A Fetus-in-Fetu (FIF) is defined as an embryonic structure possessing a spinal column and internal organs arranged along this axis, enclosed within a normally developing fetus (1, 2). The occurrence of FIF is extremely rare, with studies suggesting an overall prevalence of approximately 1 in 500,000 (3). About 80% cases of FIF have been found in the retroperitoneum, with additional instances identified in the cranial cavity, oropharynx, neck, mediastinum, spine, and scrotum (4). The diagnosis of FIF relies on radiological examination. Typically, FIF diagnosis culminates in surgical removal as the primary treatment approach, with a generally favorable prognosis (5). However, this study represents the first documented case of intraperitoneal testicular FIF in an infant.
Case dataA male infant at four months of age presented with a mass in the left pelvic cavity detected during maternal prenatal screening at 29 weeks of gestation. The mother had no history of past miscarriages. There were no familial occurrences of twins or other abnormalities. The child was born naturally in our hospital and showed no postnatal vomiting or other gastrointestinal problems. Surgical intervention was advised by our department upon consultation, however, due to familial reasons, the guardian declined the surgery. Subsequent color Doppler ultrasonography revealed progressive enlargement of the mass, with persistent absence of the left testis and the mass shifting from the left to the right. Urgent surgical intervention was recommended. After deliberation, the guardian agreed to the surgery, but the child developed fever and respiratory tract infection symptoms. The child was hospitalized upon recovery, where a physical examination revealed a mass approximately 4.0 × 3.0 cm in the right lower abdomen with clear boundaries, good mobility, and normal bowel sounds. No palpable testicular tissue was identified in the left scrotum or inguinal area, meanwhile the right testis was palpable in the right scrotum. The child's serum levels of α-fetoprotein (AFP), carcinoembryonic antige (CEA), and β-human chorionic gonadotropin (β-HCG) were within normal ranges.
Abdominal ultrasound revealed a slightly hyperechoic mass measuring approximately 4.30 × 3.08 × 3.30 cm in the lower right abdomen, with an elongated bone fragment measuring around 2.48 cm (Figure 1A). Additionally, an irregular strong spinal echo, measuring approximately 1.91 × 1.02 cm, raised suspicions of fetal intra-abdominal calcifications (Figure 1B). Magnetic resonance imaging (MRI) of the abdominal and pelvic regions showed a cystic-solid mass with distinct boundaries measuring around 3.60 × 3.10 × 4.40 cm, containing fatty tissue. The left scrotum appeared empty (Figure 1C).
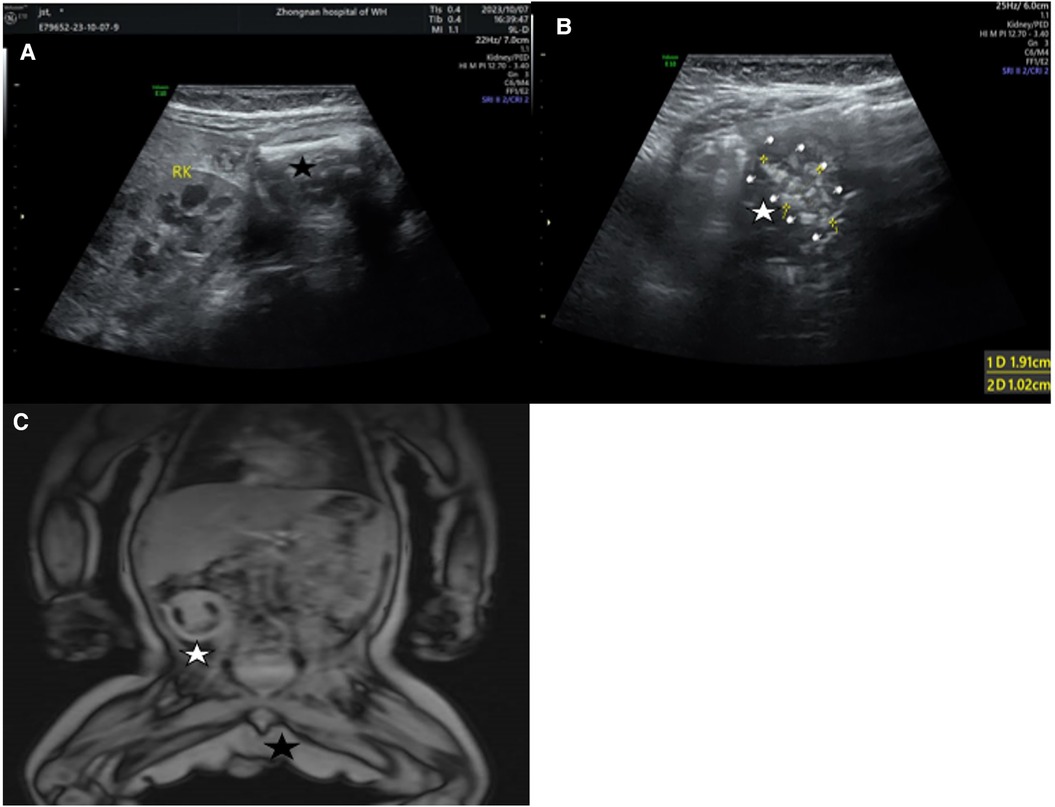
Figure 1. (A) Hyperechoic mass in the lower right abdomen and a long bone (black pentagram). (B) Irregular spinal hyperechoic area (White pentagram). (C) a cystic-solid mass in the right abdomen (White pentagram), left scrotal emptiness (Black pentagram).
Following completion of the preoperative evaluation, surgical resection of the suspected FIF was performed under general anesthesia. Intraoperatively, the tumor was found to be connected with the left spermatic cord and vas deferens, with observed torsion (Figure 2). No obvious testicular tissue was identified upon attempting tumor removal. Finally, the resection of the left testis and spermatic cord tissue was performed, while preserving the right testis and spermatic cord tissue. Postoperative pathological assessment confirmed the diagnosis of testicular FIF.
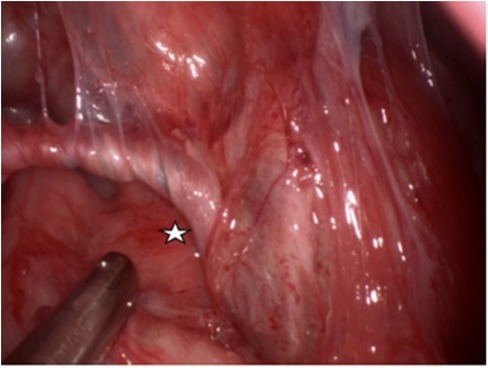
Figure 2. The left wall of the tumor is connected with the left spermatic cord and vas deferens, and the spermatic cord and vas deferens are twisted. (White pentagram).
The postoperative histopathological findings revealed a 4.5 × 3.5 × 3.5 cm gray-brown specimen resembling intact testicular tissue with sections of spermatic cord tissue measuring about 2.5 cm (Figure 3A). After dissection of the specimen, taupe and grayish-yellow tissues were observed, along with identifiable cartilage and long bone tissues. Furthermore, the presence of the spinal structure was also confirmed (Figure 3B). Microscopic examination with HE staining displayed irregular bone and cartilage proliferation (Figure 3C). Microscopic analysis showed presence of cartilage, intact bone cortex, and bone marrow cavity within the long bone. Although skin layers (epidermis, dermis), subcutaneous tissue, and digestive tract structures were observable, clear testicular parenchymal structures such as seminiferous tubules were absent.
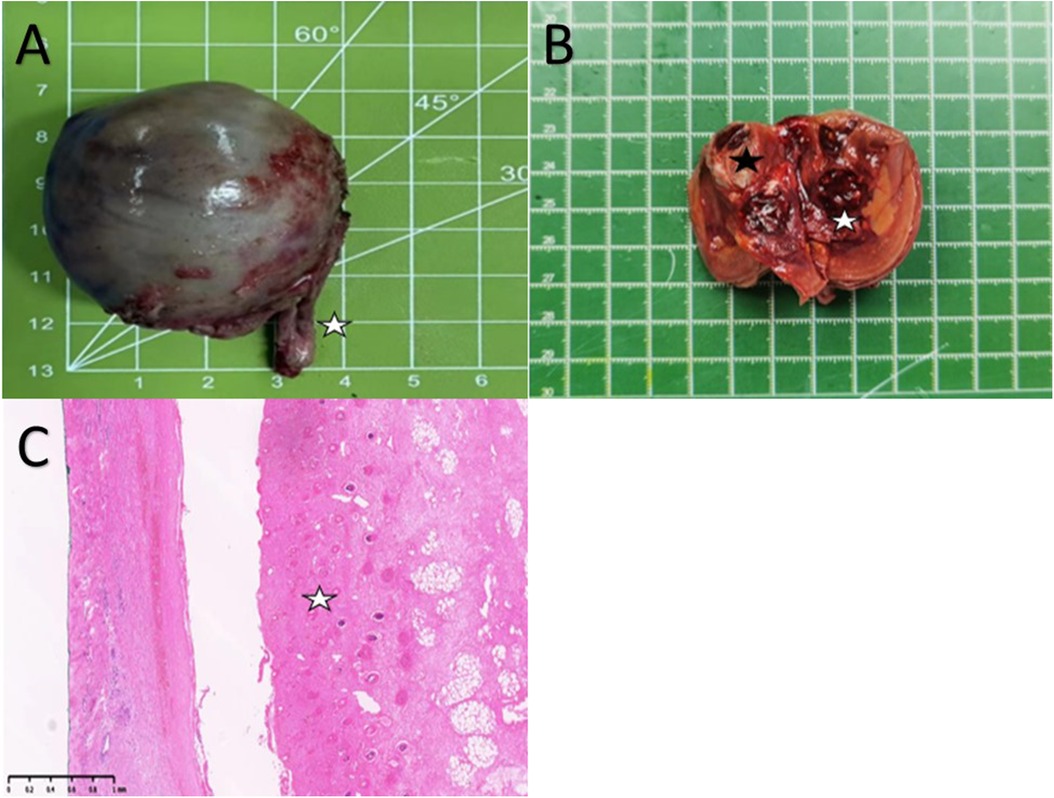
Figure 3. (A) A Spermatic cord tissue (white pentagram). (B) Cartilage-like tissue (Black pentagram) and local spinal osseous tissue (White pentagram). (C) Cartilage hyperplasia (White pentagram).
Following the surgery, the child had a smooth recovery and was discharged on the 6th postoperative day. During the 6-month follow-up period, no postoperative complications were reported.
DiscussionFIF is an extremely uncommon condition, characterized by a unique type of conjoined twins in monozygotic twins, which can be classified into endogenous FIF and exogenous FIF. Endogenous FIF is seen more commonly than exogenous fetus (6). FIF cases are more prevalent in singleton pregnancies, few in multiple pregnancies, and are typically identified in infants and young children, with very few cases occurring in adulthood (7).
The prevailing view among scholars is that FIF stems from the degeneration of twins or teratomas. The mainstream view is the monozygotic twin theory, proposing that unequal division of the fertilized egg at the early embryonic stage, resulting in the smaller inner cell mass being enveloped by the larger cell mass, which leading to the differentiation and development of the fetus. This theory can be substantiated by determining if the FIF individual and the host share the same gender, blood type, and DNA alleles (8). However, the teratoma degeneration theory suggests that teratomas could transform into highly differentiated FIF structures.
The clinical presentations of FIF are primarily associated with the space-occupying effects of masses, leading to symptoms such as vomiting, dyspnea, abdominal distension, jaundice, intestinal obstruction, and compressive damage to the kidney. The predominant clinical feature is the presence of abdominal masses during infancy and early childhood (9).
The diagnosis of FIF heavily relies on imaging studies. Spines and vertebrae are identified in only about 50% of reported cases (10). However, ultrasound is capable of directly visualizing the number of fetuses in the amniotic sac and the site of fusion of conjoined fetuses (11). CT and MRI imaging offer exceptional tissue resolution and advanced image processing capabilities, enabling precise assessment of the anatomy, fusion site, bone structure, blood supply, and vascular anatomy of early conjoined twins, facilitating surgical planning (12, 13). Considering the patient's young age and the potential radiation risks associated with CT scans, MRI was chosen for preoperative evaluation. The diagnosis of FIF necessitates specific characteristics, such as the mass being encapsulated, partially or fully covered by skin, displaying distinct anatomical structures, and being connected to the host through a pedicle containing blood vessels (14).
Distinguishing FIF from teratoma is primarily achieved through pathological examination. Teratomas typically contain hair, teeth, and some intestinal components, but lack fully developed organs like long bones, limbs, and spine that are commonly found in FIF. Compared to the differential diagnosis of teratoma, meconium pseudocyst can be differentiated through imaging studies, showcasing abdominal calcification and predominantly ascites, whereas calcification in FIF occurs in the spine and long bones, with the absence of ascites (15).
Once FIF is diagnosed, prompt surgical intervention is essential. Whether opting for traditional laparotomy or minimally invasive surgery, it is crucial to adequately expose the surgical field, identify the supplying vessels, and approach the FIF as a neoplastic entity to ensure complete excision of both the FIF and its capsule. Despite FIF being characterized as a benign condition, there exists a certain risk of malignant transformation. A documented case of malignant FIF has been reported (16). Hence, prolonged post-operative monitoring and regular reassessment are imperative for children with FIF.
ConclusionIntraperitoneal testicular FIF is a highly uncommon condition, and the underlying causes are not yet fully understood. This research is the initial attempt to extensively outline the diagnosis and management of intraperitoneal testicular FIF. Upon FIF diagnosis, prompt surgical removal is imperative, followed by routine post-operative monitoring through color ultrasound and tumor marker assessments.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by the Medical Ethics Committee of Zhongnan Hospital of Wuhan University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributionsML: Formal Analysis, Writing – original draft. GL: Investigation, Supervision, Writing – review & editing. HG: Conceptualization, Supervision, Validation, Writing – review & editing. WZ: Conceptualization, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This paper was supported by grants from Project for Team Development in the Sub-specialty of the Women's and Children's Hospital at Zhongnan Hospital of Wuhan University (XKJS202001).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AbbreviationsFIF, Fetus in fetu; MRI, magnetic resonance imaging; AFP, α-fetoprotein; CEA, serum carcinoembryonic antigen; β-HCG, serum carcinoembryonic antigen; Fig, figure.
References3. Aoki K, Matsumoto Y, Hamazaki M, Sano M, Fukumoto K, Fukaya T, et al. MRI Reveals Fetus in fetu in the mediastinum. Pediatr Radiol. (2004) 34(12):1017–9. doi: 10.1007/s00247-004-1295-4
PubMed Abstract | Crossref Full Text | Google Scholar
4. Tofigh AM, Kavyani A, Abdollahi SM, Kazemzadeh G, Salman DN. Fetus in fetu: report of a case and literature review. Int J Surg. (2008) 6(6):e94–6. doi: 10.1016/j.ijsu.2007.04.010
PubMed Abstract | Crossref Full Text | Google Scholar
5. Taher HMA, Abdellatif M, Wishahy AMK, Waheeb S, Saadeldin Y, Kaddah S, et al. Fetus in fetu: lessons learned from a large multicenter cohort study. Eur J Pediatr Surg. (2019) 30(04):343–9. doi: 10.1055/s-0039-1698765
PubMed Abstract | Crossref Full Text | Google Scholar
7. Hoeffel CC, Nguyen KQ, Phan HT, Truong NH, Nguyen TS, Tran TT, et al. Fetus in fetu: a case report and literature review. Pediatr. (2000) 105(6):1335–44. doi: 10.1542/peds.105.6.1335
PubMed Abstract | Crossref Full Text | Google Scholar
8. Chua JH, Chui CH, Sai Prasad TR, Jabcobsen AS, Meenakshi A, Hwang WS. Fetus-in-fetu in the pelvis: report of a case and literature review. Ann Acad Med Singap. (2005) 34(10):646–9.16382253
PubMed Abstract | Google Scholar
10. Gangopadhyay AN, Srivastava A, Srivastava P, Gupta DK, Sharma SP, Kumar V. Twin Fetus in fetu in a child: a case report and review of the literature. J Med Case Rep. (2010) 4:96. doi: 10.1186/1752-1947-4-96
PubMed Abstract | Crossref Full Text | Google Scholar
12. Hong SS, Goo HW, Jung MR, Kim HJ, Kim EA, Kim KS, et al. Fetus in fetu: three-dimensional imaging using multidetector CT. AJR Am J Roentgenol. (2002) 179(6):1481–3. doi: 10.2214/ajr.179.6.1791481
PubMed Abstract | Crossref Full Text | Google Scholar
14. Alekrashy MA, Khodary AR, AaA A, Elsammak AA, Elgebaly SM. A fetus excised from the small intestinal mesentery of a neonate (fetus in fetu): a case report. Ann Pediatr Surg. (2022) 18(1):5. doi: 10.1186/s43159-021-00137-0
Crossref Full Text | Google Scholar
15. Ji Y, Song B, Chen S, Jiang X, Yang G, Gao X, et al. Fetus in fetu in the scrotal sac: case report and literature review. Med. (2015) 94(32):e1322. doi: 10.1097/MD.0000000000001322
PubMed Abstract | Crossref Full Text | Google Scholar
16. Hopkins KL, Dickson PK, Ball TI, Ricketts RR, O’shea PA, Abramowsky CR. Fetus-in-fetu with malignant recurrence. J Pediatr Surg. (1997) 32(10):1476–9. doi: 10.1016/s0022-3468(97)90567-4
留言 (0)