Colorectal cancer (CRC) is the fourth most commonly diagnosed cancer and the second leading cause of cancer-related deaths (1). Approximately 15–30% of patients present with metastases at diagnosis, and 20–50% of initially localized cases eventually develop metastases (2). Management of metastatic CRC involves various active drugs used in combination or as single agents. Standard first-line treatment includes a backbone of 5-fluorouracil (5-FU) with the addition of oxaliplatin or irinotecan, often combined with EGFR-targeted antibodies like cetuximab or panitumumab or antiangiogenic therapies like bevacizumab, ramucirumab, or aflibercept. Second-line treatment is determined based on the first-line treatment received (3). Despite failure to respond to these treatments, many patients remain medically fit; however, the survival efficacy of subsequent treatments after second-line treatment for CRC is relatively limited, indicating an unmet need for new treatment options (4).
TAS-102 (trifluridine/tipiracil) is an oral combination drug consisting of a cytotoxic thymidine analog, trifluridine, and a thymidine phosphorylase inhibitor, tipiracil hydrochloride, which prevents the degradation of trifluridine (5). RECOURSE, a phase 3 trial, demonstrated that TAS-102 significantly prolonged overall survival compared with placebo in patients with metastatic CRC who had undergone extensive prior treatments, even in patients with disease refractory to fluoropyrimidines, across patient subgroups of age, geographical origin, or RAS mutation status (7.1 vs. 5.3 months; HR, 0.68; 95% CI, 0.58–0.81; p <.001) (6).
Bevacizumab is a humanized monoclonal antibody that blocks the activity of VEGF, important in tumor angiogenesis (7). Bevacizumab has been proven to improve outcomes when combined with cytotoxic chemotherapy as a first-line treatment for metastatic CRC (8–10). Moreover, continuation of bevacizumab following progression has also been shown to provide a survival benefit (11, 12).
The combination of TAS-102 with bevacizumab has been clinically demonstrated to provide a survival benefit compared to either drug alone after failure of standard therapy in metastatic CRC, and this combination is used in clinical practice. C-TAST FORCE, a phase 2 trial, evaluated the survival efficacy of TAS-102 plus bevacizumab and reported a 16-week progression-free survival rate of 42.9% (80% CI, 27.8–59.0%) in CRC patients who were refractory or intolerant to fluoropyrimidine, irinotecan, oxaliplatin, anti-VEGF therapy, and anti-EGFR therapy (for tumors with wild-type KRAS) and who had no previous treatment with regorafenib (13). Consistent with these results, SUNLIGHT, a phase 3 trial, showed a significant survival benefit in patients who had received no more than two previous chemotherapy regimens for the treatment of advanced CRC. The median overall survival was 10.8 months (HR, 0.61; 95% CI, 0.49–0.77; p<0.001), and the median progression-free survival was 5.6 months (HR, 0.44; 95% CI, 0.36– 0.54; p <0.001) (14).
Regimens comprising TAS-102 with and without bevacizumab are considered standard salvage treatment options in patients with refractory metastatic CRC. In this study, we evaluated the efficacy and safety of TAS-102 with and without bevacizumab in patients with refractory metastatic CRC in regular clinical practice.
2 Materials and methods2.1 Study design and participantsThis analysis included patients with metastatic colorectal cancer who received either TAS-102 plus bevacizumab or TAS-102 between July 2022 to November 2023 at Samsung Medical Center. We reviewed electronic clinical records to gather information including age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, histopathology, MSI status, RAS mutation status, BRAF mutation status, tumor mutation burden (TMB), carcinoembryonic antigen (CEA) level, primary tumor location, sites of metastatic disease, and previous treatments. MSI status, RAS mutation status, BRAF mutation status, and TMB were identified using next-generation sequencing (NGS) with the TruSight Oncology 500 assay (Illumina, San Diego, CA, USA) and Oncomine Focus assay (ThermoFisher, Waltham, MA, USA). Patients were treated with TAS-102 35 mg/m² orally twice a day on day 1-5 and 8-12 in a 28-day cycle with or without bevacizumab (5 mg/kg intravenously) every two weeks.
2.2 OutcomesClinical outcomes evaluated were objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), and overall survival (OS). Tumor response was evaluated as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD), according to the Response Evaluation Criteria in Solid Tumor, version 1.1. ORR was defined as the percentage (%) of patients with confirmed CR or PR. DCR was defined as the percentage (%) of patients with confirmed CR, PR, or SD. PFS was measured from the start of the treatment to the date of disease progression or death from any cause using RECIST 1.1. OS was calculated from the start of the treatment to the date of death from any cause. Safety objectives were evaluated according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.
2.3 Statistical analysisCategorical and continuous variables were summarized using descriptive statistics. Survival analyses were performed using Kaplan-Meier curves and compared using log-rank tests. Cox proportional hazards regression models were used to obtain estimates of hazard ratios (HRs) based on multivariate analysis. All P values were two-sided and confidence intervals (CI) were at the 95% level, with statistical significance defined as P ≤ 0.05. All statistical analyses were performed using IBM SPSS Statistics 27 and R version 4.3.0. The data cutoff date was February 28, 2024.
3 Results3.1 Clinical characteristics83 patients received TAS-102 plus bevacizumab (TAS-102 + Bev) and 56 received TAS-102 alone (TAS-102).
Baseline characteristics of analyzed patients are presented in Table 1. The median age was 60.8 years, and 56.8% of patients were male. Almost all patients (95.0%) were MSS, while only two (1.4%) were MSI-H, both of whom received TAS-102 + Bev. Among patients, 56.8% had RAS mutations, and six (4.3%) had BRAF mutations. The median TMB was 6.9, with 24 patients (17.3%) having a TMB-H status (>10 mutations). Primary tumor location was on the left side in 80.6% of patients, of whom 55 (39.6%) had tumors in the rectum. Metastases to the liver, lung, bone, peritoneum, and other sites were observed in 67.6%, 73.4%, 5.8%, 29.5%, and 42.4% of patients, respectively. The median number of previous lines of therapy was four (range: 2.45–6.55). All patients had previously received 5-FU, with 96.4% and 98.6% having received oxaliplatin and irinotecan, respectively. One hundred thirty-four patients had received anti-VEGF therapy (106 patients received bevacizumab only, 27 received both bevacizumab and aflibercept, and one received aflibercept only). Anti-EGFR therapy had been administered to 36 patients (25.9%). Fourteen patients in the TAS-102 + Bev group had prior regorafenib treatment compared to five in the TAS-102 group.
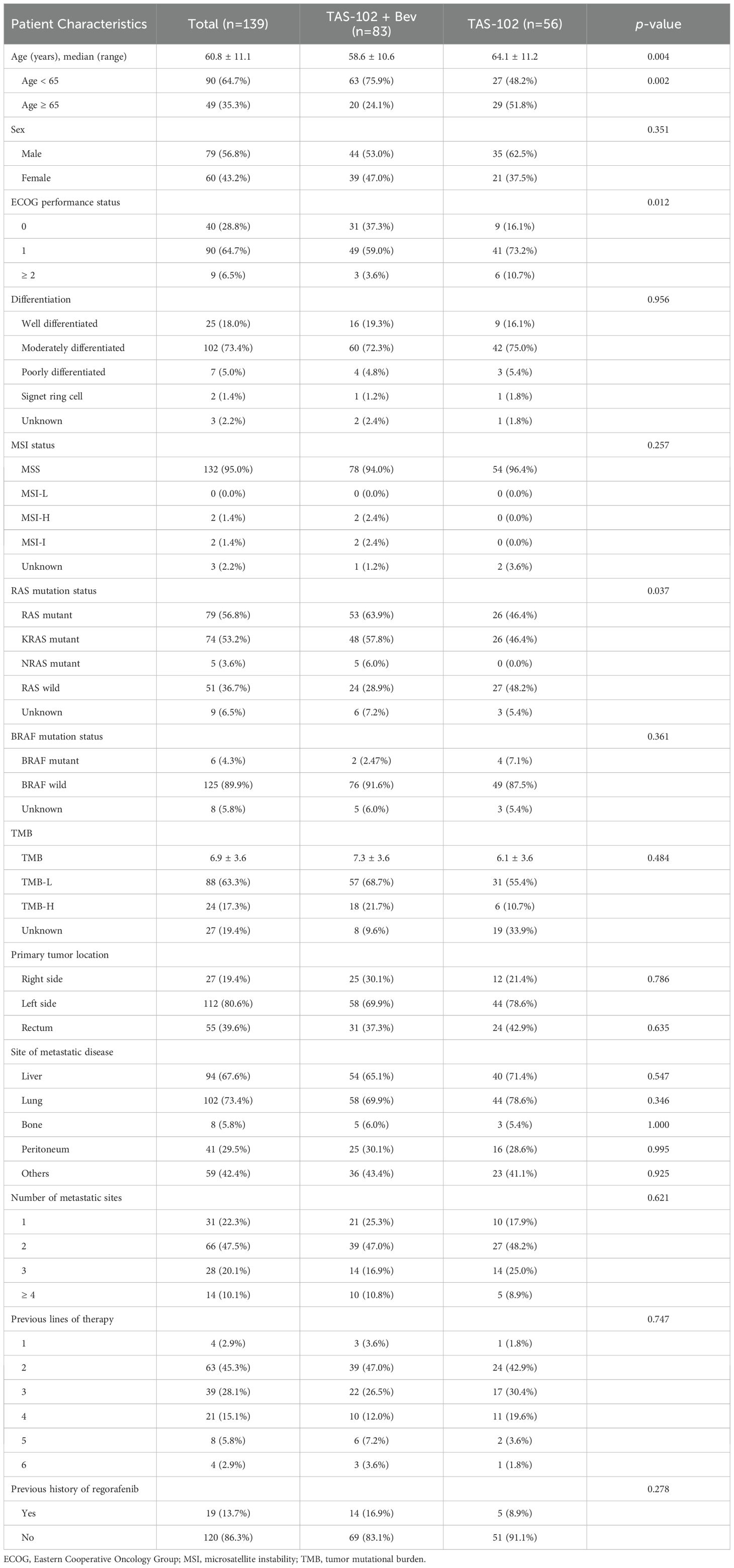
Table 1. Baseline Characteristics.
The median age of patients in the TAS-102 + Bev group was 58.6 years, which was younger compared to 64.1 years in the TAS-102 group. The proportion of patients with poor performance status (ECOG 2 or higher) was smaller in the TAS-102 + Bev group (3.6% vs. 10.7%), which contained a larger number of patients with RAS mutations (63.9% vs. 46.4%). Other baseline characteristics were similar between the two groups.
3.2 Efficacy of TAS-102 with and without bevacizumabDuring the median follow-up duration of 13.8 months (range: 10.0–16.0 months), the median number of treatment cycles was three (range: 1–13) in the TAS-102 + Bev group and two (range: 1–11.5) in the TAS-102 group.
Seven patients achieved PR (8.4%), while 36 achieved SD (43.4%), resulting in an ORR of 8.4% and a DCR of 51.8% in the TAS-102 + Bev group. In the TAS-102 group, three patients (5.4%) had PR, 15 patients (26.5%) had SD, while 28 patients, which is half of the group, achieved PD, resulting in an ORR of 5.4% and a DCR of 32.1% (Table 2).
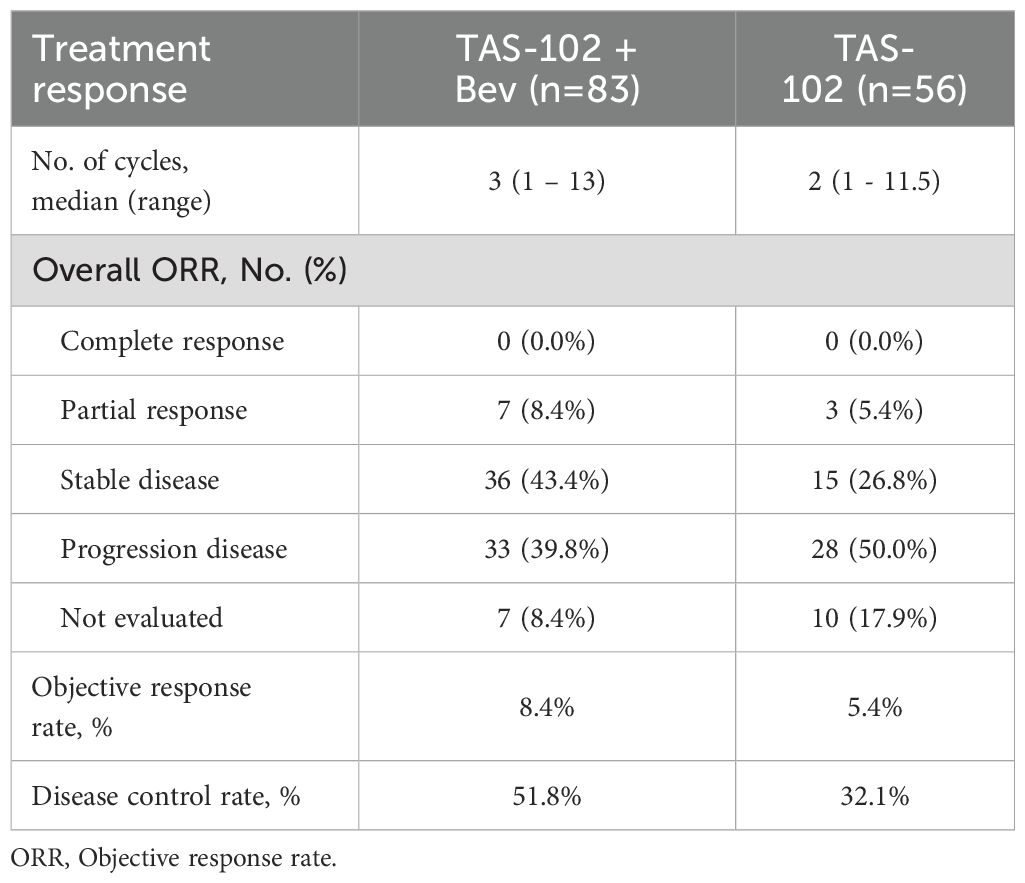
Table 2. Objective response rates by treatments.
The median PFS of all patients was 3.0 months (95% CI, 2.5–4.0), with values of 3.3 months (95% CI, 2.7–6.6) in the TAS-102 + Bev group and 2.5 months (95% CI, 2.0–3.8) in the TAS-102 group (HR, 0.56; 95% CI, 0.38–0.82; p=0.003) (Figure 1A). The median OS was 8.4 months (95% CI, 6.4–11.1) in all patients, with values of 10.8 months (95% CI, 8.4–NA) in the TAS-102 + Bev group and 6.0 months (95% CI, 4.8–9.8) in the TAS-102 group (HR, 0.62; 95% CI, 0.40–0.97, p=0.033) (Figure 1B).
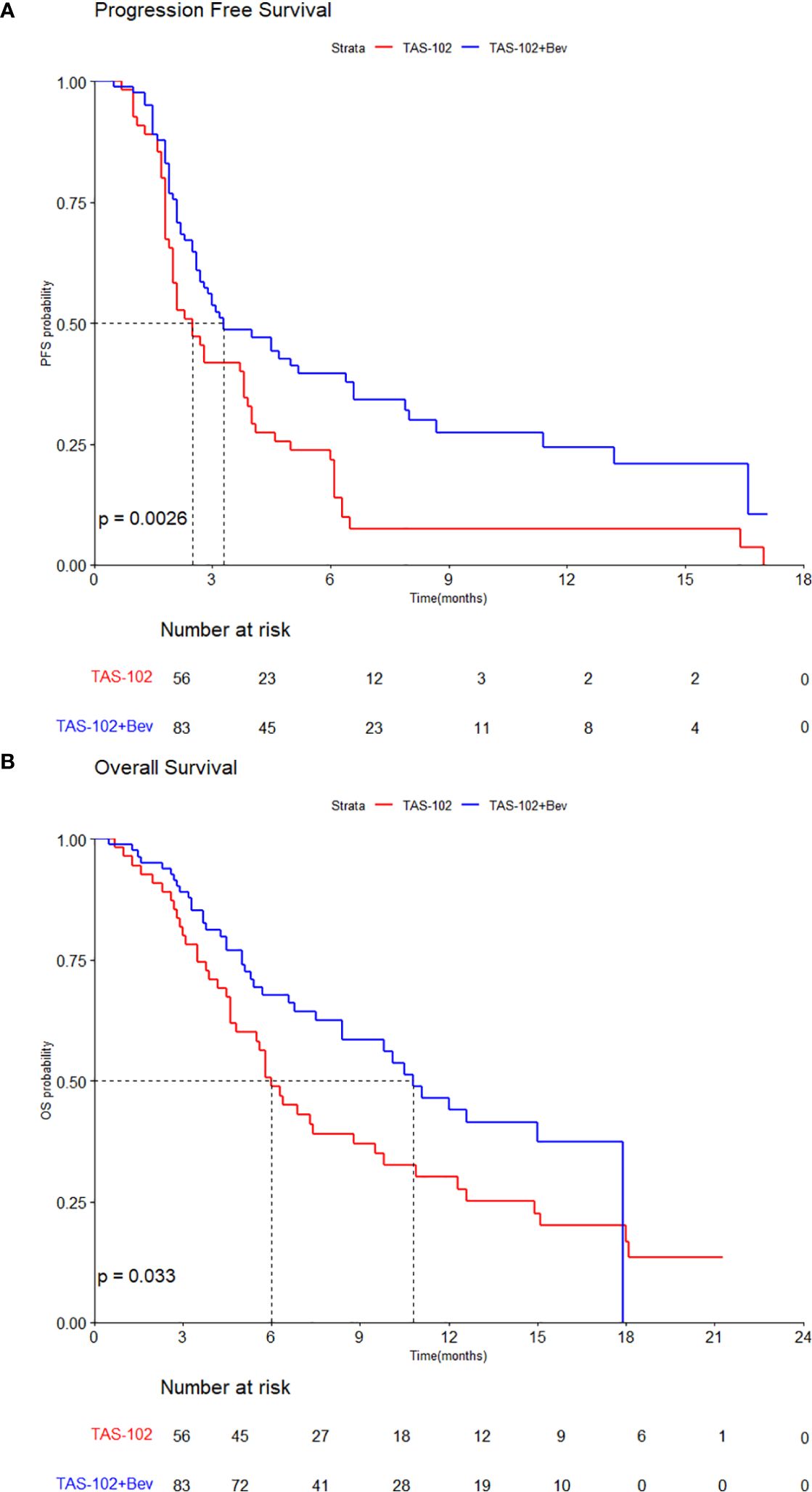
Figure 1. Kaplan–Meier curves by treatments for (A) progression-free survival and (B) overall survival.
Subgroup analyses of PFS revealed favorable survival outcomes for most subgroups in the TAS102 + Bev compared to TAS102 group, except for patients with an ECOG PS ≥ 2 (Figure 2A). Similar to PFS, longer OS was observed in all subgroups who received TAS102 + Bev treatment, except for the subgroup with ECOG PS ≥ 2 and a primary tumor location in the rectum (Figure 2B).
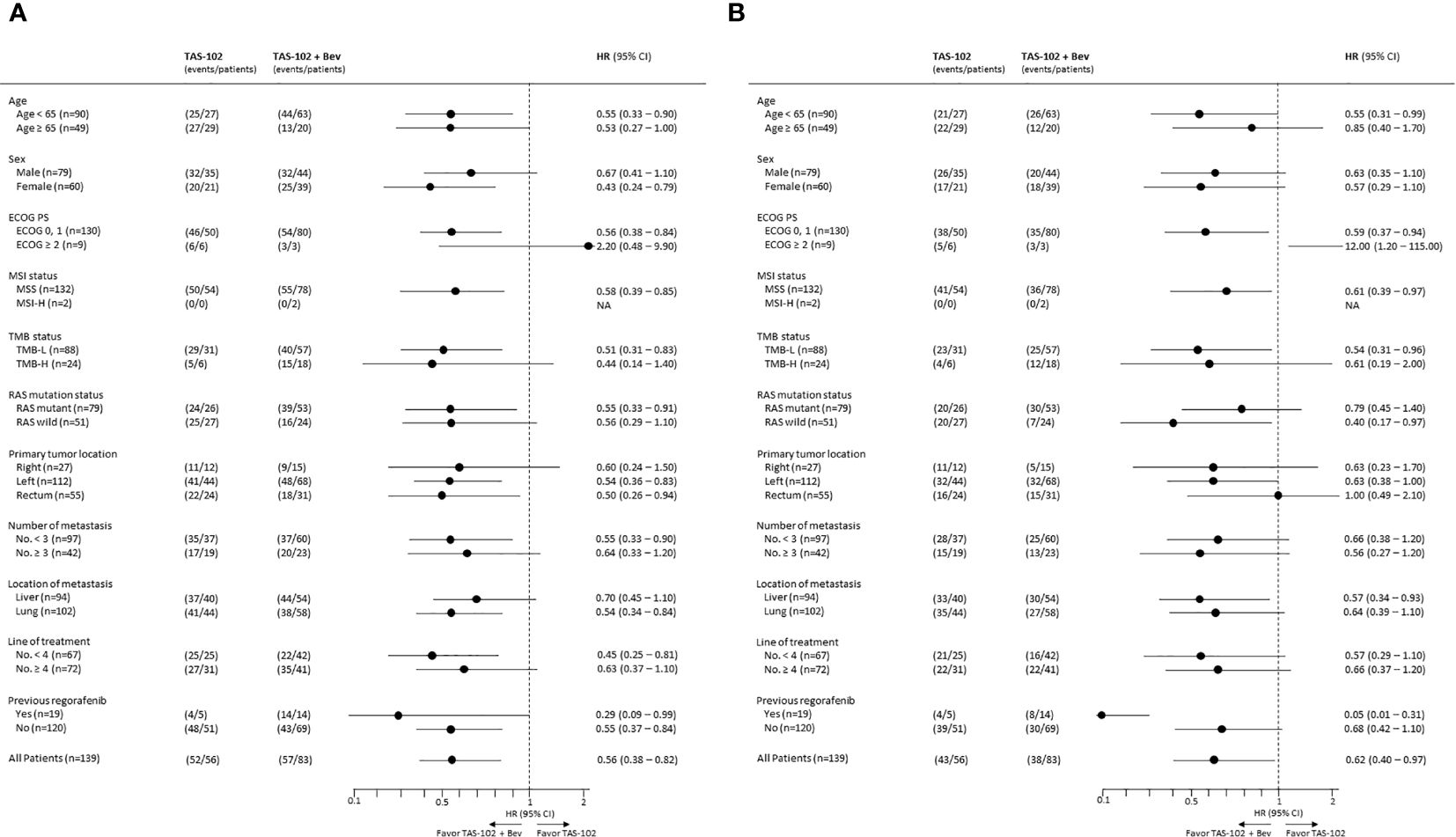
Figure 2. Forest plots of subgroup analyses of (A) progression-free survival and (B) overall survival.
3.3 Exploratory analysis of TAS-102 + Bev by KRAS G12 mutationAn exploratory analysis was conducted in the TAS-102 + Bev group to determine whether KRAS mutation status affects the clinical outcome of TAS-102 + Bev. Out of the total 83 patients treated with TAS-102 + Bev, 5 patients whose KRAS mutation status was unknown were excluded. Of these, 35 patients had KRAS G12 mutation (KRAS G12) while 43 had no KRAS G12 mutation (No KRAS G12). Among No KRAS G12, 13 had other KRAS mutation status: 11 had the KRAS G13 mutation, and 2 had other uncommon KRAS mutations.
The median PFS of total was 3.3 months (95% CI, 2.7–6.6), with 3.2 months (95% CI, 2.7–NA) in KRAS G12 group and 3.3 months (95% CI, 2.3–8.0) in the No KRAS G12 group. The PFS of TAS-102 + Bev did not differ significantly by KRAS mutation status (HR, 1.02, 95% CI, 0.60–1.75, p=0.94) (Figure 3A).
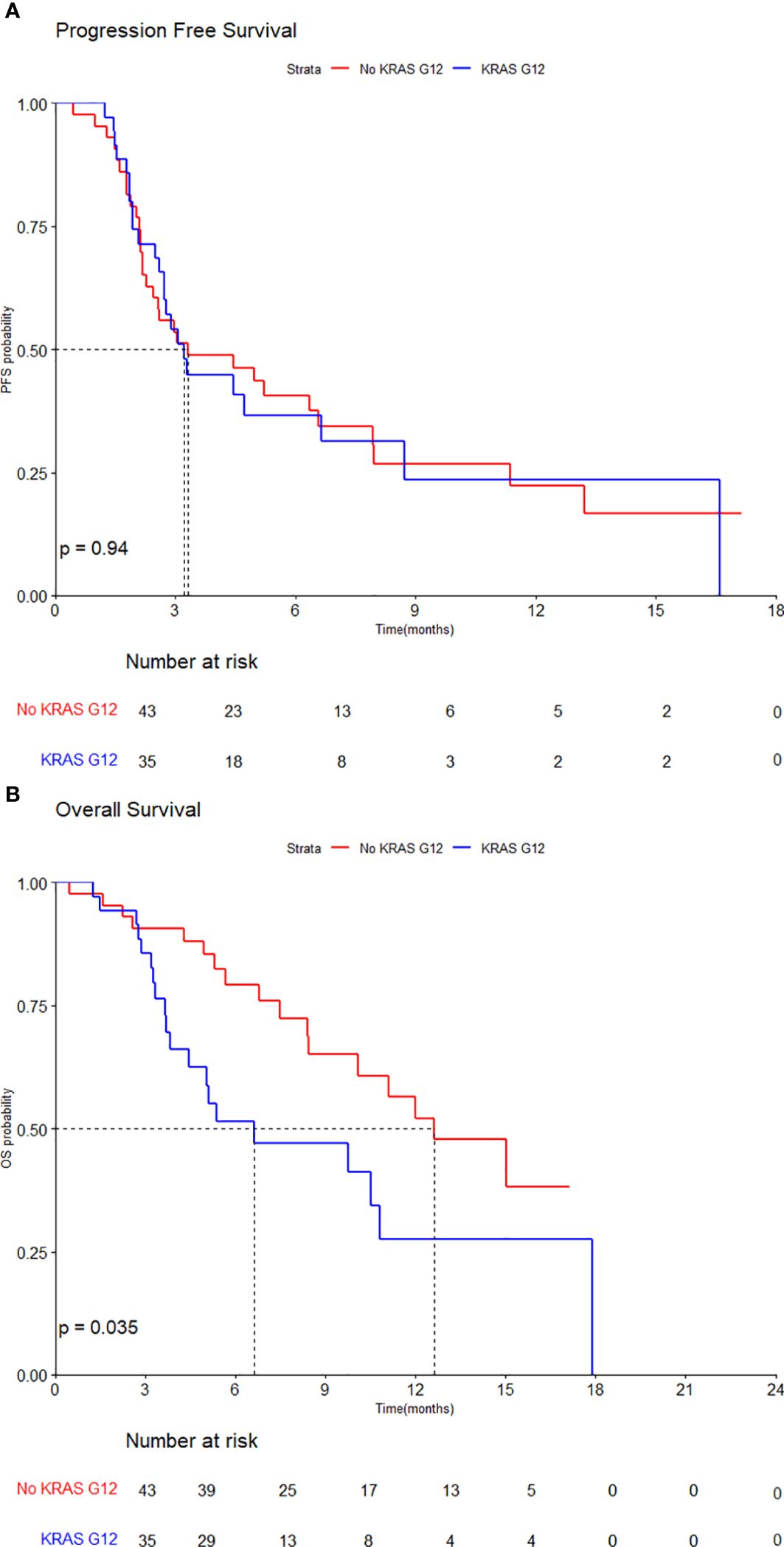
Figure 3. Kaplan–Meier curves by KRAS G12 mutation for (A) progression-free survival and (B) overall survival.
However, the median OS of total population was 10.8 months (95% CI, 10.1–NA), with the median OS of 6.6 months (95% CI, 4.5–NA) in KRAS G12 group and 12.6 months (95% CI, 10.1–NA) in No KRAS G12 group. The OS of TAS-102 + Bev was statistically significantly inferior in patients with the KRAS G12 mutation (HR, 2.01, 95% CI, 1.04–3.90, p=0.035) (Figure 3B).
3.4 Safety profile of TAS-102 with and without bevacizumabIn the two groups, commonly observed adverse events (AEs) were hematologic in nature, including neutropenia, anemia, and thrombocytopenia, as well as nausea (Table 3). While any grade neutropenia was observed at similar frequencies in the groups (57.8% and 57.1%), grade 3 or higher neutropenia was more frequently observed in the TAS-102 + Bev group than the TAS-102-alone group (31.3% vs. 17.9%).
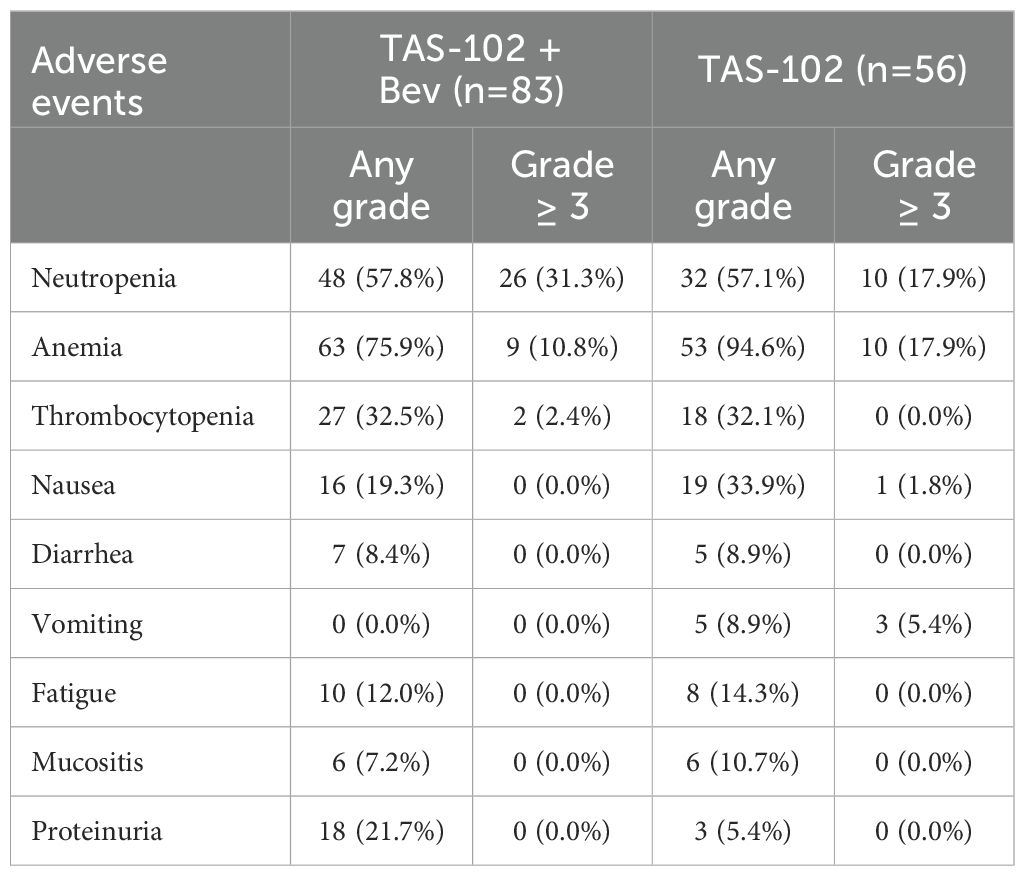
Table 3. Adverse events by treatments.
For non-hematologic AEs, incidence rates were similar between the two groups, except for proteinuria. Grade 3 or higher non-hematologic AEs were rare. Proteinuria was infrequent in the TAS-102 group but occurred in 21.7% of the TAS-102 + Bev group, although all cases were lower than grade 3.
3.5 Subsequent treatments after response failure to TAS-102 with and without bevacizumab treatmentAmong the 83 patients who received TAS-102 + Bev, 36 underwent subsequent anticancer therapy. Excluding nine patients who received prior regorafenib, 20 of the remaining 27 patients received regorafenib as the subsequent therapy. Of nine patients who had received regorafenib previously, eight were treated with a 5FU-based regimen (five received capecitabine, two received 5-FU/LV, and one received irinotecan/capecitabine), while the remaining patient was re-treated with regorafenib.
4 DiscussionIn this study, we demonstrated that TAS-102 plus bevacizumab provided a meaningful survival benefit and had a manageable safety profile compared to TAS-102 alone as a salvage regimen in patients with refractory metastatic CRC, consistent with the findings of previous studies. The median PFS in this analysis was relatively shorter than that reported in the SUNLIGHT study. In SUNLIGHT, 92.1% of patients had received only two prior therapies, whereas 51.8% of patients in this analysis had received at least three prior therapies and 23.7% had received four or more prior therapies (14). The differences in patient populations analyzed in the two studies may have contributed to the differences in PFS between these two studies.
TAS-102 with anti-VEGF therapy has been shown to have an anti-tumor effect in preclinical studies. The combination of TAS-102 with bevacizumab increases phosphorylated trifluridine levels within tumors while avoiding an increase in systemic trifluridine exposure (15). This enhances antitumor efficacy and extends survival, highlighting the superiority of TAS-102 plus bevacizumab over TAS-102 alone.
TAS-102 plus bevacizumab had survival benefits in all subgroups except patients with ECOG PS ≥2. Among nine patients with ECOG PS of 2 or higher, three received TAS-102 + Bev treatment, whereas six received TAS-102 only. Most of these patients received the treatment for two or fewer cycles, with a PFS shorter than two months and OS shorter than five months. However, one patient in the TAS-102 group was a notable long survivor with a PFS of 3.8 months and an OS of 18.6 months, which is believed to be the cause of the hazard ratio inversion. In subgroup analysis, patients, irrespective of their RAS mutation status, showed better survival outcomes when treated with TAS-102 + Bev. This finding aligns with previous prospective studies (16). Additionally, patients with prior bevacizumab and regorafenib treatment had favorable survival outcomes after TAS-102 + Bev treatment.
In previous preclinical studies, it has been confirmed that CRC can have different disease entities depending on their codon-specific KRAS mutation (17). Furthermore, recent studies showed that the KRAS G12 mutation can act as a biomarker for reduced OS benefit from TAS-102 treatment in CRC patients (18). In this study, the OS of TAS-102 + Bev was inferior in patients with the KRAS G12 mutation compared to those without the mutation. Similar to its role as a potential biomarker for poor outcomes in TAS-102 treatment of CRC, this study demonstrates that the KRAS G12 mutation can also serve as a biomarker when TAS-102 is combined with bevacizumab. Considering that TAS-102 + Bev is used as a salvage option in metastatic CRC and can be recommended with other options such as regorafenib or capecitabine, selecting patients who would benefit more from TAS-102 + Bev through codon-specific KRAS mutation analysis would be highly advantageous in clinical practice. However, given the small sample size of this cohort, these findings should be interpreted with caution and further prospective study with large cohort will be necessary.
Safety profiles were comparable between the two groups, although a higher incidence of grade 3 or higher neutropenia was observed in the TAS-102 + Bev group than the TAS-102 group. This might be related to increased phosphorylated trifluridine accumulation facilitated by bevacizumab, as suggested by preclinical studies (15). Furthermore, the risk of severe neutropenia in the TAS-102 + Bev group might potentially have been increased by the longer duration of therapy and by concentration of phosphorylated trifluridine in hemopoietic cells.
TAS-102 plus bevacizumab is recommended as one of the standard treatment options in metastatic CRC patients that have progressed following first- and second-line therapies involving 5-FU plus oxaliplatin or irinotecan along with anti-EGFR or VEGFR therapies when excluding biomarker-directed and immunotherapy. Regorafenib is also considered a standard option in this clinical setting. Regorafenib was demonstrated to have efficacy in the CORRECT trial (OS of 6.4 months for regorafenib vs. 5.0 months for placebo; HR, 0.77; 95% CI, 0.64–0.94; p= .005; PFS of 1.9 months vs. 1.7 months; HR, 0.49; 95% CI, 0.42–0.58; p <.000001) and the CONCUR trial (OS of 8.8 months for regorafenib vs. 6.3 months for placebo; HR, 0.55; 95% CI, 0.40–0.77; p <.001) (19, 20). TAS-102 alone and regorafenib have shown similar survival outcomes as third-line or later treatments in heavily pretreated metastatic CRC populations (21–24). However, the common adverse events of the two agents are different, and TAS-102 is better tolerated with fewer severe adverse effects than regorafenib, which can lead to higher adherence and compliance among patients (25). In this study, 74.1% of patients received regorafenib after failure with TAS-102 plus bevacizumab. Further research is needed to evaluate the optimal sequence of salvage therapies with regard to TAS-102 plus bevacizumab and regorafenib (26–28).
This study had several limitations. First, data were obtained from a single center and were retrospective in nature, which could have resulted in bias. In particular, AEs were determined from EMRs, where they may not have been well documented, leading to a possibility of underestimation. Second, there were differences in patient populations between the TAS-102 + Bev and TAS-102 groups. These differences could have affected the survival outcomes. Third, potential interventions such as palliative primary tumor resection, metastasectomy, or radiation therapy during treatment may have interfered with survival outcomes.
The combination of TAS-102 and bevacizumab resulted in better survival outcomes than TAS-102 monotherapy, consistent with previous studies. Our findings support the use of TAS-102 and bevacizumab in combination as the best therapeutic option for patients with refractory metastatic colorectal cancer in clinical practice.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by Institutional Review Board at Samsung Medical Center (IRB No. 2024-03-116). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributionsJS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. SL: Supervision, Validation, Writing – review & editing. JL: Supervision, Validation, Writing – review & editing. HL: Supervision, Validation, Writing – review & editing. YP: Supervision, Validation, Writing – review & editing. SK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. (2020) 70:145–64. doi: 10.3322/caac.21601
PubMed Abstract | Crossref Full Text | Google Scholar
3. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. (2016) 27:1386–422. doi: 10.1093/annonc/mdw235
PubMed Abstract | Crossref Full Text | Google Scholar
4. Nielsen DL, Palshof JA, Larsen FO, Jensen BV, Pfeiffer P. A systematic review of salvage therapy to patients with metastatic colorectal cancer previously treated with fluorouracil, oxaliplatin and irinotecan +/– targeted therapy. Cancer Treat Rev. (2014) 40:701–15. doi: 10.1016/j.ctrv.2014.02.006
PubMed Abstract | Crossref Full Text | Google Scholar
6. Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. New Engl J Med. (2015) 372:1909–19. doi: 10.1056/NEJMoa1414325
PubMed Abstract | Crossref Full Text | Google Scholar
7. Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, et al. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev. (2020) 86:102017. doi: 10.1016/j.ctrv.2020.102017
PubMed Abstract | Crossref Full Text | Google Scholar
8. Cunningham D, Lang I, Marcuello E, Lorusso V, Ocvirk J, Shin DB, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomized phase 3 trial. Lancet Oncol. (2013) 14:1077–85. doi: 10.1016/S1470-2045(13)70154-2
PubMed Abstract | Crossref Full Text | Google Scholar
9. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New Engl J Med. (2004) 350:2335–42. doi: 10.1056/NEJMoa032691
PubMed Abstract | Crossref Full Text | Google Scholar
10. Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. (2008) 26:2013–9. doi: 10.1200/JCO.2007.14.9930
PubMed Abstract | Crossref Full Text | Google Scholar
11. Cartwright TH, Yim YM, Yu E, Chung H, Halm M, Forsyth M. Survival outcomes of bevacizumab beyond progression in metastatic colorectal cancer patients treated in US community oncology. Clin Colorectal Cancer. (2012) 11:238–46. doi: 10.1016/j.clcc.2012.05.005
PubMed Abstract | Crossref Full Text | Google Scholar
12. Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomized phase 3 trial. Lancet Oncol. (2013) 14:29–37. doi: 10.1016/S1470-2045(12)70477-1
PubMed Abstract | Crossref Full Text | Google Scholar
13. Kuboki Y, Nishina T, Shinozaki E, Yamazaki K, Shitara K, Okamoto W, et al. TAS-102 plus bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (C-TASK FORCE): an investigator-initiated, open-label, single-arm, multicenter, phase 1/2 study. Lancet Oncol. (2017) 18:1172–81. doi: 10.1016/S1470-2045(17)30425-4
PubMed Abstract | Crossref Full Text | Google Scholar
14. Prager GW, Taieb J, Fakih M, Ciardiello F, Van Cutsem E, Elez E, et al. Trifluridine-tipiracil and bevacizumab in refractory metastatic colorectal cancer. N Engl J Med. (2023) 388:1657–67. doi: 10.1056/NEJMoa2214963
PubMed Abstract | Crossref Full Text | Google Scholar
15. Tsukihara H, Nakagawa F, Sakamoto K, Ishida K, Tanaka N, Okabe H, et al. Efficacy of combination chemotherapy using a novel oral chemotherapeutic agent, TAS-102, together with bevacizumab, cetuximab, or panitumumab on human colorectal cancer xenografts. Oncol Rep. (2015) 33:2135–42. doi: 10.3892/or
PubMed Abstract | Crossref Full Text | Google Scholar
16. Zhou M, Yu P, Qu J, Chen Y, Zhou Y, Fu L, et al. Efficacy of bevacizumab in the first-line treatment of patients with RAS mutations metastatic colorectal cancer: a systematic review and network meta-analysis. Cell Physiol Biochem. (2016) 40:361–9. doi: 10.1159/000452551
PubMed Abstract | Crossref Full Text | Google Scholar
18. van de Haar J, Ma X, Ooft SN, van der Helm PW, Hoes LR, Mainardi S, et al. Codon-specific KRAS mutations predict survival benefit of trifluridine/tipiracil in metastatic colorectal cancer. Nat Med. (2023) 29:605–14. doi: 10.1038/s41591-023-02240-8
PubMed Abstract | Crossref Full Text | Google Scholar
19. Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicenter, randomized, placebo-controlled, phase 3 trial. Lancet. (2013) 381:303–12. doi: 10.1016/S0140-6736(12)61900-X
PubMed Abstract | Crossref Full Text | Google Scholar
20. Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2015) 16:619–29. doi: 10.1016/S1470-2045(15)70156-7
PubMed Abstract | Crossref Full Text | Google Scholar
21. Nevala-Plagemann C, Sama S, Ying J, Shen J, Haaland B, Florou V, et al. A real-world comparison of regorafenib and trifluridine/tipiracil in refractory metastatic colorectal cancer in the United States. J Natl Compr Cancer Netw. (2023) 21:257–64. doi: 10.6004/jnccn.2022.7082
Crossref Full Text | Google Scholar
22. Masuishi T, Taniguchi H, Hamauchi S, Komori A, Kito Y, Narita Y, et al. Regorafenib versus trifluridine/tipiracil for refractory metastatic colorectal cancer: A retrospective comparison. Clin Colorectal Cancer. (2017) 16:e15–22. doi: 10.1016/j.clcc.2016.07.019
PubMed Abstract | Crossref Full Text | Google Scholar
23. Moriwaki T, Fukuoka S, Taniguchi H, Takashima A, Kumekawa Y, Kajiwara T, et al. Propensity score analysis of regorafenib versus trifluridine/tipiracil in patients with metastatic colorectal cancer refractory to standard chemotherapy (REGOTAS): A Japanese society for cancer of the colon and rectum multicenter observational study. Oncologist. (2018) 23:7–15. doi: 10.1634/theoncologist.2017-0275
PubMed Abstract | Crossref Full Text | Google Scholar
24. Abrahao ABK, Ko YJ, Berry S, Chan KKW. A comparison of regorafenib and TAS-102 for metastatic colorectal cancer: A systematic review and network meta-analysis. Clin Colorectal Cancer. (2018) 17:113–20. doi: 10.1016/j.clcc.2017.10.016
PubMed Abstract | Crossref Full Text | Google Scholar
25. Patel AK, Duh MS, Barghout V, Yenikomshian MA, Xiao Y, Wynant W, et al. Real-world treatment patterns among patients with colorectal cancer treated with trifluridine/tipiracil and regorafenib. Clin Colorectal Cancer. (2018) 17:e531–e9. doi: 10.1016/j.clcc.2018.04.002
PubMed Abstract | Crossref Full Text | Google Scholar
26. Bekaii-Saab T, Kim R, Kim TW, O’Connor JM, Strickler JH, Malka D, et al. Third- or later-line therapy for metastatic colorectal cancer: reviewing best practice. Clin Colorectal Cancer. (2019) 18:e117–e29. doi: 10.1016/j.clcc.2018.11.002
PubMed Abstract | Crossref Full Text | Google Scholar
27. Walter T, Hawkins NS, Pollock RF, Colaone F, Shergill S, Ross PJ. Systematic review and network meta-analyses of third-line treatments for metastatic colorectal cancer. J Cancer Res Clin Oncol. (2020) 146:2575–87. doi: 10.1007/s00432-020-03315-6
PubMed Abstract | Crossref Full Text | Google Scholar
28. Fernandez-Montes A, Gravalos C, Pericay C, Safont MJ, Benavides M, Elez E, et al. Current options for third-line and beyond treatment of metastatic colorectal cancer. Spanish TTD Group Expert Opinion. Clin Colorectal Cancer. (2020) 19:165–77. doi: 10.1016/j.clcc.2020.04.003
留言 (0)