macrophages with ASC subunit restores the ability of pro-caspase 1 cleavage with subsequent production and secretion of mature IL-β (Bryan et al., 2010). In addition, RAW264.7 macrophages have fluctuating expression of several genes and proteins with increasing passage numbers. More than 50 passages increased the gene expression of hypoxia-inducible factor 1-alpha (Hif1a), integrin subunit alpha L (Itgal), cluster of differentiation 86 (Cd86), and others in RAW264.7 macrophages while the expression of arginase 1 (Arg1), transferrin receptor 2 (Trf2), and interferon regulatory factor 8 (Irf8) changed already after 15 passages (Taciak et al., 2018).
Moreover, transfection with DNA, which is possible in RAW264.7 cells, but induces cell death in primary macrophages, suggests that this cell line is not comparable to primary macrophages (Hornung et al., 2009; Roberts et al., 2009; Liu and Guan, 2018; Paludan et al., 2019). Notably, J774 cells, similar to primary macrophages but unlike RAW264.7 cells, experience cell death following transfection with plasmid DNA, whereas they do not when transfected with mRNA (Van De Parre et al., 2005).
Validating results from macrophage-like cell lines with studies on primary macrophages is important for characterizing macrophage functions. Moreover, conducting animal experiments could further elucidate the in vivo relevance compared with the in vitro results of a single cell type.
5.1.1 RAW264.7The macrophage-like cell line RAW264.7 (Raschke et al., 1978) was isolated from BALB/c mice injected with the Abelson murine leukemia virus (A-MuLV), a replication-impaired virus carrying the v-abl tyrosine kinase oncogene (Hartley et al., 2008). When combined with a suitable type C helper virus, A-MuLV can transform embryo fibroblasts in vitro and trigger swift B cell lymphoid leukemia development in vivo (Twardzik et al., 1982). RAW264.7 cells are therefore an immortalized cancer cell line with macrophage-like capabilities (Taciak et al., 2018).
A-MuLV is a biosafety level 2 (BSL-2) agent (Feroz et al., 2021) posing a “moderate hazard” to lab personnel and the public, requiring specific safety protocols (Ta et al., 2019). Although macrophage cell lines originated from A-MuLV-induced tumors, it’s unclear if the A-MuLV genome contributed to cell transformation (Raschke et al., 1978). A-MuLV preparations often include a helper virus, but tests for replication-competent virus were negative when initially described (Raschke et al., 1978). RAW264.7 cells, as currently provided by the American Type Culture Collection (ATCC), express both ecotropic MuLV, which exhibits the biological characteristics of the Moloney isolate, and polytropic MuLV (Hartley et al., 2008). Polytropic viruses have a broad host range, allowing them to infect multiple species or various types of cell culture lines (Stoye and Coffin, 1987). The ecotropic virus, on the other hand, is characterized by its integration into cellular DNA and cell surface expression of A-MuLV antigens. Ecotropic refers to a virus with a limited host range, capable of infecting only one or a small number of species or cell culture lines. These findings, warrant caution in experimental design and data interpretation when using RAW264.7 cells (Hartley et al., 2008).
The RAW264.7 cell line is widely used to characterize macrophage phagocytosis (Kassas et al., 2012). The biggest advantage of RAW264.7 cells is that they can be transfected using various methods (electroporation, lipofection) and that they are also relatively easy to use for CRISPR-Cas9 lentiviral dropout screens. This facilitates mechanistic genetic unbiased screening studies (Smale, 2010; Cheung et al., 2015; Napier and Monack, 2017; Scinicariello et al., 2023). However, continuous passaging might even increase the accumulation of mutations and separate the cell line further from the definition of a primary macrophage (Stacey et al., 1993; Thompson et al., 1999; Cemma et al., 2015; Cheung et al., 2015; Paludan et al., 2019). Therefore, although this cell line serves as a practical tool for the initial screening of potentially important factors and functions in macrophages, it is important to consider continued passages of this cell lines could lead to gene depletion and compromise the immune functions of macrophages compared with primary macrophages and in vivo studies.
5.1.2 J774Similar to the RAW264.7 cell line (Raschke et al., 1978) the macrophage-like cell line J774 originally was discovered during cancer research (Ralph and Nakoinz, 1975). The J774 cell line is derived through re-cloning from the primary ascites and solid tumor J774.1 cell line. J774.1 cells were firstly described as cells derived from a murine reticulum sarcoma that exhibited macrophage-like morphology, presence of immunoglobulin receptors, phagocytic capacity and antibody mediated lysis of target cells (Ralph and Nakoinz, 1975). Notably, these original studies never claimed to have isolated macrophages but only described cells that display some typical macrophage properties (Ralph and Nakoinz, 1975; 1977). Nevertheless, similar to the RAW264.7 cell line, J774 cells were further characterized in terms of motility, phagocytosis, and antibacterial activity (Portnoy et al., 1988; Kant et al., 2002; Lam et al., 2009; Kresinsky et al., 2016). Notably, J774 cells, unlike RAW264.7 cells but similar to primary macrophages, undergo cell death after transfection with plasmid DNA, but not with mRNA (Van De Parre et al., 2005).
5.2 Primary mouse macrophagesThe cultivation and utilization of primary macrophages offer several advantages over long-term cultured immortalized cell lines (Kaur and Dufour, 2012). They have a shorter lifespan than immortalized cell lines, leading to less accumulation of genetic mutations owing to long-term cultivation (He et al., 2023). Moreover, the origin of the primary macrophages may influence the results (Sreejit et al., 2020). It is of utmost importance to understand which primary macrophages are used in in vitro studies, as they differ due to differences in cultivation protocols and cultivation conditions.
5.2.1 Peritoneal macrophagesPeritoneal macrophages (PMs) are a common in vitro model used to investigate tissue-resident macrophage response (Liu et al., 2018). Analysis of peritoneal macrophages provides insights into general macrophage biology and ex vivo behavior in response to various stimuli and disease models (Cassado Ados et al., 2015).
While working with the PMs, the isolation protocol should be considered. PMs can be obtained as unstimulated resident cells. The cells are isolated through peritoneal lavage (Ray and Dittel, 2010) and represent one of the largest sources of naïve, resident tissue macrophages (Yang et al., 2012b; Cassado Ados et al., 2015; Davies and Taylor, 2015; Davies et al., 2017; Gluschko et al., 2018). However, sorting with a macrophage-specific marker of choice (e.g., CD11b or F4/80) is recommended as the peritoneal lavage contains all cells present in the peritoneal cavity, mainly B cells and to a very small percentage also monocytes and neutrophils (Ray and Dittel, 2010; Gluschko et al., 2018). Relying solely on culture well adherence as the “sorting” step for macrophage purity may not be sufficient to achieve a completely pure peritoneal macrophage culture (Ponte-Sucre et al., 2007; Johnston et al., 2017), which is why authors suggest using the term peritoneal exudate cells for cells that have not been further purified (Johnston et al., 2017).
In this context, however, it must also be mentioned that a further flow cytometric analysis can influence the macrophage biology. Although a higher purity is achieved through purification, this in turn comes at the cost of influencing the biology of the cells. Moreover, extracting naïve peritoneal macrophages from mice has a significant drawback, as only a limited number of cells (1x106 cells per mouse) are obtained, and only around 40% - 50% are macrophages (Ray and Dittel, 2010; Rios et al., 2017). Therefore, a greater number of mice must be sacrificed to obtain an adequate number of cells for experimental procedures (Zhang et al., 2008).
Consequently, to augment macrophage production, a sterile eliciting agent (such as thioglycollate) can be administered into the peritoneal cavity prior to cell collection (Misharin et al., 2012; Layoun et al., 2015). Thioglycollate consists of an infusion from beef, proteose peptone, sodium chloride, dextrose, sodium thioglycolate, bacto agar, methylene blue (Brewer, 1940; Schleicher and Bogdan, 2009). Composition of thioglycollate was shown to determine killing capacity of intracellular pathogens compared to naïve PMs. Reduction of agar and methylene blue in the eliciting agent increased killing capacity (Leijh et al., 1984).
Following thioglycollate administration, the macrophage yield per mouse increased by 10-fold (Layoun et al., 2015). Research has indicated that Brewer’s thioglycollate elicits significant recruitment of macrophages and leads to increased levels of activation compared to naïve PMs (Pavlou et al., 2017). Despite the increased macrophage yield, Brewer’s thioglycollate medium serves as an irritant, triggering an inflammatory response that leads to a swift decrease in overall resident monocyte/macrophage counts. Subsequently, there is an increase of peripheral inflammatory monocytes/macrophages, accompanied by a gradual restoration of resident macrophages (Davies et al., 2011, 2013b). This recruitment may or may not affect gene expression (Layoun et al., 2015). Furthermore, they exhibited an increase in lysosomal enzyme activity (Dy et al., 1978) and in phagocytic uptake (Pavlou et al., 2017).
Two studies have identified multiple leukocyte populations present in both the naïve and thioglycollate-elicited peritoneal cavities (Schleicher et al., 2005; Ghosn et al., 2010). However, maintaining high purity of macrophage culture is crucial for in vitro experiments. While freshly isolated thioglycollate-elicited peritoneal cells reportedly contain a high percentage of macrophages (86–95%) (Vodovotz et al., 1993; Schindler et al., 2001), their purity increases to nearly 99% through adherence (Zhang et al., 2008; Schleicher and Bogdan, 2009). It is worth noting that assessing macrophage percentage solely based on antigens like CD11b or F4/80, previously considered “macrophage specific”, might be misleading. This is because other myeloid cells such as neutrophils, eosinophils, and dendritic cells also express these antigens. Including markers against dendritic cells (CD11c), eosinophils (Siglec-F) and neutrophils (Ly6G) (Zhang et al., 2004; Connelly et al., 2022; Pfeifhofer-Obermair et al., 2022) might be useful because even minor levels of contaminating cells could impact the results of in vitro assays, leading to data misinterpretation (Schleicher et al., 2005; Martinez-Pomares and Gordon, 2008).
A separate study revealed that the proportion of macrophages from the peritoneal cavity was lower than previously reported (Schleicher et al., 2005; Ghosn et al., 2010), with a significant presence of eosinophils (Misharin et al., 2012). Furthermore, there are reports on eosinophil contamination in adherent cultures of peritoneal macrophages (Ruiz-Alcaraz et al., 2020). The contaminating cells affect the functional readouts of standard assays performed on macrophages (Tateyama et al., 2019). Therefore, eosinophils can hinder accurate interpretation of findings from in vitro studies using cultured thioglycollate-elicited peritoneal macrophages (Misharin et al., 2012; Tateyama et al., 2019). Eosinophils exhibit several cell surface markers, such as CD45 and CD11b, which are commonly found on other hematopoietic cells typically present in inflammatory sites, such as alveolar macrophages and neutrophils (Stevens et al., 2007). Siglec-F, a member of the sialic acid binding immunoglobulin-like lectin (Siglec) family, has been identified on murine eosinophils’ surface (Stevens et al., 2007). Siglec-F is predominantly expressed solely on eosinophils in blood and their precursors in the bone marrow of mice (Zhang et al., 2004). In addition to eosinophils, contamination of natural killer cells from peritoneal exudate cells can markedly influence conclusions regarding macrophage regulatory circuits (Misharin et al., 2012). To exclude the possible influence of NK cells in analyses, peritoneal macrophages, for example, can be induced in recombination activating gene 2 (RAG2)-gamma chain knock-out mice (Schleicher et al., 2005). To minimize the influence of eosinophils, double GATA-site (ΔdblGATA) knock-out mice could be used, for example, in which the development of eosinophils is severely impaired (Yu et al., 2002).
Another important point to consider is the changes in the metabolic activity of macrophages due to differences in the isolation protocols. Resident peritoneal macrophages in their naïve state exhibited lower metabolic activity than the elicited macrophages (Pavlou et al., 2017). Elicited macrophages display elevated levels of glycolysis and oxidative phosphorylation, potentially correlated with their enhanced phagocytic capacity and heightened maturation and activation levels (Pavlou et al., 2017). Gaining deeper insights into the molecular connections between metabolic pathways and cellular function is essential for devising strategies to regulate macrophage function through metabolic reprogramming.
5.2.2 Bone marrow-derived macrophagesCompared to PM, BMDM are not a model for tissue-resident macrophages, as they are differentiated in vitro (Fejer et al., 2015). They are generated by flushing myeloid progenitor cells from the bone marrow of the hind legs of mice and stimulating with either recombinant M-CSF or L-929-cell-conditioned media (L929, source of M-CSF) to gain differentiated macrophages. M-CSF induces proliferation and differentiation of the progenitor cells into BMDM via M-CSF receptor-mediated signaling (Becker et al., 1987; Brugger et al., 1991; Klappacher et al., 2002; Stanley and Chitu, 2014; Ushach and Zlotnik, 2016).
The main advantage of using BMDM as a macrophage model is the amount of cells that are generated after isolation and differentiation (4-6 x 106 cells per mouse) (Brigo et al., 2022). Differences in culture conditions (medium type, amount of M-CSF, and L929 usage) led to high variability in BMDM generation. However, clear description of the methodology is necessary for a high reproducibility of the results (Murray et al., 2014). In numerous research facilities, the L929 supernatant is favored over the use of recombinant M-CSF due to its cost-effectiveness and ability to produce significantly greater quantities of differentiated macrophages, as fewer animals need to be euthanized (Boltz-Nitulescu et al., 1987; Heap et al., 2021).
Before the availability of recombinant M-CSF, the traditional method, which remains prevalent in many laboratories (Yang et al., 2012b; Bringmann et al., 2013; Johnston et al., 2017; Koster et al., 2017; Nazir et al., 2017; Abuaita et al., 2018; Javmen et al., 2018; McTiernan et al., 2020), involved differentiating BMDM using the supernatant of the L929 immortalized fibroblast cell line, in which M-CSF and a variety of other factors are provided to the developing BMDM precursor (Tomida et al., 1984; Burgess et al., 1985; Portnoy et al., 1988; Pang et al., 2001). While certain strains of mouse L929 cells have the capability to generate significant levels of M-CSF (Rice et al., 2020), the composition of L929 supernatant can exhibit variability between batches, potentially leading to inconsistencies in experimental results (Warren and Vogel, 1985).
Despite exposure to other substances in the L929 supernatant (Trouplin et al., 2013), L929-derived macrophages exhibit similar phagocytic and pathogen eliminating abilities to M-CSF-derived macrophages (Rice et al., 2020). However, L929-derived macrophages showed distinct cytokine secretion patterns, with lower inflammatory cytokine levels and higher IL-10 secretion (de Brito Monteiro et al., 2020; Heap et al., 2021). They also display heightened metabolic activity and increased accumulation of dysfunctional mitochondria compared with M-CSF-derived macrophages. Although differences in metabolism and cytokine secretion exist, both types of macrophages demonstrate comparable microbicidal effectiveness (de Brito Monteiro et al., 2020).
Using mass spectrometry, the examination of L929 supernatant revealed 2,193 proteins, including notable amounts of M-CSF and other immune-regulating proteins like migration inhibitory factor (MIF), osteopontin, and chemokines such as CC-chemokine ligand (CCL) 2 and CCL 7. When differentiated with L929, macrophages showed a more robust anti-inflammatory M2-like phenotype compared to differentiation with M-CSF. Moreover, macrophages grown in L929 supernatant exhibited reduced responses to oxidative stress, as well as decreased activity in cell division and mitotic machinery (Heap et al., 2021).
In summary, these findings indicate that the choice of differentiation agent influences BMDM phenotypes and proteomes, leading to variations in biological functions. Consequently, it is crucial to recognize the biological implications of different BMDM differentiation methods and the resulting in vitro outcomes. Conversely, employing defined concentrations of M-CSF may alleviate experimental variability and improve the standardization of laboratory methodologies (Murray et al., 2014).
5.3 Cell line generation from mouse bone marrow-derived macrophagesSeveral methods for immortalizing mouse BMDM for generation of macrophage cell lines have been described; however, the low rates of transduction and transfection observed in macrophages pose challenges to these procedures (Zhang et al., 2009; Alamuru-Yellapragada et al., 2017; Warwick and Usachev, 2017; Poltavets et al., 2020). Some potential approaches for immortalizing primary BMDM are outlined in the next section of this review. However, one must carefully consider the advantages and disadvantages of these methods.
Genetic modulation of primary macrophages can lead to changes in their phenotype compared to the initial cell population (Xie et al., 2024). Nevertheless, immortalizing BMDM can lead to a reduction in animal usage (Spera et al., 2021). BMDM from genetically modified mice are frequently employed to analyze mechanisms of the immune system and having a reservoir of these immortalized BMDM readily available facilities the conduction of in vitro studies and reduces reliance on live animals (De Nardo et al., 2018; Spera et al., 2021).
5.3.1 Overexpressing oncogenes or a transcription factor in bone marrow-derived macrophagesA method for immortalizing macrophage populations from particular mouse strains by utilizing the Cre-J2 retroviral infection technique (De Nardo et al., 2018). This method of immortalization involves infecting cells with the J2 recombinant retrovirus, which is derived from a replication-defective 3611-Moloney sarcoma virus (MSV) and contains the v-raf and v-myc oncogenes (Spera et al., 2021).
The J2 virus itself lacks the essential viral packaging proteins (gag-pol, env), rendering it replication-deficient. Therefore, a viral packaging helper cell line called Psi-Cre-J2 (derived from NIH 3T3 fibroblasts) is utilized to generate recombinant Cre-J2 retrovirus. The Cre-J2 retroviral infection technique for immortalization has proven effective on various murine macrophage populations, such as those derived from bone marrow, fetal liver, spleen, and microglia (Blasi et al., 1985; Roberson and Walker, 1988; Cox et al., 1989; Blasi et al., 1990).
A commercially available immortalized murine macrophage cell line, designated as BM A3.1A7, originates from adherent macrophages extracted from the bone marrow of adult female C57BL/6 mice. These cells have been rendered immortal by the introduction of elevated levels of raf and myc oncogenes (Kovacsovics-Bankowski and Rock, 1994).
Currently only one study analyzed the immortalized murine macrophage cell line, BM A3.1A7 for their macrophage capabilities. They demonstrated that BM A3.1A7 polarize into either M1-like macrophages, identified by their secretion of inflammatory cytokines such as IL-1β, IL-6, IL-12, and tumor necrosis factor (TNF), along with increased expression of inducible nitric oxide (NO) synthase (iNOS), or M2-like macrophages characterized by their typical elevated ARG1 activity (Banete et al., 2015). Further studies and comparisons with other primary macrophages or other relevant models are needed to better assess the value of the model.
Moreover, hematopoietic precursors are immortalized by retroviral transduction with an estrogen-inducible form of the transcription factor homeobox B8 (Hoxb8) (Wang et al., 2006). Hoxb8 promotes self-renewal and halts differentiation (Leithner et al., 2018). In the presence of elevated levels of the hormone β-estradiol that exceed the physiological range, Hoxb8 is transcriptionally active. By removing β-estradiol, Hoxb8 is inactivated, leading to differentiation of the immortalized progenitor cells depending on the applied cytokine cocktail for the desired myeloid subset (Wang et al., 2006; Odegaard et al., 2007). Therefore, these cells can be grown in cell culture for weeks, providing them with sufficient time to be genetically modified, while still remaining capable of maturing into DCs, macrophages, or granulocytes (Redecke et al., 2013). Macrophages derived from the Hoxb8 lineages exhibited comparable phenotypic and functional attributes and similarly elevated the expression of activation-related genes when stimulated with LPS, as observed in primary macrophages (Wang et al., 2006; Odegaard et al., 2007).
Advantages of these cells are that they can be readily produced from the bone marrow or fetal liver of any mouse strain (Hammerschmidt et al., 2018). Additionally, Hoxb8 cells can be efficiently modified using viral transduction and CRISPR-mediated genome editing (Di Ceglie et al., 2017; Hammerschmidt et al., 2018). They are a convenient tool for protein overexpression or knockdown experiments (Bromberger et al., 2022). One disadvantage of these cells is the heterogeneity, as the initial bone marrow population can be diverse, potentially impacting the uniformity of the resulting cells (Yu and Scadden, 2016).
5.3.2 Cas9+-immortalized macrophagesGenetic manipulation of macrophages is in general cumbersome (Zhang et al., 2009). There are for instance protocols for RNAi delivery into macrophages (Siegert et al., 2014) or retroviral transduction of bone marrow progenitors (De Veerman et al., 1999). The discovery of the RNA-guided endonuclease Cas9 and its use as gene scissors (‘genome editing’) has opened new technical possibilities to search genome-wide with CRISPR (clustered regularly interspaced short palindromic repeats)-Cas9 for regulators of biological processes starting from the phenotype with so-called ‘forward genetic screens’ (Joung et al., 2017). Introducing Cas9 protein into cells poses a difficulty within the CRISPR-Cas9 process (Liang et al., 2015). To overcome this challenge Cas9 knock-in mice facilitate the production of various knock-outs in immortalised cells, which was already shown in immortalised DCs of Cas9 expressing mice (Parnas et al., 2015).
As previously mentioned, hematopoietic precursors immortalized by retroviral transduction with Hoxb8 can be cultured for weeks, allowing genetic modifications and maturation into DCs, macrophages, or granulocytes (Wang et al., 2006; Redecke et al., 2013), as Hoxb8 expression facilitates self-renewal and halts differentiation (Leithner et al., 2018). To overcome the inducibility of the Hoxb8 overexpression via removal of β-estradiol, bone marrow of the Cas9-transgenic mice was immortalized by lentiviral transduction, introducing a doxycycline-regulated version of the transcription factor Hoxb8. These cells can be cultured consistently for weeks when doxycycline and puromycin are added. Moreover, these cells facilitate CRISPR/Cas9 technology, as the Cas9 gene is expressed in the cells (Hammerschmidt et al., 2018).
A recent study focused on the notable differences and constraints observed between primary macrophages and immortalized cell lines by creating a new immortalized macrophage cell lines using the CRISP/Cas9 technique (Roberts et al., 2019). Roberts and colleagues introduced ER-HoxB8 by retroviral transduction into hematopoietic stem cells from Cas9 expressing mice, and performed a comprehensive comparison between Cas9+-immortalized macrophages (CIMs) and BMDM, the primary macrophage type. Through a series of meticulously designed experiments, they examined various macrophage functions, including iNOS expression, NO generation, bacterial phagocytosis, and antibacterial activity. CIMs were similar to BMDM, the only significant difference was that killing of Listeria monocytogenes was enhanced, which they hypothesize is a result of improved early elimination through the phago-lysosomal pathway. The growth rate of M. tuberculosis was similar in CIMs to that observed in BMDM. CIMs and BMMs showed increased expression of iNOS and produced comparable levels of NO when stimulated with the combination of LPS and interferon-γ (Roberts et al., 2019).
6 Immortalized versus primary mouse macrophages: advantages and disadvantages of in vitro modelsAs previously mentioned, numerous studies concentrate exclusively on the primary role of macrophages, which is phagocytosis. Nevertheless, macrophages are not merely scavengers; they respond to diverse environmental cues by generating complex reactions, such as generating reactive oxygen and nitrogen species (ROS and RNS) as well as pro- or anti-inflammatory cytokines and chemokines, which depend on their polarization (Arango Duque and Descoteaux, 2014; Mosser et al., 2021).
It is becoming evident that macrophage-like cell lines differ from primary macrophages, while primary macrophages vary based on isolation and cultivation methods. The subsequent section of this review will illustrate these distinctions.
6.1 Differences between naïve PM and BMDMPrimary mouse macrophages, including BMDM and PM, are commonly employed in vitro to investigate various mechanisms such as infection control (Schatz et al., 2016; Brigo et al., 2021), phagocytosis (Herb et al., 2018; Gluschko et al., 2022), and cytokine production (Sasse et al., 2022; Brigo et al., 2023). Interestingly, these two macrophage types exhibit distinct functional differences.
Macrophages in various anatomical locations may exhibit diverse degrees of heterogeneity (Geissmann et al., 2010; Gautier et al., 2012; Gordon et al., 2014; Komohara et al., 2014; Hume et al., 2023). Employing flow cytometry, the observations revealed that BMDM formed tight clusters in both forward scatter and side scatter data, contrasting with naïve PMs, which appeared dispersed among at least two distinct populations (Zajd et al., 2020). These findings suggest that PMs display notable heterogeneity. Conversely, BMDM exhibited a higher degree of uniformity, likely attributable to their induction by M-CSF (Zhao et al., 2017). Moreover, PMs exhibit larger cell sizes compared to BMDM due to containing more cytoplasm and increased lysosomal content (Wang et al., 2013). In addition, proliferation is enhanced in BMDM, cell number increased from day 4 and continued to rise until day 14, reaching a 60-fold increase over baseline compared to PMs, which showed no proliferation during the 14-day culture period (Wang et al., 2013).
BMDM, unlike PM, fail to activate an antimicrobial phagocytosis variant known as LC3-associated Phagocytosis (LAP) (Heckmann and Green, 2019; Herb et al., 2020). Not only do BMDM fail to induce LAP during bacterial infection, but they also exhibit significantly reduced capacities in phagosomal ROS production and intracellular bacteria killing (Gluschko et al., 2022). ROS pr
留言 (0)