Immune checkpoint inhibitors (ICIs) have changed the landscape of management of several cancers harnessing anti-tumor adaptive immunity by inhibiting key immune system inactivators such as CTLA-4, PD-1, and PDL-1. However, the robust immune response could act against self-antigens leading to significant toxicity. Cardiovascular toxicity is one of the primary forms of toxicity that often leads to treatment discontinuation. Most of the evidence on cardiovascular toxicity of ICIs has been limited to case series and systematic reviews, and little was known about ICI re-challenge post-cardiotoxicity. This review will highlight the available evidence on the most common ICI-related cardiotoxicity and their reintroduction.
Types of CardiotoxicityIpilimumab (CTLA-4 inhibitor), Pembrolizumab/Nivolumab/Durvalumab (PD-1 inhibitors), and Atezolizumab (PD-L1 inhibitor) are the commonly used immune checkpoint inhibitors [1]. More recent ICIs include PD-1 inhibitors such as Dostarlimab [2] and Cemiplimab [3]. ICI-related cardiotoxicity has varied clinical manifestations, with myocarditis, pericarditis, cardiomyopathy, and arrhythmia being the commonly described ones. Other toxicities include vasculitis, hypertension, and atherosclerosis. A study has shown a three-fold higher risk of aortic plaque progression and coronary atherosclerosis leading to myocardial infarction and coronary revascularization [4]. Although myocarditis is potentially fatal, the treatment limitations of other cardiac manifestations have implications for further treatment continuation. According to a study, the most common cardiac complication was heart failure (17%), followed by myocarditis (15%) and pericarditis (13%) with myocarditis carrying the highest mortality rate [5]. Coronary artery vasculitis was reported in a few studies.
MyocarditisCTLA-4 and PD-1, which are co-inhibitory molecules on T cells [6], are presumed to have a homeostatic role in the myocardium based on the pre-clinical data. For example, loss of CTLA-4 or PD-1 in mice induces spontaneous myocarditis [7, 8]. CTLA-4 is innately present on regulatory T cells, and inhibiting it promotes T cell stimulation [9]. Evolving data suggests that blocking PD-1 from binding with PD-L1 on cardiac myocytes triggers T cells against the myocardium [9]. Johnson et al hypothesized that a selective T-cell clone might attack the myocardium due to a common antigen between the tumor and myocardium, similar to the shared antigen theory [10]. Another theory is the development of autoantibodies causing myocarditis while on ICIs, as evidenced by pathology showing autoantibodies in a study [11, 12]. An autopsy study demonstrated predominant CD4+ T cell infiltration in the heart of patients treated with CTLA-4 inhibitor compared to CD8+ T cell infiltration in those treated with a PD-1 inhibitor [13]. It was proposed that pre-exposure to chemotherapy or radiotherapy could liberate cardiac antigens leading to enhanced ICI-related cardiotoxicity [14].
Incidence and prevalence of myocarditis varied greatly between studies owing to misclassification bias and lack of timely cardiac monitoring. The true incidence is difficult to estimate. For instance, a study has shown that concomitant treatment with Nivolumab and Ipilimumab causes myocarditis in 0.27% of patients vs. 0.06% in patients receiving only Ipilimumab [10]. In contrast, another study estimated the prevalence to be 1.14% [15]. A study estimated that there has been a 42% increase in the patient pool who qualify for ICIs from 2011 to 2018 [16]. It is unclear if this widened patient pool leads to an increased prevalence of myocarditis. The average onset time was 17 days between receiving ICIs and the development of myocarditis [10], in contrast to another study, which found that the average onset time was 34 days ranging from 1–3 months [15]. Another retrospective study demonstrated an average emergence time of 27 days, with 76% of cases reported within 6 weeks of starting treatment [17]. It is unclear if exposure to a different ICI, before the actual ICI that triggered the myocarditis, decreases the onset time of myocarditis, as evident in a few case reports [18].
Also, concomitant administration of CTLA-4 and PD-1/PDL-1 inhibition and diabetes mellitus were distinct predisposing factors for the development of ICI-associated myocarditis [15]. It is unclear if the type of cancer, pre-existing cardiac pathology, and autoimmune diseases increased the risk of myocarditis [19–22]. In a systematic review involving 88 cases, dyspnea (49%), followed by fatigue (25%), and chest pain (17%) were the most commonly reported symptoms [23–26]. Interestingly, smoldering myocarditis with little to no symptoms was reported [27].
Diagnosis of ICI-Related MyocarditisIn most cases of myocarditis, clinical picture and cardiac MRI (CMRI) help presume the diagnosis, though a gold standard procedure, endomyocardial biopsy (EMB) is seldom done due to its invasive nature [28].
Numerous definitions were proposed for the diagnosis of ICI-related myocarditis. Common terminology criteria for adverse events (CTCAE) has been historically used until Bonaca et al [29], put forward a criteria for standard assessments. More recently, the International Cardi-Oncology Society (IC-OS) proposed a criteria as in Table 1 below [30]. IC-OS criteria are binary, do not include PET scan, and rather include immune-related adverse events (irAEs) affecting other organ systems compared to criteria by Bonaca et al. In addition, a study has proposed including an onset time of symptoms of less than 3 months in the minor criteria to improve the specificity of IC-OS definition [31].
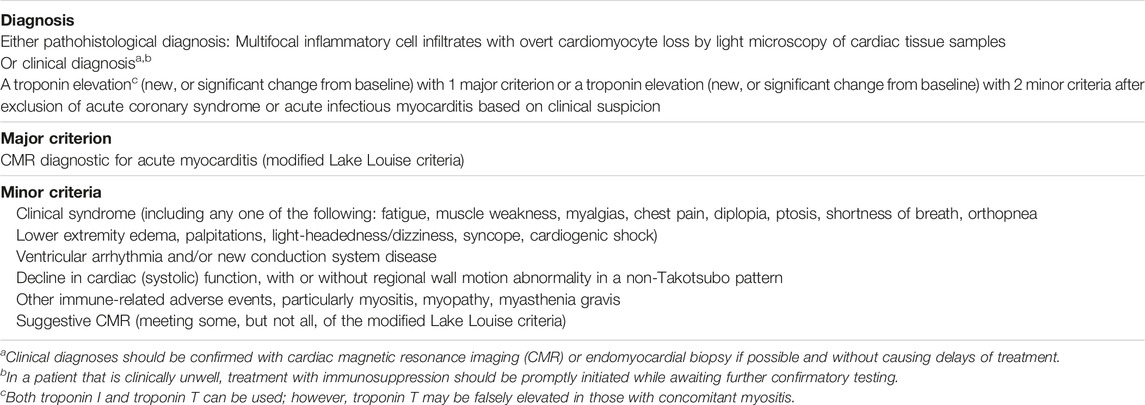
TABLE 1. [30] International Cardio-Oncology Society Consensus 2021 definition for immune checkpoint inhibitor myocarditis. Reprinted from defining cardiovascular toxicities of cancer therapies: An International Cardio-Oncology Society (IC-OS) consensus statement by Herrmann et al. (2021).
EKG: No specific finding on EKG was diagnostic of ICI myocarditis and majority of case reports showed widely variable findings like T wave inversions, sinus tachycardia, non-specific ST-T changes, QT prolongation, and even Torsades [32]. An abnormal ECG was found in 89% of myocarditis patients in a study [15]. Also, the worst prognostic features such as high grade heart block and ventricular arrhythmias were found in 28% and 22% of patients [26].
Cardiac BiomarkersTroponin is crucial in the diagnosis of ICI-related myocarditis. Obtaining baseline troponin I is recommended in all patients before initiating ICIs [1]. A systematic review has shown elevated troponin in 42 out of 43 cases [26]. Normalization of troponin levels was observed in patients who responded to immunosuppressive therapy [27, 33, 34]. In contrast, an increasing troponin level is not always suggestive of or diagnostic of myocarditis, mandating comprehensive evaluation [35]. Nevertheless, ≥1.5 ng/mL troponin is linked to major adverse cardiac events [15]. As outlined in the IC-OS definition, troponin is a requisite for clinical diagnosis of ICI-related myocarditis. While studies suggested serial troponin monitoring for early diagnosis of ICI-related myocarditis, caution is advised in clinical interpretation due to significant false positives and inadvertent interruption in therapy [36]. In addition to troponin, CK-MB and CK levels were raised in many cases [18, 25, 26, 37]. Higher CK and CK-MB levels were linked to worse mortality [36]. Elevated BNP/NT pro-BNP [15, 25, 26, 43] and anti-striated muscle antibodies [19] are associated with the onset of myocarditis. Therefore, obtaining a baseline BNP/NT pro-BNP is recommended per European Society of Cardiology (ESC) guidelines [1].
Transthoracic Echocardiogram (TTE)A multicentre registry showed preserved ejection fraction (EF) in 51% of cases [15], and it was unclear if preserved EF correlates well with a worse prognosis. A systematic review reported that 8 out of 13 cases with preserved EF died eventually [26]. It was also unclear if low EF leads to high mortality. In patients with a low risk of myocarditis, the global longitudinal strain could have a role, especially when EF is a poor diagnostic and prognostic indicator [38]. Also, a retrospective study of 101 patients showed that global longitudinal strain was lower in myocarditis compared to controls [39]. More recently, a study has shown that a reduction in longitudinal strain was linked to elevated troponin I levels [40]. A baseline TTE is recommended in patients who are at high risk of developing ICI-related myocarditis [1].
Cardiac MRI (CMRI)CMRI is widely used to diagnose myocarditis. The characteristic findings include late gadolinium enhancement (LGE) with myocardial wall edema on T2-weighted imaging. A recent original research article involving six patients showed LGE in five out of six cases [18]. In contrast, another recent international registry study showed LGE in 48% of patients with ICI-associated myocarditis which warrants caution when relying on CMRI alone [41]. This low sensitivity could be explained by cases involving scattered to low-grade [40] myocardial inflammation [42].
Fluorodeoxyglucose-Positron Emission Tomography (FDG-PET)Due to the lack of sensitivity of CMRI in cases with little to no myocardial inflammation, researchers have evaluated FDG-PET for its applicability. In a prospective study, FDG-PET complemented CMRI findings in 65 patients who were evaluated for possible myocarditis [43]. FDG-PET has been included in the diagnostic criteria by Bonaca et al.
Endomyocardial BiopsyEndomyocardial biopsy has been considered the gold standard technique in diagnosing myocarditis from ICIs; however, this may not be feasible in all clinical scenarios. In a systematic review of 26 cases (14 cases underwent biopsy during angiography and 12 cases during autopsy), the biopsy showed predominantly lymphocytic myocarditis along with other cells. CD8+ve T cells were the major group of lymphocytes, along with occasional CD4+ve T cells [26]. Autopsy in a few case reports showed CD3+ T cells [44]. An autopsy showed lymphocytic myocarditis with patchy fibrosis with a confirmation of non-infectious etiology [45]. Despite being a gold standard diagnostic study, EMB could miss the diagnosis in patients presenting with focal myocardial inflammation.
Grades of MyocarditisG1: Abnormal cardiac biomarker testing, including abnormal ECG.
G2: Abnormal screening tests with mild symptoms.
G3: Moderately abnormal testing or symptoms with mild activity.
G4: Moderate to severe decompensation, IV medication or intervention required, life-threatening conditions [46].
ManagementThe treatment of ICI-associated myocarditis is extrapolated from non-immunotherapy-related myocarditis. For example, ICI-associated myocarditis commonly presents in older populations, more likely to be associated with ventricular tachycardia/ventricular fibrillation/advanced heart block and higher mortality when compared to non-ICI myocarditis. Nevertheless, advanced age, co-morbidities, and advanced cancer could explain higher mortality [47].
Most case reports and systematic reviews initiated Methylprednisolone 1–2 mg/kg when myocarditis was suspected. In patients who worsen, few studies suggested the addition of Mycophenolate mofetil (MMF) or tacrolimus [48]. Also, in patients with limited response to initial doses of steroids, Methylprednisolone 1 gm/day can be considered [46]. Some experts and a few case reports have shown the benefit of using methylprednisolone 1 g daily upfront especially in patients in which high troponin corresponds to a higher risk of significant cardiac adverse effects [18, 19]. NCCN guidelines recommend a methylprednisolone pulse dose of 1 gm/day for G3 and G4 myocarditis, and steroid tapering over 4–6 weeks until the cardiac function returns to baseline [49]. ASCO guidelines recommend holding ICI for G1 and rechecking troponins 6 h after initial elevation. Resuming ICI could be considered after normalization of troponin. For Grade ≥2 myocarditis, ASCO recommends holding and initiating high-dose corticosteroids (1–2 mg/kg/day) within 24 h. In patients without an immediate response, pulse dose methylprednisolone 1g every day should be initiated [50]. Gradual tapering over 4–6 weeks is instituted when cardiac function returns to baseline. The flow chart (Figure 1) presented is derived from preceding data and expert consensus [50, 51].
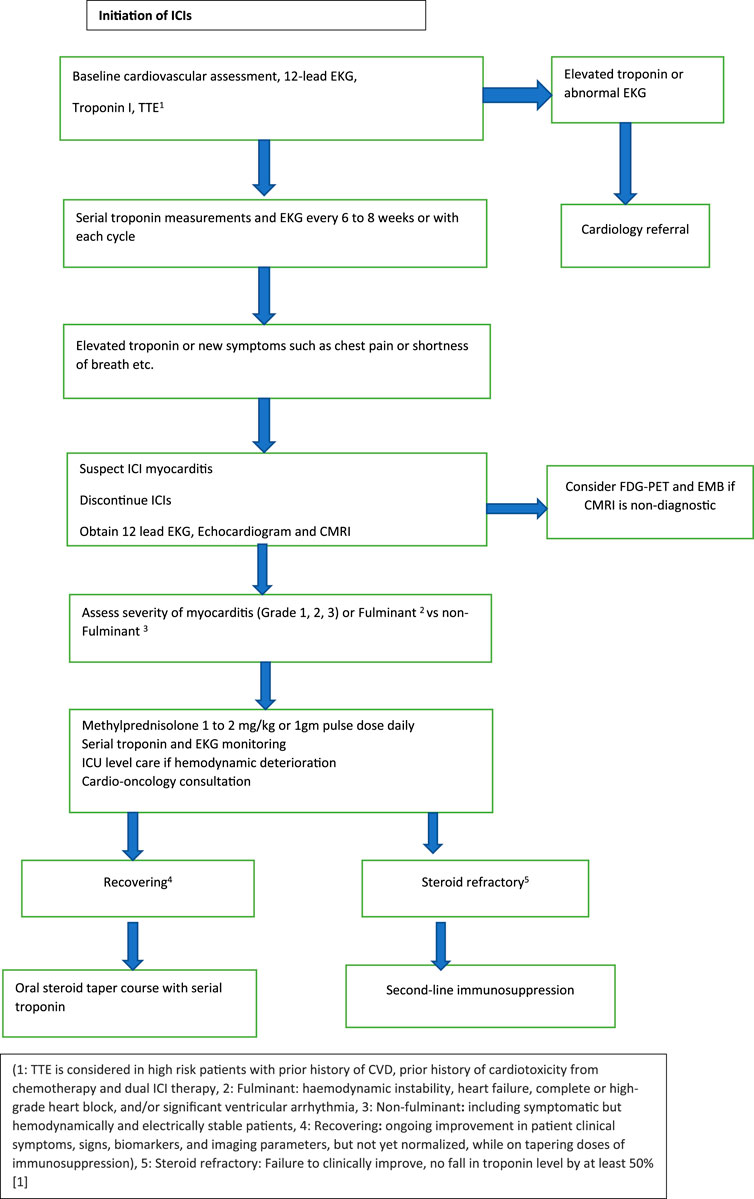
FIGURE 1. Management of ICI associated myocarditis.
Second-Line ImmunosuppressionVarious immunosuppressants have been studied for steroid-refractory cases. Steroid refractory myocarditis is defined as rising troponin or <50% reduction in troponin from peak with no clinical improvement [51]. NCCN recommends adding Antithymocyte globulin (ATG) or infliximab for G4 myocarditis if no improvement is seen within 24 h on steroids. However, it is important to note that infliximab is contraindicated in heart failure [49]. Limited evidence also suggests elevated cardiovascular death risk with infliximab [52]. In contrast, ESMO guidelines recommend adding tocilizumab or mycophenolate mofetil (MMF) as second-line agents followed by ATG or alemtuzumab or abatacept as third-line agents [51]. Additionally, ASCO guidelines recommend abatacept or alemtuzumab as additional options for life-threatening cases [50]. Resolution of steroid-refractory myocarditis from nivolumab with abatacept has been demonstrated in a case report [53]. Similarly, abatacept improved myocarditis from pembrolizumab in another case report, however, evidence is limited as the patient also received plasmapheresis [54]. Abatacept being a CTLA-4 agonist induces T cell anergy leading to suppression of inflammation, however, risks include infections and possible progression of cancer [53]. Alemtuzumab has been shown to have improved steroid refractory myocarditis from PD-1 inhibitor in a case report [55]. Alemtuzumab acts by killing peripheral immune cells however does not affect the bone marrow clone of immune cells [55]. Tocilizumab has also shown efficacy in the treatment of PD-1-associated myocarditis [56, 57]. Finally, plasmapheresis helps reduce inflammation by removing immune complexes and should be considered in life-threatening cases [58].
OutcomeIn a systematic review of 99 cases, the overall case fatality rate was 35%. Mir et al reported that complete heart block and ventricular arrhythmias are associated with poor prognosis; interestingly, steroids showed no difference in survival. These patients were managed with immunosuppressive therapies, including MMF, ATG, and intravenous immunoglobulin (IVIG) to rescue 75% (9 out of 12 cases) [59]. Another systematic review reported that 31.1% of patients did not require hospitalization and the case fatality rate was 47.2%. Normalization of EKG correlated with clinical improvement [60, 61].
Role of Re-Challenging With ICI Following ICI-Associated MyocarditisHasson et al, described three cases of lung adenocarcinoma in which patients were treated with durvalumab, pembrolizumab, and atezolizumab, respectively [37]. In this case series, all three patients were diagnosed with myocarditis and treated with Prednisone. Two patients could sustain the ICI re-challenge upon resolution of myocarditis. However, unlike the other patients who had grade 1 and grade 2 severity, the third patient suffered a fatal outcome attributed to grade 3 severity. Guo et al described a case of stage 4 melanoma where Ipilimumab and Nivolumab were reintroduced after the resolution of atezolizumab-associated myocarditis, however, it was complicated by immune-mediated nephritis and stopped [10]. Few case reports demonstrated successful reintroduction of pembrolizumab and nivolumab [32, 62, 63]. Re-challenging was suggested in the above cases based on the response of malignancy to immunotherapy, normalization of ejection fraction, and cardiac biomarkers. Most importantly, the grade of myocarditis determines the recurrence of myocarditis, and co-treatment with low-dose maintenance prednisone may have favorable outcomes [25].
Per ASCO guidelines, it is recommended to discontinue ICI after a Grade ≥2 cardiovascular toxicity [91]. ESMO guidelines suggest permanent discontinuation of ICIs for steroid-resistant myocarditis or grade 4 myocarditis [51]. However, clinicians are posed with the challenge of making the decision of re-introducing the ICI, especially in the setting of a good tumor response. A careful multidisciplinary discussion and individualized approach in each case is warranted to make the choice. If the decision is made to re-challenge a patient, monotherapy with an alternative agent could be considered. For example, a study has demonstrated a successful rechallenge of pembrolizumab in patients who developed irAEs from combined CTLA-4 + PD-1 inhibitors. In this study, only 18% had recurrent irAEs among which none had myocarditis [64]. Similarly, a patient with pembrolizumab-induced grade 4 myocarditis was re-challenged with nivolumab at a lower dose with no recurrence [65]. In contrast, a pharmacovigilance analysis among 180 patients reported that re-challenging with the same ICI or same class ICI is associated with a lower risk of recurrent irAEs [66]. Systemic monitoring for cardiovascular symptoms, coupled with surveillance for asymptomatic disease with serial troponins and periodic cardiac imaging, is recommended [58]. Recurrence of myocarditis seems to be lower than colitis and hepatitis during re-challenge as demonstrated in a pharmacovigilance cohort study [67]. Table 2 outlines case scenarios where ICIs were reintroduced after myocarditis.
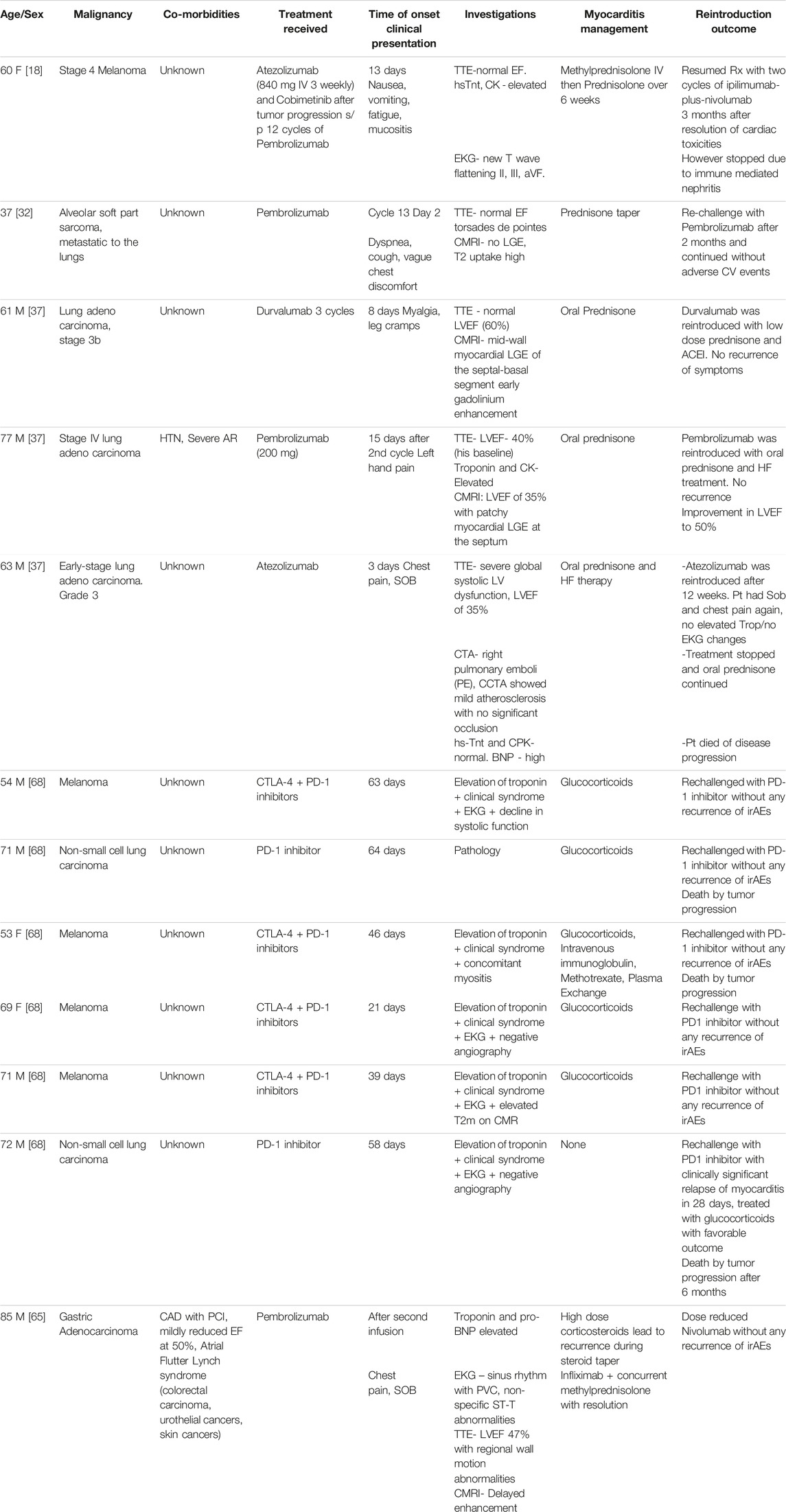
TABLE 2. ICI rechallenge for ICI-associated myocarditis.
PericarditisPericardial disease from ICIs ranges from simple inflammation to fatal tamponade. Also, peri myocarditis/myopericarditis are other presentations associated with ICI [69].
ICI-associated pericarditis probably results from ICI-stimulated T cells against the pericardium leading to inflammation. The majority of these cases are associated with lung cancer patients [70]. The hypothesis behind this disproportionately high incidence of pericardial disease in lung cancer patients is the exposure of patients previously to radiotherapy. Theoretically, radiotherapy might expose the pericardial antigens leading to enhanced T-cell binding and inflammation [71]. Furthermore, CD 68+ve cells in pericardial fluid suggested macrophage impairment as a predisposing factor [72].
Hemodynamically significant pericardial effusions were reported in 0.38% (15/3,966) of patients treated with ICI [73]. The highest prevalence was noticed with Nivolumab (0.61%) thus far followed by pembrolizumab (0.19%) and atezolizumab (0.32%) [73]. An international database of patient case reports (WHO Vigibase) showed the average time to emergence of ICI-associated pericarditis as 30 days with a mortality of 20% [71]. A pharmacovigilance study showed that clinicians diagnosed 81% of pericarditis cases as high grade on presentation [70].
Clinical presentation of pericarditis could be cardiac symptoms like chest pain and SOB or non-specific ones such as myalgia and fatigue. EKG findings are usually non-specific, and TTE/MRI shows pericardial effusion and inflammation respectively. Also important is distinguishing pericarditis or pericardial effusion from pseudo-progression. Pseudo-progression is a condition with transient worsening of tumor status before resolution [74]. This pseudo-progression likely arises from the extensive inflammation generated from activated T cells against the tumor that leads to effusion [75, 76].
HistopathologyIn most cases, pericardiocentesis revealed malignant cells along with inflammatory cells, commonly lymphocytes [77]. However, in a retrospective study on 15 patients with ICI-associated pericardial effusion, less than a third of patients had inflammatory cells in the pericardial fluid [73].
Grades of Pericarditis: (NCI-CTCAE v. 5.0)G1: Asymptomatic with EKG or physical exam (example: pericardial rub) consistent with pericarditis.
G2: Symptomatic pericarditis (example: chest pain)
G3: Pericarditis with physiologic consequences.
G4: Pericarditis with life-threatening consequences needing urgent intervention G5: Death.
ManagementA widely adopted treatment strategy that has proven effective in most instances was the temporary suspension of ICI along with the use of pericardiocentesis and corticosteroids. Additionally, successful results were reported with other treatments like MMF and TNF-alpha inhibitors. Corticosteroids alone were also associated with significant improvement [39]. Intrapericardial bleomycin (bleomycin: 15 mg/kg) [60], and pericardiectomy [55] were other commonly reported procedures. A systematic review of 28 cases of pericarditis showed that around 90% of Grade 3 to Grade 4 cases needed pericardiocentesis or pericardial window in addition to corticosteroids [78]. Based on the available evidence, ICIs could be continued for Grade 1 pericarditis. Corticosteroids are the cornerstone for Grade 2 to Grade 4 pericarditis and pericardiocentesis for moderate to large effusion. However, studying the role of non-steroidal anti-inflammatory drugs (NSAIDs) and Colchicine in ICI pericarditis is imperative. Table 3 outlines case scenarios where ICIs were reintroduced after pericarditis.
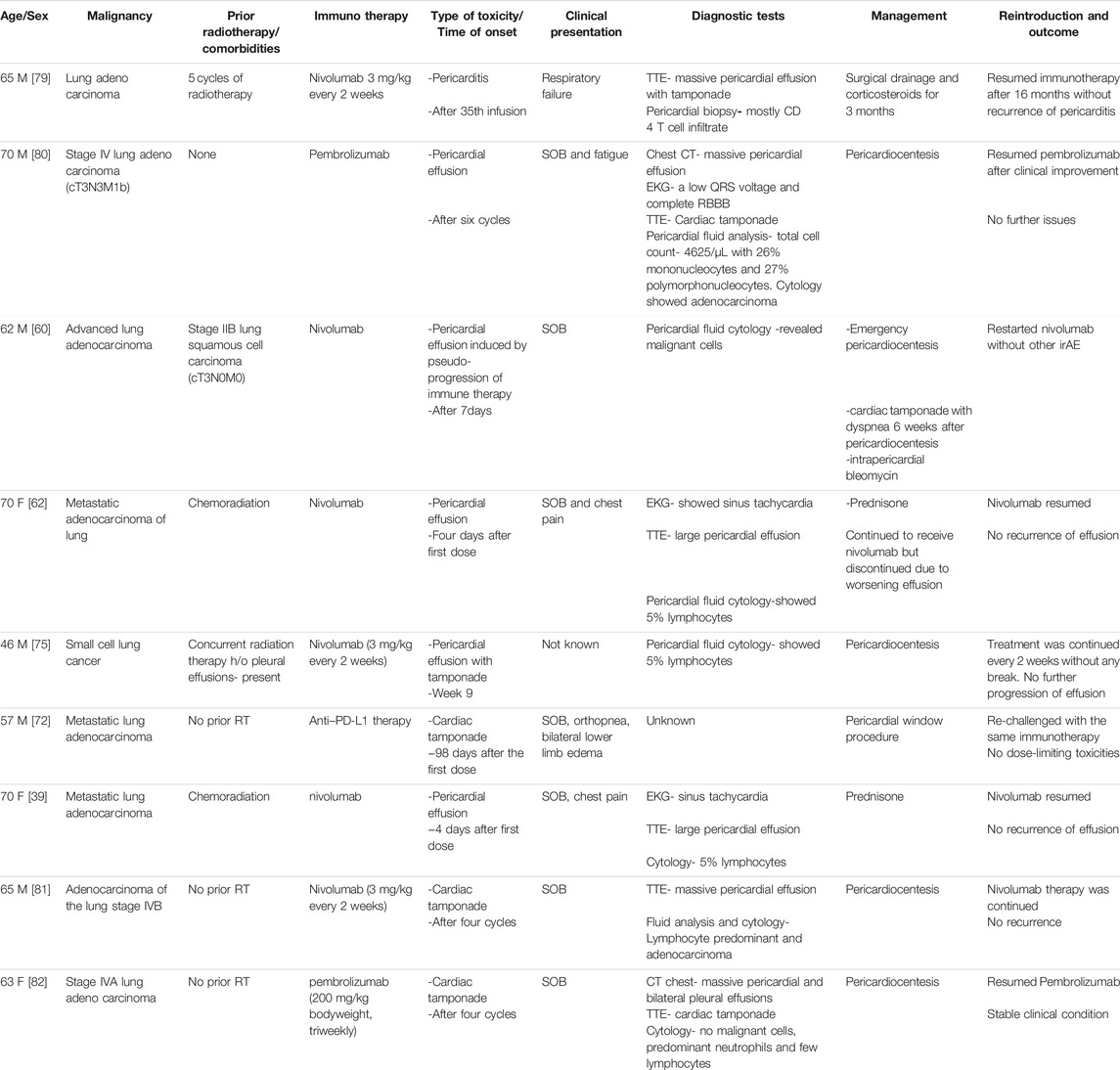
TABLE 3. ICI rechallenge for ICI associated pericarditis.
Atherosclerosis & DyslipidemiaMultiple animal and human studies have demonstrated that PD-1, PD-L1, and CTLA-4 are down regulators of atherosclerosis [83, 84]. PD-1 deficiency could potentially increase cholesterol synthesis via gene regulation [85]. A meta-analysis has shown a significant association between ICIs and dyslipidemia leading to atherosclerosis and myocardial infarction (MI). Interestingly, dyslipidemia is this study’s most common adverse effect [86]. A single-center retrospective study has shown a three-fold higher risk of aortic plaque progression and coronary atherosclerosis leading to myocardial infarction, ischemic stroke, and coronary revascularization [4]. Another study demonstrated enhanced FDG-PET uptake in large arteries after ICI treatment signifying a pro-inflammatory state [87]. The above studies did not comment on previous radiotherapy administration, making the results questionable. Due to the probable association between ICIs and inflammation leading to atherosclerosis, treatment options to reduce this risk are of priority. Drobni et al revealed that the progression of aortic plaque while on ICIs was diminished with the usage of statin [4]. Statins enhance antigen presentation to CD4+ and CD8+ T cells by reducing protein prenylation in mice models [88]. This confers an enhanced inflammatory response and might lead to a synergistic action with ICIs. Notably, omori et al and cantini et al demonstrated increased response rate, PFS, and OS with statins while on anti-PD-1 therapy [89, 90]. A single-center retrospective observational study by Buti et al evaluated the effect of concomitant medications with ICI initiation on overall response rate (ORR), progression-free survival (PFS), and overall survival (OS). Statins were associated with better OS [91]. Another observational retrospective study by cortellini et al analyzed oncologic outcomes with ICIs while on concomitant medications, statins were associated with a better objective response rate [92]. A meta-analysis of cantini et al, buti et al, cortellini et al, and two other studies where statins and ICIs were used concomitantly, revealed better overall PFS and OS. Sub-group analysis, however, showed a correlation for better OS and PFS in cases where PD-1/PD-L1 inhibitors are used [93–95]. Similar to statins, PCSK-9 inhibitors also demonstrated synergistic action with PD-1 inhibitors by suppressing tumor growth in mouse models [96]. Further studies are necessary to validate the synergistic effect of statins and PCSK-9 inhibitors with ICIs. In addition, establishing the safety profile of statins while on ICIs is crucial due to the risk of adverse effects including but not limited to myopathy.
ArrhythmiasICIs can lead to arrhythmias such as atrial fibrillation, supraventricular tachycardias, ventricular tachycardia, and heart blocks. It is important to note that these arrhythmias may arise independently due to various factors, such as concurrent myocarditis, pericarditis, or electrolyte abnormalities, and their relationship to ICIs is non-overlapping [70]. Ventricular arrhythmias are commonly seen in underlying myocarditis, making a correlation between ICIs and ventricular arrhythmias challenging [97].
Heart Failure & CardiomyopathyCardiomyopathy resulting from ICIs can take two forms: inflammatory cardiomyopathy, typically associated with underlying myocarditis and non-inflammatory cardiomyopathy. Researchers hypothesize that the non-inflammatory heart failure syndrome is a delayed side effect [1]. Non-inflammatory cardiomyopathy presents as an exclusionary diagnosis where troponin is normal and there is no inflammation on CMRI [98]. Other presentations include Takotsubo cardiomyopathy [99]. The use of corticosteroids to treat non-inflammatory heart failure syndrome appears moot due to lack of inflammation, but further studies are necessary.
VasculitisResearchers hypothesize that the etiology of vasculitis stemming from ICIs comes from augmented inflammation within the blood vessels. For instance, in a study focused on Giant Cell Arteritis (GCA), it was observed that the expression levels of PD-1 and PD-L1 were diminished in the affected temporal arteries [100]. This indicates a potential link between ICIs and the onset of vasculitis. The deficiency of PD-1 and PD-L1 contributes to excess cytokine and T-cell aggregation response [101]. A pharmacovigilance study attributed 0.2% of irAEs to vasculitis, among other cardiotoxicities. Temporal arteritis was the most commonly reported event [70]. A case series reported that ICI-related vasculitis mainly involves large arteries, especially in the central nervous system [102]. Treatment mainly involves corticosteroids [103].
Arterial and Venous ThrombosisResearchers hypothesize that a deficiency of PD-1, PD-L1, and CTLA-4 leads to enhanced inflammation culminating in arterial thrombosis (ATE) and venous thrombosis (VTE) [104]. A systematic review reported a VTE rate of 2.7% and an ATE rate of 1.1% [105]. In contrast, a recent meta-analysis did not report an increased risk of VTE in patients treated with ICIs [106]. Caution has to be exercised in attributing ICIs as the cause of thrombosis, as underlying cancer or paraneoplastic syndrome could be the primary driver of thrombosis. At this time, the role of prophylactic low-dose anticoagulation is unclear in patients treated with it. Further studies are warranted to develop a scoring system exclusively for ICI-treated patients to predict the necessity of prophylactic anticoagulation. ASCO guidelines stratify ICI-associated VTE into four grades [50].
G1: Superficial venous thrombosis.
G2: Uncomplicated deep vein thrombosis (DVT).
G3: Uncomplicated pulmonary embolism (PE).
G4: Life-threatening consequences from DVT or PE.
For G1, ASCO recommends continuing ICIs and close clinical surveillance. They recommend discontinuing ICIs and starting anticoagulation for G2 to G4 VTE. Re-initiation of ICIs might be an option after considering risks and benefits in the future for G2 to G4 toxicities [50].
ConclusionRecognizing patients at risk, their ongoing monitoring and the chance to reintroduce immunotherapy presents significant challenges, particularly for those with a terminal prognosis. The evidence supporting the re-administration of ICIs for patients who experienced cardiac toxicity is scarce, and there is a lack of expert guidance in this area. Early and continuous interdisciplinary collaboration with cardiologists and oncologists is crucial to manage this situation effectively. Further research is required to understand the roles of cardiac MRIs and FDG-PETs, particularly given the limitations associated with invasive biopsy techniques. Besides myocarditis and pericarditis, it is important to identify and manage other potential ICI-related complications such as atherosclerosis, heart failure, and myocardial infarction. This attention to detail is vital in creating a comprehensive strategy for ICI-related cardiac events.
Author ContributionsThis work was carried out in collaboration between all authors. RP gathered the literature data, wrote the initial manuscript draft. YP gathered the literature and refined the initial manuscript draft. DR put forward with the concept, reviewed and edited the final draft.
Conflict of InterestRP is a consultant and adivisor for Exelixis, Ipsen and Seagen.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary MaterialThe Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/or.2023.11456/full#supplementary-material.
Supplementary Material S1 | (A) Main messages, (B) Multiple choice questions, (C) Key references, (D) Current research questions, (E) Answers.
References1. Lyon, AR, López-Fernández, T, Couch, LS, Asteggiano, R, Aznar, MC, Bergler-Klein, J, et al. ESC Guidelines on Cardio-Oncology Developed in Collaboration With the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the Task Force on Cardio-Oncology of the European Society of Cardiology (ESC). Eur Heart J (2022) 43(41):4229–361. doi:10.1093/eurheartj/ehac244
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Mirza, MR, Chase, DM, Slomovitz, BM, dePont Christensen, R, Novák, Z, Black, D, et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. New Engl J Med (2023) 388(23):2145–58. doi:10.1056/nejmoa2216334
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Gross, ND, Miller, DM, Khushalani, NI, Divi, V, Ruiz, ES, Lipson, EJ, et al. Neoadjuvant Cemiplimab for Stage II to IV Cutaneous Squamous-Cell Carcinoma. New Engl J Med (2022) 387(17):1557–68. doi:10.1056/nejmoa2209813
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Drobni, ZD, Alvi, RM, Taron, J, Zafar, A, Murphy, SP, Rambarat, PK, et al. Association Between Immune Checkpoint Inhibitors With Cardiovascular Events and Atherosclerotic Plaque. Circulation (2020) 142(24):2299–311. doi:10.1161/circulationaha.120.049981
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Master, SR, Robinson, A, Mills, GM, and Mansour, RP. Cardiovascular Complications of Immune Checkpoint Inhibitor Therapy. J Clin Oncol (2019) 37(15):2568. doi:10.1200/jco.2019.37.15_suppl.2568
CrossRef Full Text | Google Scholar
6. Regalla, DKR, Williams, GR, and Paluri, Rk. Immune Checkpoint Inhibitors in the Management of Malignancies in Transplant Recipients. Postgrad Med J (2018) 94(1118):704–8. doi:10.1136/postgradmedj-2018-136081
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Tivol, EA, Borriello, F, Schweitzer, A, Lynch, WP, Bluestone, JA, and Sharpe, AH. Loss of CTLA-4 Leads to Massive Lymphoproliferation and Fatal Multiorgan Tissue Destruction, Revealing a Critical Negative Regulatory Role of CTLA-4. Immunity (1995) 3(5):541–7. doi:10.1016/1074-7613(95)90125-6
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Tarrio, ML, Grabie, N, Bu, D, Sharpe, AH, and Lichtman, AH. PD-1 Protects Against Inflammation and Myocyte Damage in T Cell-Mediated Myocarditis. J Immunol (2012) 188(10):4876–84. doi:10.4049/jimmunol.1200389
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Johnson, DB, Balko, JM, Compton, ML, Chalkias, S, Gorham, J, Xu, Y, et al. Fulminant Myocarditis With Combination Immune Checkpoint Blockade. N Engl J Med (2016) 375(18):1749–55. doi:10.1056/nejmoa1609214
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Xu, S, Sharma, UC, Tuttle, C, and Pokharel, S. Immune Checkpoint Inhibitors: Cardiotoxicity in Pre-Clinical Models and Clinical Studies. Front Cardiovasc Med (2021) 8:619650. doi:10.3389/fcvm.2021.619650
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Tedeschi, A, Camilli, M, Ammirati, E, Gentile, P, Palazzini, M, Conti, N, et al. Immune Checkpoint Inhibitor-Associated Myocarditis: From Pathophysiology to Rechallenge of Therapy - A Narrative Review. Future Cardiol (2023) 19(2):91–103. doi:10.2217/fca-2022-0120
PubMed Abstract | CrossRef Full Text | Google Scholar
13. Reuben, A, Petaccia de Macedo, M, McQuade, J, Joon, A, Ren, Z, Calderone, T, et al. Comparative Immunologic Characterization of Autoimmune Giant Cell Myocarditis With Ipilimumab. Oncoimmunology (2017) 6(12):e1361097. doi:10.1080/2162402x.2017.1361097
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Rubio-Infante, N, Ramírez-Flores, YA, Castillo, EC, Lozano, O, García-Rivas, G, and Torre-Amione, G. Cardiotoxicity Associated With Immune Checkpoint Inhibitor Therapy: A Meta-Analysis. Eur J Heart Fail (2021) 23(10):1739–47. doi:10.1002/ejhf.2289
PubMed Abstract | CrossRef Full Text | Google Scholar
15. Mahmood, SS, Fradley, MG, Cohen, JV, Nohria, A, Reynolds, KL, Heinzerling, LM, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol (2018) 71(16):A699–1764. doi:10.1016/s0735-1097(18)31240-3
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Haslam, A, and Prasad, V Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw Open (2019) 2(5):e192535. doi:10.1001/jamanetworkopen.2019.2535
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Moslehi, JJ, Salem, JE, Sosman, JA, Lebrun-Vignes, B, and Johnson, DB. Increased Reporting of Fatal Immune Checkpoint Inhibitor-Associated Myocarditis. The Lancet (2018) 391(10124):933. doi:10.1016/s0140-6736(18)30533-6
CrossRef Full Text | Google Scholar
18. Guo, CW, Alexander, M, Dib, Y, Lau, PK, Weppler, AM, Au-Yeung, G, et al. A Closer Look at Immune-Mediated Myocarditis in the Era of Combined Checkpoint Blockade and Targeted Therapies. Eur J Cancer (2020) 124:15–24. doi:10.1016/j.ejca.2019.09.009
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Johnson, DB, Sullivan, RJ, Ott, PA, Carlino, MS, Khushalani, NI, Ye, F, et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol (2016) 2:234. doi:10.1001/jamaoncol.2015.4368
PubMed Abstract | CrossRef Full Text | Google Scholar
22. Martinez-Calle, N, Rodriguez-Otero, P, Villar, S, Mejías, L, Melero, I, Prosper, F, et al. Anti-PD1 Associated Fulminant Myocarditis After a Single Pembrolizumab Dose: The Role of Occult Pre-Existing Autoimmunity. Haematologica (2018) 103(7):e318–e321. doi:10.3324/haematol.2017.185777
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Semper, H, Muehlberg, F, Schulz-Menger, J, Allewelt, M, and Grohé, C. Drug-Induced Myocarditis After Nivolumab Treatment in a Patient With PDL1-Negative Squamous Cell Carcinoma of the Lung. Lung Cancer (2016) 99:117–9. doi:10.1016/j.lungcan.2016.06.025
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Matson, DR, Accola, MA, Rehrauer, WM, and Corliss, RF. Fatal Myocarditis Following Treatment With the PD-1 Inhibitor Nivolumab. J Forensic Sci (2018) 63(3):954–7. doi:10.1111/1556-4029.13633
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Tadokoro, T, Keshino, E, Makiyama, A, Sasaguri, T, Ohshima, K, Katano, H, et al. Acute Lymphocytic Myocarditis With Anti-PD-1 Antibody Nivolumab. Circ Heart Fail (2016) 9(10):e003514. doi:10.1161/circheartfailure.116.003514
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Pradhan, R, Nautiyal, A, and Singh, S Diagnosis of Immune Checkpoint Inhibitor-Associated Myocarditis: A Systematic Review. Int J Cardiol (2019) 296:113–21. doi:10.1016/j.ijcard.2019.07.025
PubMed Abstract | CrossRef Full Text | Google Scholar
27. Norwood, TG, Westbrook, BC, Johnson, DB, Litovsky, SH, Terry, NL, McKee, SB, et al. Smoldering Myocarditis Following Immune Checkpoint Blockade. J ImmunoTherapy Cancer (2017) 5(1):91. doi:10.1186/s40425-017-0296-4
PubMed Abstract | CrossRef Full Text | Google Scholar
28. Kindermann, I, Barth, C, Mahfoud, F, Ukena, C, Lenski, M, Yilmaz, A, et al. Update on Myocarditis. J Am Coll Cardiol (2012) 59(9):779–92. doi:10.1016/j.jacc.2011.09.074
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Bonaca, MP, Olenchock, BA, Salem, JE, Wiviott, SD, Ederhy, S, Cohen, A, et al. Myocarditis in the Setting of Cancer Therapeutics. Circulation (2019) 140(1):80–91. doi:10.1161/circulationaha.118.034497
PubMed Abstract | CrossRef Full Text | Google Scholar
30. Herrmann, J, Lenihan, D, Armenian, S, Barac, A, Blaes, A, Cardinale, D, et al. Defining Cardiovascular Toxicities of Cancer Therapies: An International Cardio-Oncology Society (IC-OS) Consensus Statement. Eur Heart J (2021) 43(4):280–99. doi:10.1093/eurheartj/ehab674
CrossRef Full Text | Google Scholar
31. Deharo, F, Thuny, F, Cadour, F, Resseguier, N, Meilhac, A, Gaubert, M, et al. Diagnostic Value of the International Society of Cardio-Oncology Definition for Suspected Immune Checkpoint Inhibitor–Associated Myocarditis. J Am Heart Assoc (2023) 12(8):e029211. doi:10.1161/jaha.122.029211
PubMed Abstract | CrossRef Full Text | Google Scholar
32. Lee, DH, Armanious, M, Huang, J, Jeong, D, Druta, M, and Fradley, MG. Case of Pembrolizumab-Induced Myocarditis Presenting as Torsades de Pointes With Safe Re-Challenge. J Oncol Pharm Pract (2020) 26(6):1544–8. doi:10.1177/1078155220904152
PubMed Abstract | CrossRef Full Text | Google Scholar
33. Mehta, A, Gupta, A, Hannallah, F, Koshy, T, and Reimold, S. Myocarditis as an Immune-Related Adverse Event With Ipilimumab/Nivolumab Combination Therapy for Metastatic Melanoma. Melanoma Res (2016) 26(3):319–20. doi:10.1097/cmr.0000000000000251
PubMed Abstract | CrossRef Full Text | Google Scholar
34. Samara, Y, Yu, CL, and Dasanu, CA Acute Autoimmune Myocarditis and Hepatitis Due to Ipilimumab Monotherapy for Malignant Melanoma. J Oncol Pharm Pract (2019) 25(4):966–8. doi:10.1177/1078155218755868
PubMed Abstract | CrossRef Full Text | Google Scholar
35. Sarocchi, M, Grossi, F, Arboscello, E, Bellodi, A, Genova, C, Dal Bello, MG, et al. Serial Troponin for Early Detection of Nivolumab Cardiotoxicity in Advanced Non-Small Cell Lung Cancer Patients. The Oncologist (2018) 23(8):936–42. doi:10.1634/theoncologist.2017-0452
PubMed Abstract | CrossRef Full Text | Google Scholar
36. Puzanov, I, Subramanian, P, Yatsynovich, YV, Jacobs, DM, Chilbert, MR, Sharma, UC, et al. Clinical Characteristics, Time Course, Treatment and Outcomes of Patients With Immune Checkpoint Inhibitor-Associated Myocarditis. J ImmunoTherapy Cancer (2021) 9(6):e002553. doi:10.1136/jitc-2021-002553
留言 (0)