Hepatocellular carcinoma (HCC) ranks as the sixth leading cause of morbidity and the third leading cause of mortality worldwide, presenting a significant threat to public health (1). HCC is often accompanied by portal hypertension (PHT), which can lead to severe complications, including acute variceal bleeding (AVB), ascites, and hepatic encephalopathy. Notably, AVB is an independent risk factor for mortality in HCC patients (2). To reduce the risk of gastrointestinal bleeding, current guidelines recommend the use of non-selective β-receptor blockers (NSBBs) for patients with high-risk varices (HRV) (3). Therefore, accurately identifying HRV in HCC patients is essential. Although endoscopy is the standard method for diagnosing varices, it poses challenges due to patient discomfort and potential risks. This underscores the urgent need for a reliable, cost-effective, and non-invasive screening tool to help clinicians determine when to identify HCC patients with HRV who require endoscopic evaluation and to prescribe NSBBs.
The Baveno VI consensus, published in 2015, recommended that patients meeting specific criteria—namely, liver stiffness measurement (LSM) < 20 kPa and platelet count (PLT) > 150×109/L—could safely avoid endoscopy due to their low risk of developing varices requiring treatment. Instead, these patients should be monitored annually, with endoscopy reserved for situations where these parameters worsen (4). In 2022, the Baveno VII consensus reaffirmed the cutoff values established in the Baveno VI guidelines but introduced an updated definition of HRV. HRV is now characterized by the presence of large varices (≥5 mm), red spot signs, or a Child-Pugh C classification (3). Numerous studies have widely validated the accuracy of the Baveno VI criteria in screening for HRV in patients with liver cirrhosis (5–8). However, while several studies have examined the diagnostic value of the Baveno VI criteria for assessing HRV in patients with HCC, the results have been inconsistent (9, 10). Therefore, a meta-analysis is necessary to evaluate the accuracy of the Baveno VI criteria in diagnosing HRV in patients with HCC.
2 MethodsThis meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (11). The PROSPERO registration number of this study is CRD42024533946.
2.1 Literature searchWe searched for articles published in English until April 19, 2024, related to Baveno criteria and HCC across PubMed, Embase, Web of Science, and Cochrane databases. We used the following keywords both individually and in combination: “hepatocellular carcinoma,” “Baveno,” “liver stiffness measurement,” and “platelet count.” Detailed search strategies are available in the supporting information.
2.2 Literature inclusion and exclusion criteriaThe inclusion criteria for the literature were as follows: (1) studies published in English that assessed the accuracy of the Baveno criteria in diagnosing HRV in patients with HCC; (2) studies that provided sufficient data to directly or indirectly calculate true positive (TP), false positive (FP), true negative (TN), and false negative (FN) values; and (3) studies where endoscopy was used as the gold standard for diagnosing HRV in the evaluation of Baveno criteria.
The exclusion criteria were as follows: (1) studies classified as reviews, editorials, opinions, conference abstracts, or case reports; (2) studies involving animals or cellular experiments; and (3) studies lacking sufficient data to calculate TP, FP, TN, and FN values either directly or indirectly.
2.3 Quality assessmentTwo reviewers (X.Z. and H.J.) independently conducted the quality assessment of the studies using the QUADAS-2 tool (Quality Assessment of Diagnostic Accuracy Studies) (12). In cases where they disagreed and could not reach a consensus, a third reviewer (C.G.) was brought in to resolve the issue.
2.4 Data extractionData from the four studies were systematically extracted and organized into a standardized table with the following categories: (1) Patient characteristics: sample size, number of male patients, age, liver disease etiology, Child-Pugh grade, and BCLC stage; (2) Study characteristics: first author, country, year of publication, study design, and endoscopy results; (3) Favorable Baveno VI criteria data (LSM < 20 kPa and PLT > 150×109/L): TP, TN, FP, and FN values.
Two independent researchers (H.X. and H.Y.) conducted the data extraction. Any disagreements were resolved by a third researcher (H.G.).
2.5 The definition of HRVHRV was identified during upper endoscopy using the following criteria: (1) varices measuring 5 mm or more, classified as grade 2 or 3; (2) grade 1 varices displaying recent signs of bleeding, such as red markings or a fibrin clot; or (3) varices with evidence of active bleeding (4).
As established by consensus, the favorable Baveno VI criteria are defined as LSM < 20 kPa and PLT > 150×109/L (3).
2.6 Statistical analysisThis meta-analysis was performed using STATA 14.0 and MetaDisc 1.4. Meta-Disc 1.4 was utilized to test for threshold effects in the gathered four-grid table data. Spearman’s correlation coefficient served as the assessment criterion, with a P-value greater than 0.05 indicating the absence of threshold effects (13). Statistical heterogeneity among the four included studies was assessed using I² and P values, with I² > 50% or P < 0.05 indicating significant heterogeneity (14). A random-effects model was used to calculate the pooled sensitivity (SEN), specificity (SPE), diagnostic odds ratio (DOR), positive likelihood ratio (LR+), and negative likelihood ratio (LR-), all with 95% confidence intervals (CI) (15). Subgroup and meta-regression analyses were performed to identify potential sources of heterogeneity (16). We constructed a summary receiver operating characteristic curve (SROC) to summarize diagnostic accuracy and calculated the area under the curve (AUC).
3 Results3.1 Search resultsAfter a comprehensive search across multiple databases, we initially identified 1,232 articles published in English. After removing 295 duplicates, 937 articles remained for title and abstract screening. We then excluded 468 irrelevant publications, 401 conference abstracts, 10 meta-analyses, 4 case reports, and 4 reviews, leaving 50 reports for further consideration. Upon full-text review, 46 articles were excluded due to lack of relevance to the topic. Ultimately, 4 studies, comprising a total of 1,277 patients, were included in evaluating the Baveno VI criteria (9, 10, 17, 18) (Figure 1).
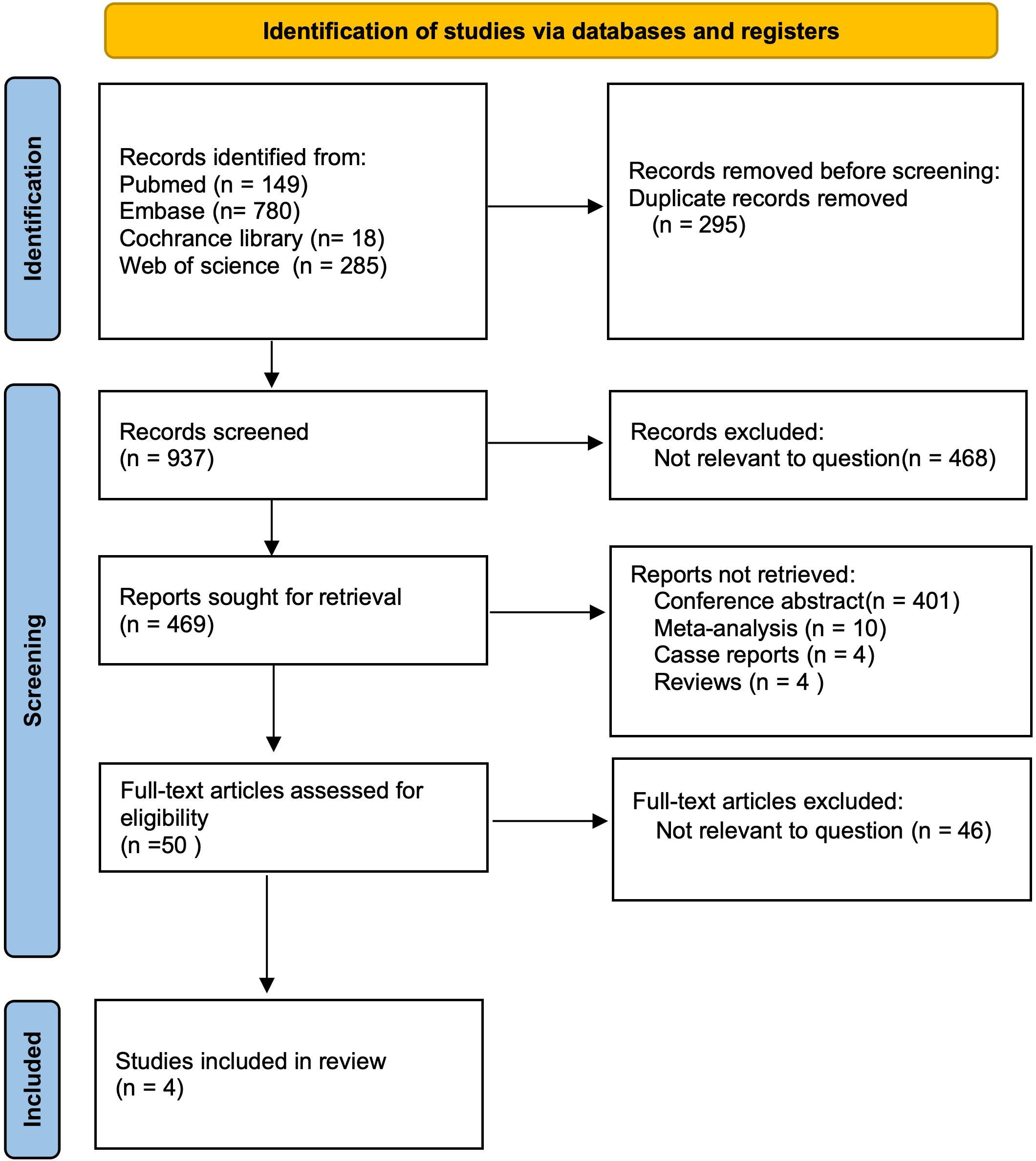
Figure 1. PRISMA flow diagram of the study selection.
3.2 Study characteristics and qualityThe four studies analyzed in this meta-analysis were all published in 2023. The patient population included 1,277 individuals, 1,079 (84.5%) were male and 198 (15.5%) were female, with a mean age ranging from 54 to 63 years. The leading causes of liver disease among these patients were chronic hepatitis B and C infections, representing 70.6% and 9.6% of cases, respectively. Among the patients, 819 (64.1%) were classified as Child-Pugh grade A, and 458 (35.9%) were classified as grade B. Regarding HCC staging, 670 (52.5%) were classified as BCLC stage 0/A, and 607 (47.5%) as BCLC stage B/C. Diagnostic accuracy metrics such as TP, FP, TN, and FN were calculated based on the original data from these studies. Table 1 presents the key characteristics of the included studies. The quality and applicability of the four studies were deemed appropriate, as assessed using the QUADAS-2 tool (Supplementary Figure S1).
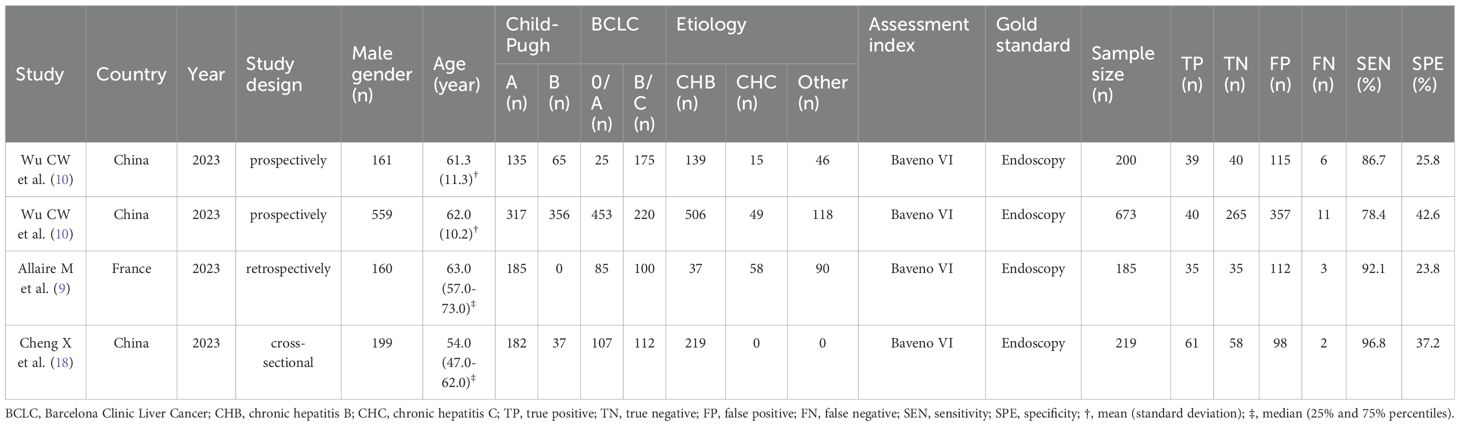
Table 1. Characteristics of the included studies.
3.3 Detecting heterogeneityFigure 2 displays the forest plot, revealing significant heterogeneity among the four studies analyzed in the Meta-analysis when considering sensitivity (P = 0.01; I2 = 71.98) and specificity (P = 0.00; I2 = 90.96). The Spearman’s correlation coefficient was 0.400, with a P-value of 0.600, indicating no evidence of a threshold effect.
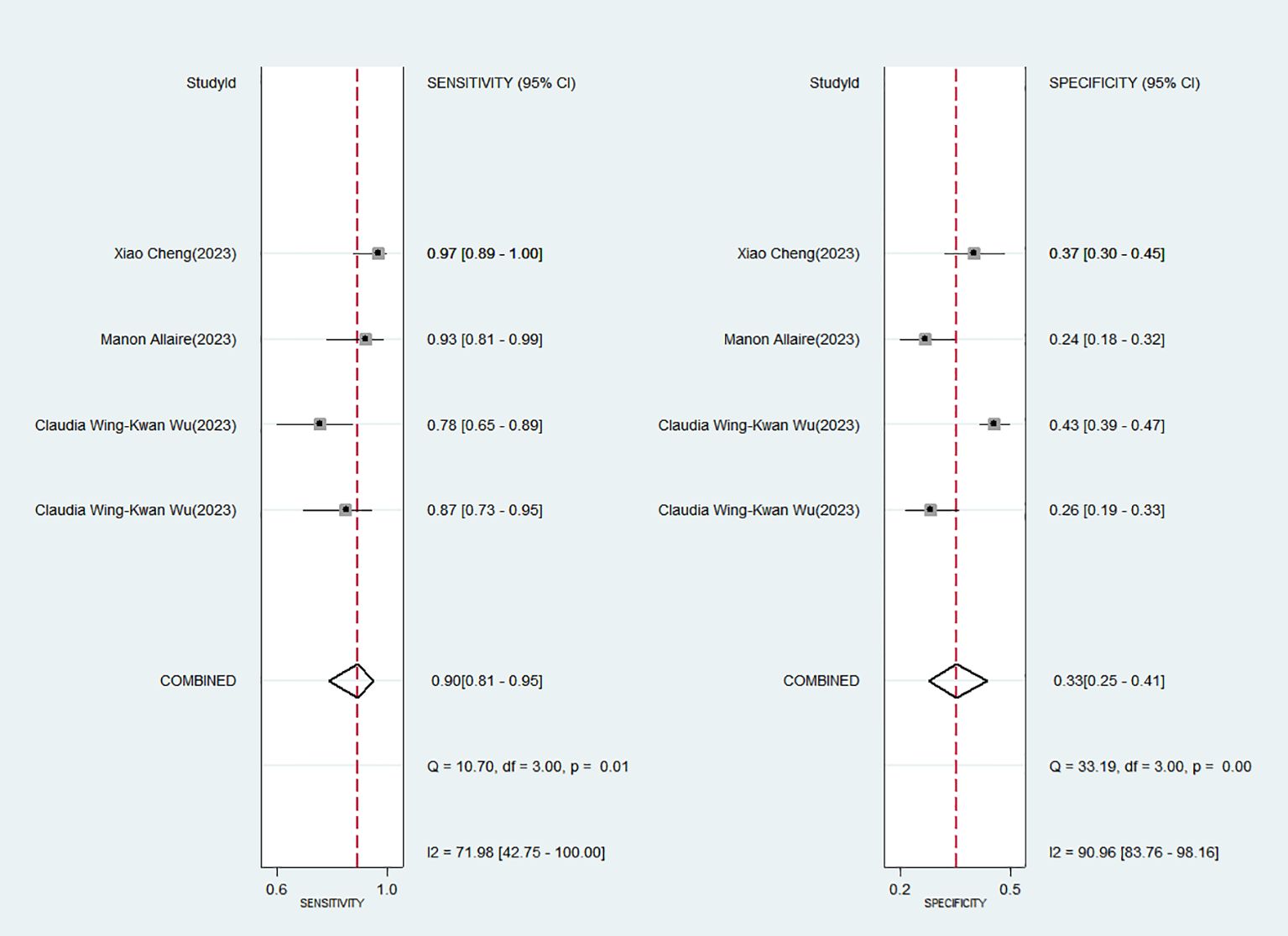
Figure 2. Forest plot of the sensitivity and specificity of Baveno VI criteria for detecting high-risk varices (HRV) in patients with hepatocellular carcinoma (HCC).
3.4 Diagnostic accuracy of favorable Baveno VI criteria for HRV in HCCThe favorable Baveno VI criteria for screening HRV in patients with HCC showed a SEN of 0.90 (95% CI: 0.81–0.95) and a SPE of 0.33 (95% CI: 0.25–0.41) (Figure 2). The LR+, LR-, and DOR were 1.34 (95% CI: 1.19–1.50), 0.30 (95% CI: 0.16–0.58), and 4.44 (95% CI: 2.14–9.22), respectively. Additionally, the AUC for the criteria in screening HRV was 0.59 (95% CI: 0.55–0.64) (Figure 3).
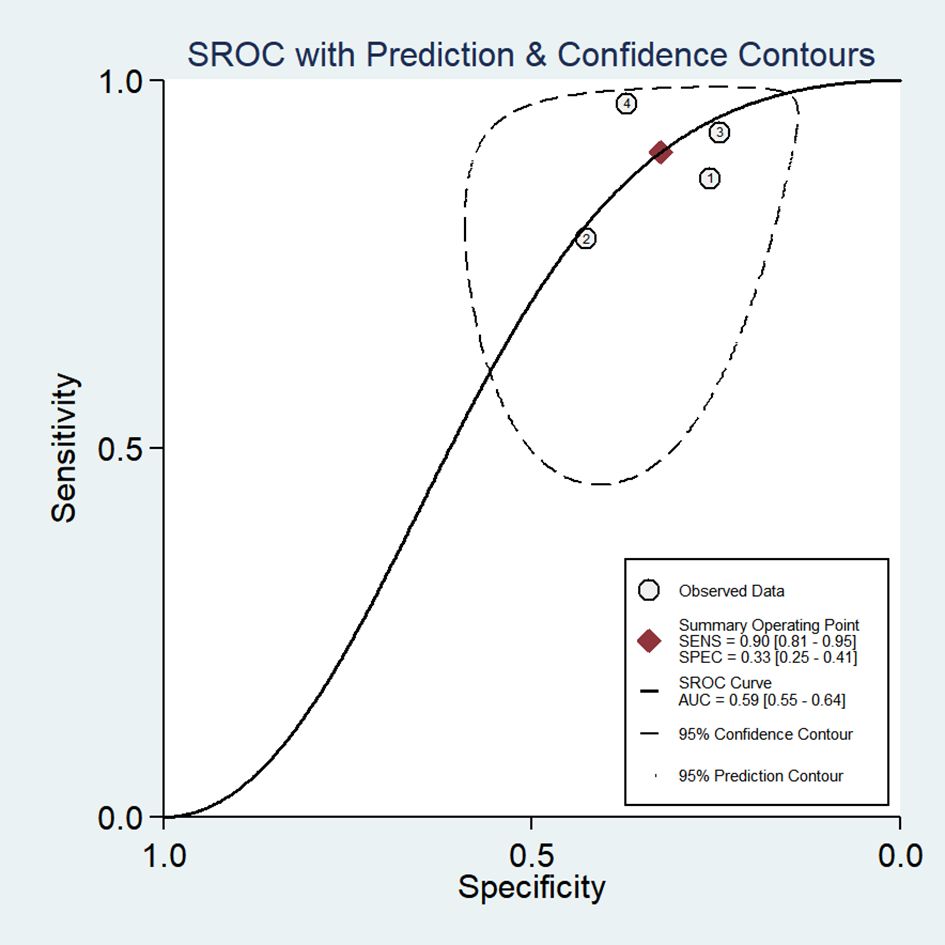
Figure 3. Summary receiver operating characteristic (SROC) curve of the diagnostic performance of Baveno VI criteria in detecting HRV in patients with HCC.
3.5 Subgroup analysis and meta-regressionThe study categorized patients into two groups based on BCLC staging: BCLC 0/A (n=3) and BCLC B/C (n=3), as well as two groups based on Child-Pugh classification: Child-Pugh A (n=3) and Child-Pugh B (n=2). The aim was to assess the impact of BCLC and Child-Pugh classifications on the accuracy of the Baveno VI criteria in screening HRV in patients with HCC. Subgroup and meta-regression analyses indicated that BCLC and Child-Pugh stages were likely the main contributors to the heterogeneity in the SPE of the Baveno VI criteria for identifying HRV in patients with HCC (P < 0.05) (Table 2).
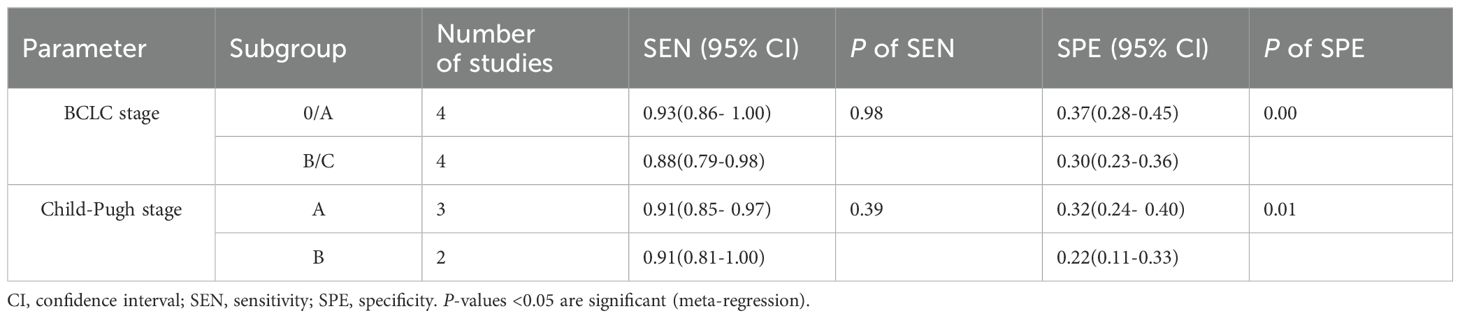
Table 2. Subgroup and Meta-regression analyses of favorable Baveno VI criteria screening HRV in HCC.
4 DiscussionThis meta-analysis focused on the accuracy of Baveno VI criteria screening for HRV in patients with HCC. We analyzed data from four studies involving 1,277 HCC patients. The findings suggest that the favorable Baveno VI criteria may not be effective for HRV screening in this population.
The Baveno VI criteria, widely used for screening HRV in patients with liver cirrhosis, are based on two simple parameters: LSM and PLT. The criteria offers several advantages over endoscopy for screening HRV in cirrhotic patients. First, endoscopy is technically demanding and requires highly skilled physicians, while the Baveno VI criteria rely on easily obtainable measures like PLT and LSM, making them suitable for use in community hospitals and remote areas. Second, the Baveno VI criteria are more cost-effective compared to endoscopy. Third, endoscopy is often uncomfortable for patients, who may resist the procedure, whereas PLT and LSM are non-invasive and generally more acceptable. Lastly, the Baveno VI criteria avoid the complications and risks associated with endoscopy, such as gastrointestinal bleeding. While these criteria have been thoroughly validated in cirrhotic patients without HCC (19), our meta-analysis and other studies (9, 18) suggest that their effectiveness is limited when applied to patients with HCC. This limitation may be attributed to the influence of HCC on LSM, PLT, and varices. First, patients with both liver cirrhosis and HCC generally exhibit higher LSM values compared to those without HCC. LSM, a non-invasive diagnostic tool for liver fibrosis, is commonly measured using transient elastography and is extensively utilized in clinical practice (20). However, LSM readings can be affected by elevated alanine aminotransferase levels, obesity, congestion, and cholestasis, all of which can result in higher values (21). Additionally, the presence of liver tumors, particularly large or multiple tumors in the right hepatic lobe, may further elevate LSM. For example, a study by Diana Feier and colleagues found that LSM values in HCC patients were significantly higher than in those with only liver cirrhosis (42 KPa vs. 27 KPa) (22). LSM has also been incorporated into various models for the early prediction of HCC, further confirming the impact of HCC on LSM readings (23). Second, PLT levels may be elevated in HCC patients compared to those without HCC, especially in cases involving large tumors (24). The interaction between HCC and PLT is well-documented. Tumor cells can activate PLT by releasing soluble stimulants and surface molecules, which, in turn, contribute to tumor progression by promoting cancer angiogenesis (25). Consequently, HCC patients with higher PLT levels tend to have a worse prognosis than those with lower levels (26). Beyond these two factors, other HCC-related elements also contribute to HRV in HCC patients. For instance, specific targeted therapy and immunotherapy drugs, such as bevacizumab and sorafenib, have been reported to exacerbate varices and bleeding in HCC patients (27). This may be due to the adverse effects of vascular endothelial growth factor receptor inhibitors on the integrity of microvascular endothelial cells in HCC (28). Additionally, portal vein tumor thrombosis (PVTT) can worsen HRV and esophageal variceal bleeding in HCC patients (29). As observed in our meta-analysis, these factors likely explain why the Baveno VI criteria demonstrated relatively high sensitivity but low specificity for screening HRV in patients with HCC. In conclusion, the accuracy of the favorable Baveno VI criteria for screening HRV in HCC patients is compromised due to the various factors discussed.
Heterogeneity analysis revealed significant heterogeneity across the studies. To further investigate the sources of this heterogeneity, we conducted subgroup analyses based on the BCLC stage and Child-Pugh stage to assess the impact of different tumor stages and liver function grades. The meta-regression analysis results suggest that the BCLC and Child-Pugh stages may be the primary factors contributing to the heterogeneity in the SPE of the Baveno VI criteria for screening HRV with HCC. Factors such as tumor number, size, and liver function may influence LSM, PLT, or portal hypertension, thereby contributing to this heterogeneity. Conducting studies with more precisely defined patient populations and larger sample sizes may help reduce this heterogeneity.
The Baveno VI criteria were applied to patients with compensated cirrhosis. Among the four articles analyzed, while both Child-Pugh A and B liver function patients were included, none of the Child-Pugh B patients had experienced liver decompensation events such as ascites, gastrointestinal bleeding, or hepatorenal syndrome at baseline. Thus, the study population aligns with the characteristics outlined in the Baveno VI criteria. Additionally, despite the varying causes of HCC across different countries, chronic hepatitis B or C virus infections remain the primary risk factors, particularly in males over the age of 40 (30). The patients included in this meta-analysis were predominantly middle-aged males with HCC related to hepatitis B or C, which may be due to the fact that most of the studies included in this paper were conducted in China.
One limitation of our study is that we were unable to conduct a more detailed subgroup analysis due to the insufficient data from the included studies. In the future, conducting detailed subgroup analyses—such as those focusing on etiology, systemic therapy, and PVTT—could provide a more comprehensive validation of the Baveno VI criteria for screening HRV in HCC.
In conclusion, the favorable Baveno VI criteria may not effectively screen HRV in patients with HCC. However, the current evidence is limited, and further research with larger sample sizes and more detailed patient subgroups is necessary to confirm these findings.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributionsXZ: Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. TM: Formal analysis, Visualization, Writing – original draft. HX: Data curation, Writing – original draft. HY: Data curation, Writing – original draft. HJ: Investigation, Writing – original draft. CG: Writing – original draft. XW: Writing – review & editing. HD: Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Capital Medical Development and Research Fund (2022–1–2181), Beijing Municipal Science and Technology Commission (Z221100007422002), State Administration of Traditional Chinese Medicine of People's Republic of China (ZYYZDXK-2023002), and the Scientific Research Project of Beijing Youan Hospital, CCMU, 2019 (BJYAYY CY2019-12).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1482290/full#supplementary-material
References1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
PubMed Abstract | Crossref Full Text | Google Scholar
2. Giannini EG, Risso D, Testa R, Trevisani F, Di Nolfo MA, Del PP, et al. Prevalence and prognostic significance of the presence of esophageal varices in patients with hepatocellular carcinoma. Clin Gastroenterol Hepatol. (2006) 4:1378–84. doi: 10.1016/j.cgh.2006.08.011
PubMed Abstract | Crossref Full Text | Google Scholar
3. de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. (2022) 76:959–74. doi: 10.1016/j.jhep.2021.12.022
PubMed Abstract | Crossref Full Text | Google Scholar
4. de Franchis R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. (2015) 63:743–52. doi: 10.1016/j.jhep.2015.05.022
PubMed Abstract | Crossref Full Text | Google Scholar
5. Gaete MI, Diaz LA, Arenas A, Gonzalez K, Cattaneo M, Fuster F, et al. Baveno VI and Expanded Baveno VI criteria successfully predicts the absence of high-risk gastro-oesophageal varices in a Chilean cohort. Liver Int. (2020) 40:1427–34. doi: 10.1111/liv.14373
PubMed Abstract | Crossref Full Text | Google Scholar
6. Pizzamiglio M, Weicker A, de Terwangne C, Henrion J, Descamps OS, De Vos M. Validation of Baveno VI and Expanded-Baveno VI Criteria for predicting gastroesophageal varices in patients with alcoholic and non-alcoholic fatty liver disease. Acta Gastroenterol Belg. (2022) 85:321–29. doi: 10.51821/88.2.9553
PubMed Abstract | Crossref Full Text | Google Scholar
7. Asesio N, Pollo-Flores P, Caliez O, Munteanu M, Ngo A, Ngo Y, et al. Baveno VI criteria as a prognostic factor for clinical complications in patients with compensated cirrhosis. Dig Liver Dis. (2022) 54:645–53. doi: 10.1016/j.dld.2021.09.004
PubMed Abstract | Crossref Full Text | Google Scholar
8. Wong YJ, Zhaojin C, Tosetti G, Degasperi E, Sharma S, Agarwal S, et al. Baveno-VII criteria to predict decompensation and initiate non-selective beta-blocker in compensated advanced chronic liver disease patients. Clin Mol Hepatol. (2023) 29:135–45. doi: 10.3350/cmh.2022.0181
PubMed Abstract | Crossref Full Text | Google Scholar
9. Allaire M, Campion B, Demory A, Larrey E, Wagner M, Rudler M, et al. Baveno VI and VII criteria are not suitable for screening for large varices or clinically significant portal hypertension in patients with hepatocellular carcinoma. Aliment Pharmacol Ther. (2023) 58:346–56. doi: 10.1111/apt.17599
PubMed Abstract | Crossref Full Text | Google Scholar
10. Wu CW, Lui RN, Wong VW, Yam TF, Yip TC, Liu K, et al. Baveno VII criteria is an accurate risk stratification tool to predict high-risk varices requiring intervention and hepatic events in patients with advanced hepatocellular carcinoma. Cancers (Basel). (2023) 15:2480. doi: 10.3390/cancers15092480
PubMed Abstract | Crossref Full Text | Google Scholar
11. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
PubMed Abstract | Crossref Full Text | Google Scholar
12. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
PubMed Abstract | Crossref Full Text | Google Scholar
13. Deville WL, Buntinx F, Bouter LM, Montori VM, de Vet HC, van der Windt DA, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. (2002) 2:9. doi: 10.1186/1471-2288-2-9
PubMed Abstract | Crossref Full Text | Google Scholar
15. Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. (2005) 58:982–90. doi: 10.1016/j.jclinepi.2005.02.022
PubMed Abstract | Crossref Full Text | Google Scholar
17. Wu CW, Wong GL, Wong VW, Yam TF, Yip TC, Wong AC, et al. Baveno VII criteria identify varices needing treatment in patients with hepatocellular carcinoma of different Barcelona Clinic Liver Cancer stages. J Gastroenterol Hepatol. (2023) 38:1381–88. doi: 10.1111/jgh.16218
PubMed Abstract | Crossref Full Text | Google Scholar
18. Cheng X, Tang Y, He Q, Song J, Wang K, Li H, et al. Spleen-dedicated stiffness measurement performed well to rule out high-risk varices in HBV-related hepatocellular carcinoma: Screening for high-risk varices in HCC. Aliment Pharmacol Ther. (2024) 59:680–91. doi: 10.1111/apt.17850
PubMed Abstract | Crossref Full Text | Google Scholar
19. Szakacs Z, Eross B, Soos A, Matrai P, Szabo I, Petervari E, et al. Baveno criteria safely identify patients with compensated advanced chronic liver disease who can avoid variceal screening endoscopy: A diagnostic test accuracy meta-analysis. Front Physiol. (2019) 10:1028. doi: 10.3389/fphys.2019.01028
PubMed Abstract | Crossref Full Text | Google Scholar
20. European Association For Study Of Liver, Asociacion Latinoamericana Para El Estudio Del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. (2015) 63:237–64. doi: 10.1016/j.jhep.2015.04.006
PubMed Abstract | Crossref Full Text | Google Scholar
21. Oeda S, Tanaka K, Oshima A, Matsumoto Y, Sueoka E, Takahashi H. Diagnostic accuracy of fibroScan and factors affecting measurements. Diagnostics (Basel). (2020) 10:940. doi: 10.3390/diagnostics10110940
PubMed Abstract | Crossref Full Text | Google Scholar
22. Feier D, Lupsor PM, Stefanescu H, Badea R. Transient elastography for the detection of hepatocellular carcinoma in viral C liver cirrhosis. Is there something else than increased liver stiffness? J Gastrointestin Liver Dis. (2013) 22:283–89. Available at: there something else than increased liver stiffness? - PubMed (nih.gov)
PubMed Abstract | Google Scholar
23. Lin H, Li G, Delamarre A, Ahn SH, Zhang X, Kim BK, et al. A liver stiffness-based etiology-independent machine learning algorithm to predict hepatocellular carcinoma. Clin Gastroenterol Hepatol. (2024) 22:602–10. doi: 10.1016/j.cgh.2023.11.005
PubMed Abstract | Crossref Full Text | Google Scholar
26. Lu L, Su Z, Zheng P, Wu Z, Zhang Y, He H, et al. Association between platelet count and hepatocellular carcinoma overall survival: a large retrospective cohort study. BMJ Open. (2020) 10:e38172. doi: 10.1136/bmjopen-2020-038172
Crossref Full Text | Google Scholar
27. Larrey E, Campion B, Evain M, Sultanik P, Blaise L, Giudicelli H, et al. A history of variceal bleeding is associated with further bleeding under atezolizumab-bevacizumab in patients with HCC. Liver Int. (2022) 42:2843–54. doi: 10.1111/liv.15458
PubMed Abstract | Crossref Full Text | Google Scholar
28. Duffy A, Wilkerson J, Greten TF. Hemorrhagic events in hepatocellular carcinoma patients treated with antiangiogenic therapies. Hepatology. (2013) 57:1068–77. doi: 10.1002/hep.26120
PubMed Abstract | Crossref Full Text | Google Scholar
29. Lim J, Kim HI, Kim E, Kim J, An J, Chang S, et al. Variceal bleeding is aggravated by portal venous invasion of hepatocellular carcinoma: a matched nested case-control study. BMC Cancer. (2021) 21:11. doi: 10.1186/s12885-020-07708-1
PubMed Abstract | Crossref Full Text | Google Scholar
30. Wen N, Cai Y, Li F, Ye H, Tang W, Song P, et al. The clinical management of hepatocellular carcinoma worldwide: A concise review and comparison of current guidelines: 2022 update. Biosci Trends. (2022) 16:20–30. doi: 10.5582/bst.2022.01061
留言 (0)