Pectus excavatum (PE), characterized by varying degrees of depression in the ribs and sternum on the anterior surface of the chest wall, is the most common major congenital anomaly, occurring in approximately one out of every 300 live births (1, 2). The PE diagnosis affects mostly male patients with an approximate 3–5:1 male to female ratio (1, 3).Children with moderate or severe PE may experience disruptions in cardiac and respiratory functions as well as reduced capacity for vigorous cardiovascular activities. Additionally, psychosocial stress related to physical appearance often arises during adolescence (4). In cases where the degree of pectus deformity does not immediately necessitate surgery, nonsurgical treatment options are required for patients (5). Furthermore, some patients may be hesitant to undergo surgery due to postoperative pain and potential risks associated with imperfect outcomes (6). The VB serves as an alternative treatment option for children with PE who do not require surgical intervention or refuse it (7).
The effectiveness and success of VB therapy in children are associated with the duration of VB usage (6). Several studies have investigated treatment compliance and factors influencing the effectiveness of VB therapy in children with PE (8–10). These studies have used clinical outcome measurements to evaluate the effect of VB treatment, reporting successful outcomes ranging from 13% to 43%. Achieving successful results in treating children with PE using VB requires active involvement from parents or primary caregivers who must fully adhere to the treatment technique. However, there is currently a gap in the literature regarding parental experiences. Therefore, it is recommended to employ a combination of subjective and objective measures for assessing therapy success (5, 11).
The objective of this study was to investigate the experiences of parents with children diagnosed with PE who underwent VB treatment, and to identify factors influencing the effectiveness of VB treatment. This manuscript adheres to the guidelines outlined in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement, ensuring comprehensive reporting of observational studies (12).
2 Materials and methods 2.1 Study designThe effectiveness of nonsurgical treatment for PE with the VB was evaluated through a cross-sectional study using questionnaires conducted at Hunan Children's Hospital in Hunan, China. This study received approval from the Institutional Ethics Committee of Hunan Children's Hospital (No. HCHLL-2024-83), and informed consent was obtained from all participants and their parents.
2.2 Setting 2.2.1 Indications for VB therapyThe indications for VB therapy included moderate to severe PE as determined by inspection or CT scan, the Haller index (13, 14) is the ratio of the transverse (coronal) internal thoracic measurement divided by the AP (sagittal) measurement at its deepest point. Mild is categorized as <3.2. Moderate includes Haller indices of 3.2 ± 3.5. Severe encompasses all number >3.5, in cases where the patient was deemed too young for surgery, had experienced recurrence after surgery, or expressed aversion to surgical intervention. Contraindications to VB therapy were limited to coagulopathies. All potential candidates for VB treatment underwent evaluation and approval or disapproval by a single surgeon.
2.2.2 Treatment protocolDuring the initial treatment with VB (Guangzhou Yikang Medical Technology Co., Ltd.) (Figure 1), patients and their parents received comprehensive training from the surgeon at our clinic. The choice of VB sizes is contingent upon the degree and scope of concavity in patients with PE. Our treatment initiation protocol involves a gradual increase in daily usage time, starting with two 10–15 min sessions per day, which progressively increases by 5–10 min per week until reaching two 60-min sessions daily. Subsequently, usage time is flexible and patients are encouraged to extend it as much as possible. The recommended vacuum pressure ranges are determined based on patient age (Table 1), while the negative pressure of VB is established according to individual tolerance levels without causing discomfort. The entire course of treatment lasts for one year and may be extended if necessary.
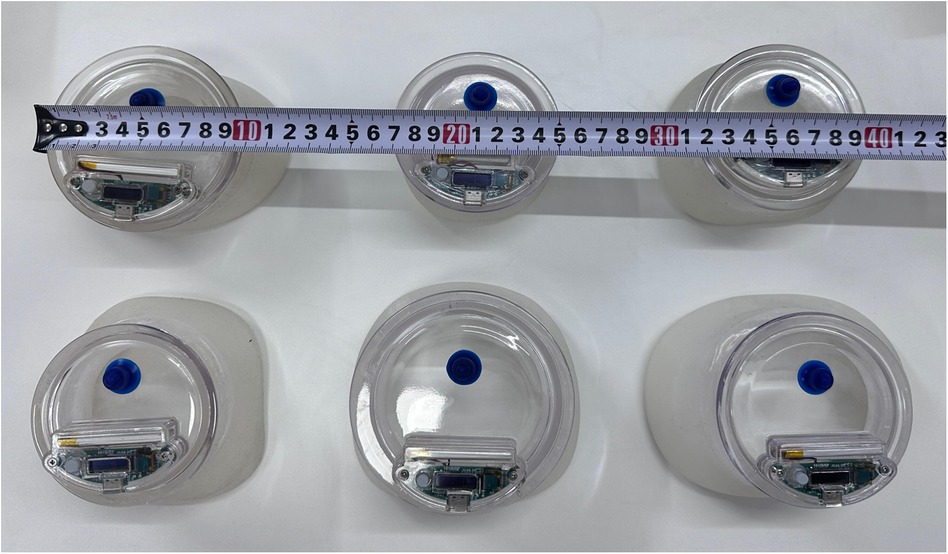
Figure 1. Different sizes of the VBs.
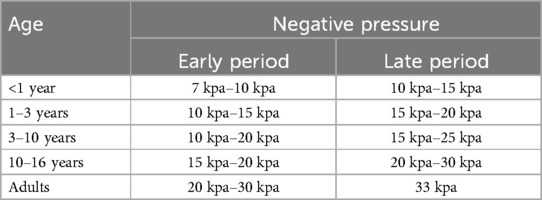
Table 1. Recommended negative pressures of different age groups.
2.2.3 Design of follow-up questionnaireTwo experts in PE independently compiled questions from previously validated questionnaires used in other deformative diseases [such as pectus carinatum (PC) and scoliosis] and developed additional items to quantitatively assess compliance with VB therapy and evaluate potential influencing factors in patients with PE. The questionnaire comprises 20 items, which measure participants' characteristics, daily VB usage (average duration in minutes and frequency), treatment duration (accumulated months of use), vacuum pressures (kPa), complications, and the effectiveness of VB therapy.
2.3 Participants and study sizeThe questionnaires were distributed to the parents between September and October 2021. Parents whose children received VB treatment from January 2018 to December 2019 were included in this study. Inclusion criteria comprised of: VB treatment for PE, age ≥1 year, treatment duration ≥6 months, and consent provided by the patient's father, mother, or guardian for accessing their data for research purposes. Exclusion criteria consisted of: absence of chest sinking determination at the start of treatment, osteogenic syndrome presence, and withdrawal from clinical follow-up.
2.4 VariablesThe parents were invited to participate in the study and complete online questionnaires 6 months to one year after the commencement of the research. The caregivers provided reports on daily VB usage (average duration in minutes and frequency per day), treatment duration (accumulated months of use), vacuum pressures (kPa), among other factors. Apart from subjective assessment, we also used the CT scan and 3D scanner to record treatment outcomes. After approval, every patient underwent initial CT scan upon ordering of a VB and later follow-ups every 3 months to half a year during use of the device. The 3D scanner was used to acquire body surface data on the standing position, which was used to record treatment outcomes, the collected body surface data were used for deformity documentation in order to compare with follow-up scans during the course of treatment.
2.5 Statistical methodsThe primary variables were analyzed using descriptive statistics and frequencies. Continuous data with a normal distribution were presented as mean ± standard deviation, while non-normally distributed data were reported as median and interquartile range. Categorical data were expressed in frequencies and percentages. Univariate analysis and multivariate logistic regression were conducted to analyze the therapeutic effect and identify factors associated with its effectiveness. Statistical analysis was performed using SPSS 27.0 (SPSS Inc., Chicago, IL, USA, 2020) software for Windows.
3 Results 3.1 ParticipantsA total of 77 questionnaires were distributed, and 65 valid responses were obtained (Figure 2). In this study, a cohort of 65 children (50 males, 15 females) was enrolled. The mean age was (6.37 ± 3.28) years, the average height was (120.18 ± 20.20) cm, and the mean weight was (23.80 ± 10.22) kg (Table 2).
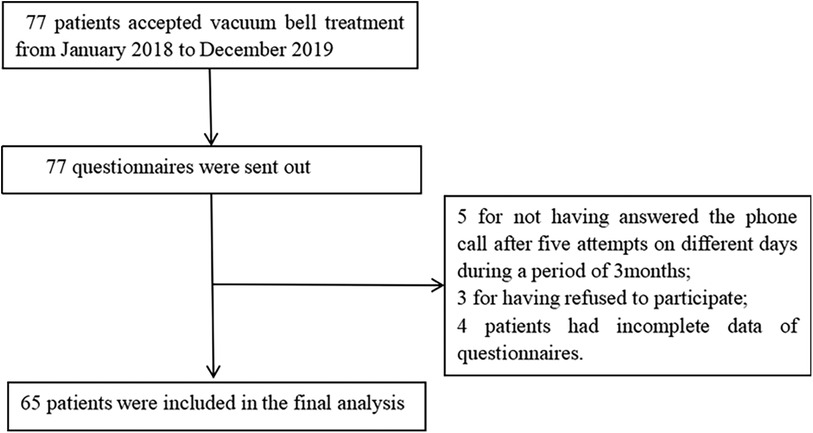
Figure 2. Study flow chart.
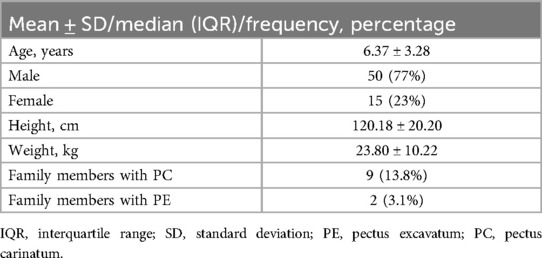
Table 2. Demographics of 65 patients.
3.2 Descriptive dataThe mean duration of VB usage was 23.20 ± 9.86 months. Regarding complications, petechiae was reported by 29 patients (44.6%) during VB use, while blistering was reported by 14 patients (21.5%), along with some cases of pain and/or discomfort. All symptoms resolved after a short pause in treatment, without any permanent sequelae from side effects. In terms of effectiveness, based on subjective evaluations by caregivers, the majority considered the therapeutic effect to be moderate (27 patients; 41.5%) or good (30 patients; 46.2%), while 8 patients (12.3%) rated it as excellent, resulting in an overall effective rate of 100% for VB treatment. Among the participants, 52 patients (80%) continued using the VB unless instructed otherwise to pause or terminate its use, whereas 39 patients (60%) experienced at least one pause lasting more than two weeks (Table 3).
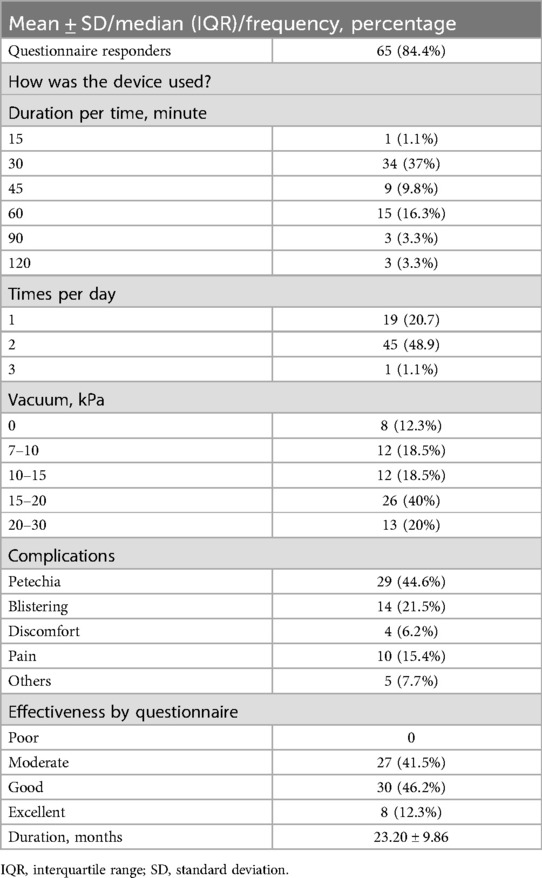
Table 3. Follow-up and questionnaire results.
3.3 Outcome data 3.3.1 The results of variance analysisThe analysis of variance revealed no statistically significant differences (P > 0.05) in age, height, weight, duration of usage (months) and the effectiveness of VB therapy. The results of univariate analysis showed that there were no significant differences (P > 0.05) between the effectiveness of VB therapy and the frequency of use per day, treatment duration for each use and the vacuum pressure. However, significant differences were observed (P < 0.05) in the occurrence of petechiae during VB treatment and the need to return to the manufacturer for repair (Table 4).
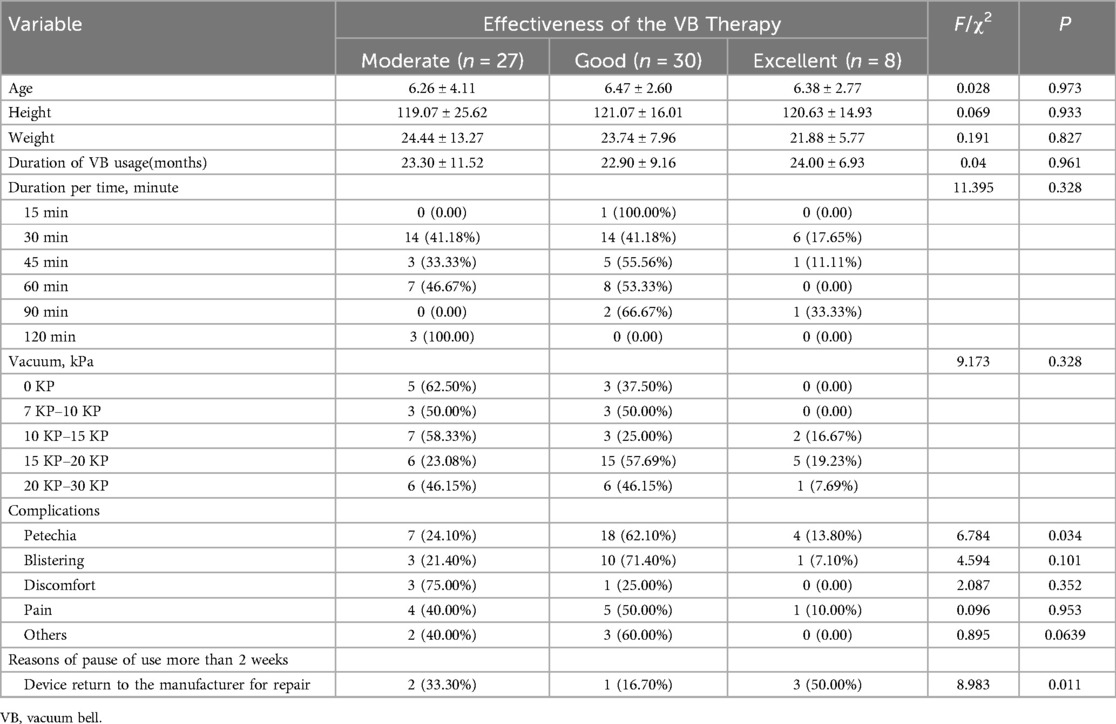
Table 4. Univariate analysis of the factors for effectiveness of the VB therapy.
3.3.2 The results of multivariate logistic regressionThe effect of VB therapy after treatment was assessed as the dependent variable, while the occurrence of petechiae during the VB treatment process and equipment return for repair were considered as independent variables in a multivariate linear regression analysis. Based on the F-test results (F = 3.717, p = 0.030 < 0.05), it can be concluded that model construction is statistically significant. The R-squared value was 0.107, indicating that the combined influence of petechiae occurrence during VB treatment and equipment return for repair explains 10.7% of the variation in post-VB treatment effect evaluation (Table 5). Analysis from the regression coefficient reveals that petechiae occurrence during VB treatment significantly affects post-treatment effect evaluation (P < 0.05). Therefore, assessment of VB therapy effectiveness primarily relies on monitoring petechiae occurrence during treatment (Table 6).
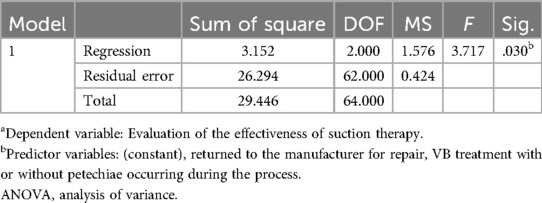
Table 5. ANOVAa.
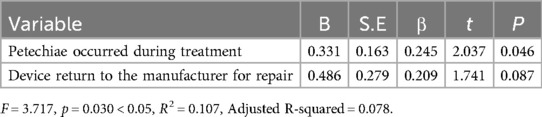
Table 6. Multivariate logistic analysis results on influencing factors of the VB therapy.
4 Discussion 4.1 Key findingsThe objective of our study was to investigate the therapeutic efficacy and influencing factors of VB therapy in pediatric patients with PE. Our findings demonstrate a 100% effective rate for VB therapy, with an average duration of vacuum bell usage being (23.20 ± 9.86) months. Patients who experienced reduced incidence of petechiae complications were more likely to achieve favorable treatment outcomes, suggesting that proactive complication prevention and intensive management may be necessary in these individuals.
The non-surgical conservative treatment of PE with VB therapy was initially described by Schier et al. in 2005 (6). Since then, it has gained increasing popularity as a viable alternative to surgical intervention for carefully selected patients (15–17). However, the efficacy of VB therapy heavily relies on patient compliance and motivation. Therefore, it is crucial to conduct rigorous follow-up assessments to evaluate the long-term stability of correction achieved through VB therapy (17). Obermeyer (10) reported that nearly one third of their patients were either lost to follow-up or exhibited poor compliance.
In our study, all patients expressed satisfaction with the use of the VB, although subjective assessment of improvement in PE varied among individuals. The subjective evaluation questionnaire revealed a 100% effective rate for VB therapy, with 41.5% showing moderate correction, 46.2% showing good correction, and 12.3% showing excellent correction. These findings are consistent with Haecker's (18) results from a study involving 133 patients, where sternum flattening was observed in 79%, despite a treatment discontinuation rate of 10%. However, the effectiveness rate in our study was significantly higher than the reported range of successful corrections in literature (13.5%–43.6%) (9, 10, 18). This discrepancy may be attributed to variations in evaluation tools used; some studies employed absolute depression depth measurements that did not account for patient body size or necessitated specialized measurement tools to quantify correctness, while others utilized Haller Index, Correction Index or modified percentage depth (19). In our questionnaire-based assessment conducted by caregivers who monitored long-term home treatments without hospital visits or during review intervals, treatment effectiveness was subjectively evaluated based on improvements in the degree of depression, though this approach is subjective it does provide insights into treatment progress and efficacy.
Contrary to previous literature, this study found no statistically significant association between age at treatment and outcomes. Obermeyer et al. reported better results in children under 11 years of age, possibly due to inclusion of both adult and pediatric patients (10). Therefore, the variation in complete correction rates among different age groups may be attributed to differences in chest wall flexibility. Our study included children with a mean age of (6.37 ± 3.28) years, all younger than 10 years. As children grow older, chest wall becomes stiffer and less flexible, which can influence treatment outcomes. All patients with available follow-up data demonstrated improved chest appearance as objectively and subjectively confirmed. Thus, the impact of age on the treatment outcome was limited in our study.
The effects of height, weight, duration of usage (months), duration per time and negative pressure on the treatment outcome were not statistically significant. This finding is consistent with Dengke Luo (8), who reported that daily usage time, initial negative pressure, sex, comorbidities, and family history also did not show statistical significance in relation to the treatment outcome.
The identification of influencing factors on treatment outcomes in this study has direct clinical applicability. Side effects, such as petechiae, blistering, discomfort or pain were reported at a similar rate to that found in literature. The occurrence of side effects may be attributed to excessive negative pressure regulation; however, all observed side effects were reversible and resolved by reducing suction pressure or pausing treatment for no more than two weeks (20). The utilization of VB was discontinued due to the occurrence of complications, which had the potential to impact treatment efficacy. This highlights the importance of educating patients and their families on managing complications throughout the entire treatment process, such as providing one-to-one guidance via Wechat, which proves to be an effective approach.
Another factor influencing therapeutic efficacy is equipment maintenance, which involves returning it to the manufacturer. As VB therapy is relatively new in the hospital where the study was conducted, there may be instances of equipment damage due to factors such as device malfunctions or improper use during treatment. Repairing the equipment requires time for it to be sent back and forth from the manufacturer, potentially affecting treatment effectiveness. In future treatments, we will establish communication with the manufacturer to ensure strict control over VB quality, thereby reducing product quality issues and minimizing treatment interruptions caused by objective reasons.
4.2 LimitationsThe limitations of this study include its retrospective design and the presence of patients lost to follow-up or with incomplete data. Additionally, it is important to note that the survey subjects were excluded from a single tertiary pediatric hospital, resulting in a relatively small sample size. Moreover, the self-reported questionnaire data on daily usage and pressure may be susceptible to memory-related or other errors. Furthermore, due to the young age of our patients and their exposure to winter during treatment, several pauses occurred for weeks or even months. Although parents were required to report these pauses, there is a possibility of errors when calculating the total duration. Furthermore, while treatment outcomes were assessed based on caregivers' measurements, aspects such as quality of life and changes in self-belief were not evaluated. Future studies should incorporate patient evaluations of outcomes and measure chest depression.
5 ConclusionThe subjective evaluations reported by parents indicated the clear effectiveness of VB, although with varying degrees. It is imperative to enhance the product and mitigate complications in order to optimize compliance and further improve its efficacy. Additionally, conducting subsequent follow-up studies is essential for evaluating the effectiveness of this therapeutic tool.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Review board of Hunan Children's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributionsLZ: Writing – original draft, Writing – review & editing. FD: Writing – review & editing, Data curation. YT: Writing – review & editing, Validation. JL: Writing – review & editing, Resources. GY: Resources, Writing – review & editing. YL: Writing – review & editing, Validation. JL: Writing – review & editing, Supervision. XD: Writing – review & editing, Conceptualization, Writing – original draft.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Health Commission of Hunan Province (C202304028427) and Hunan Provincial Science and Technology Department (2021SK50521).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AbbreviationsVB, vacuum bell; PE, pectus excavatum; PC, pectus carinatum.
References1. Kelly RE Jr. Pectus excavatum: historical background, clinical picture, preoperative evaluation and criteria for operation. Semin Pediatr Surg. (2008) 17(3):181–93. doi: 10.1053/j.sempedsurg.2008.03.002
PubMed Abstract | Crossref Full Text | Google Scholar
3. Doucha M, Kučerová B, Newland N, Vyhnánek M, Rygl M, Koucky V, et al. Treatment of the congenital thoracic deformity pectus excavatum. Rozhl Chir. (2023) 102(9):352–5. doi: 10.33699/pis.2023.102.9.352-355
PubMed Abstract | Crossref Full Text | Google Scholar
4. Prada Arias M, Gómez Veiras J, Rodríguez Iglesias P, Aneiros Castro B, Fernández Eire P, Montero Sánchez M. Treatment of pectus excavatum with vacuum bell during puberty. Cir Pediatr. (2023) 36(3):116–21. doi: 10.54847/cp.2023.03.11
PubMed Abstract | Crossref Full Text | Google Scholar
6. Schier F, Bahr M, Klobe E. The vacuum chest wall lifter: an innovative, nonsurgical addition to the management of pectus excavatum. J Pediatr Surg. (2005) 40(3):496–500. doi: 10.1016/j.jpedsurg.2004.11.033
PubMed Abstract | Crossref Full Text | Google Scholar
7. Haecker FM, Mayr J. The vacuum bell for treatment of pectus excavatum: an alternative to surgical correction? Eur J Cardiothorac Surg. (2006) 29(4):557–61. doi: 10.1016/j.ejcts.2006.01.025
PubMed Abstract | Crossref Full Text | Google Scholar
8. Luo D, Cheng K, Yuan M, Xu C, He T. Efficacy and determinants of vacuum bell treatment in preschool children with pectus excavatum. Front Pediatr. (2022) 10:1008437. doi: 10.3389/fped.2022.1008437
PubMed Abstract | Crossref Full Text | Google Scholar
9. Lopez M, Patoir A, Costes F, Varlet F, Barthelemy JC, Tiffet O. Preliminary study of efficacy of cup suction in the correction of typical pectus excavatum. J Pediatr Surg. (2016) 51(1):183–7. doi: 10.1016/j.jpedsurg.2015.10.003
PubMed Abstract | Crossref Full Text | Google Scholar
10. Obermeyer RJ, Cohen NS, Kelly RE Jr., Ann Kuhn M, Frantz FW, McGuire MM, et al. Nonoperative management of pectus excavatum with vacuum bell therapy: a single center study. J Pediatr Surg. (2018) 53(6):1221–5. doi: 10.1016/j.jpedsurg.2018.02.088
PubMed Abstract | Crossref Full Text | Google Scholar
11. Toselli L, Chinni E, Nazar-Peirano M, Vallee M, Sanjurjo D, Martinez J, et al. Determinants of success associated with vacuum bell treatment of pectus excavatum. J Pediatr Surg. (2022) 57(11):550–4. doi: 10.1016/j.jpedsurg.2022.04.010
PubMed Abstract | Crossref Full Text | Google Scholar
12. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370(9596):1453–7. doi: 10.1016/s0140-6736(07)61602-x
PubMed Abstract | Crossref Full Text | Google Scholar
13. Sidden CR, Katz ME, Swoveland BC, Nuss D. Radiologic considerations in patients undergoing the Nuss procedure for correction of pectus excavatum. Pediatr Radiol. (2001) 31(6):429–34. doi: 10.1007/s002470100455
PubMed Abstract | Crossref Full Text | Google Scholar
15. St-Louis E, Miao J, Emil S, Baird R, Bettolli M, Montpetit K, et al. Vacuum bell treatment of pectus excavatum: an early North American experience. J Pediatr Surg. (2019) 54(1):194–9. doi: 10.1016/j.jpedsurg.2018.10.011
PubMed Abstract | Crossref Full Text | Google Scholar
19. Snyder CW, Farach SM, Litz CN, Danielson PD, Chandler NM. The modified percent depth: another step toward quantifying severity of pectus excavatum without cross-sectional imaging. J Pediatr Surg. (2017) 52(7):1098–101. doi: 10.1016/j.jpedsurg.2017.01.053
PubMed Abstract | Crossref Full Text | Google Scholar
20. Deng X, Huang P, Luo J, Wang J, Yi L, Yang G, et al. A novel three-dimensional printed vacuum bell for pectus excavatum treatment: a preliminary study. J Cardiothorac Surg. (2020) 15(1):240. doi: 10.1186/s13019-020-01276-y
留言 (0)