Necrotizing enterocolitis (NEC) is a severe postnatal complication most commonly seen in preterm and low birth weight newborns and is associated with a high risk of morbidity and mortality (1). NEC is an acute inflammatory disease usually affecting parts of the small intestine. It presents with clinical findings including bloody stool, abdominal distension, and sepsis and may deteriorate with bowel necrosis and bowel perforation resulting in devasting disease (2). The etiology of NEC is multifactorial and not completely understood (2, 3). Risk factors for NEC include prematurity, low birth weight, formula feeding, hypoxic/ischemic insults, and microbial dysbiosis (1). Populations at risk for NEC include neonates with severe types of congenital heart disease (CHD) and then considered cardiogenic NEC, which has been differentiated from classical NEC of preterm newborns (4). In terms of localization, cardiogenic NEC tends to be mainly present in the colon, and NEC in preterm newborns tends to mainly involve the small intestine. In neonates with cardiogenic NEC, most frequently due to a complex type of CHD, either an unbalanced ratio of pulmonary (Qp) to systemic (Qs) perfusion or perioperative low cardiac output syndrome can lead to mesenteric hypoperfusion with hypoxic and/or ischemic results and the clinical start of NEC (5).
Besides this mesenteric hypoperfusion, the low systemic cardiac output (Qs) may also lead to cerebral hypoperfusion. Cerebral hypoxia/ischemia by itself, independent of NEC, has been described as a potential risk factor for altered brain development before and after birth (6, 7), including altered brain growth and postnatal structural alterations, of which white matter lesions and liquor space enlargement play a crucial role for impaired long-term neurodevelopmental (ND) outcome (8).
The link between NEC and impaired ND outcome may either be due to comparable hemodynamics of low cerebral and low mesenteric blood perfusion with its described consequences and/or secondary to clinical consequences of NEC such as impaired enteral diet and caloric intake and prolonged intensive care unit (ICU) and hospital length of stay. Therefore, we aimed to discriminate the impact of NEC as an additional risk factor for neurodevelopmental disorders in infants with congenital heart disease, using data from the Swiss Neurodevelopmental Outcome Registry for Children with Complex Congenital Heart Disease (ORCHID) registry (9).
Patients and methods Data sourceThe analysis is based on data from the Swiss Neurodevelopmental Outcome Registry for Children with Complex Congenital Heart Disease (ORCHID), a nationwide registry (founded in 2018) on the ND outcome of patients undergoing early neonatal cardiac surgery or hybrid palliation at <6 weeks of age (9).
PatientsWe included all consecutively registered patients of the ORCHID registry born and treated between 2019 and 2021. We grouped into those with or without the diagnosis of postoperative cardiogenic NEC according to Bell's stage ≥2, including infants with abdominal distension, blood in stool combined with bowel edema, pneumatosis, pneumoperitoneum determined by abdominal radiograph (x-ray), or abdominal ultrasound (10).
Clinical variablesWe collected and analyzed baseline characteristics, patient information, presurgical data such as type and classification of CHD, anthropometric data, sex, and periprocedural medical, surgical, and intensive care data for the first main surgical or catheter-based stage I procedure. We also documented the need for reinterventions including surgery and catheter-based procedures, complications, and clinical and ND outcome at 1 year of age [for details see also (9)].
A risk score was calculated for each patient as a cumulative score including NEC occurrence, complications as defined as “other complications” in Table 1, and need for ECMO and resuscitation. Socioeconomic status (SES) was defined as the mother's highest education level plus the father's current occupation (11).
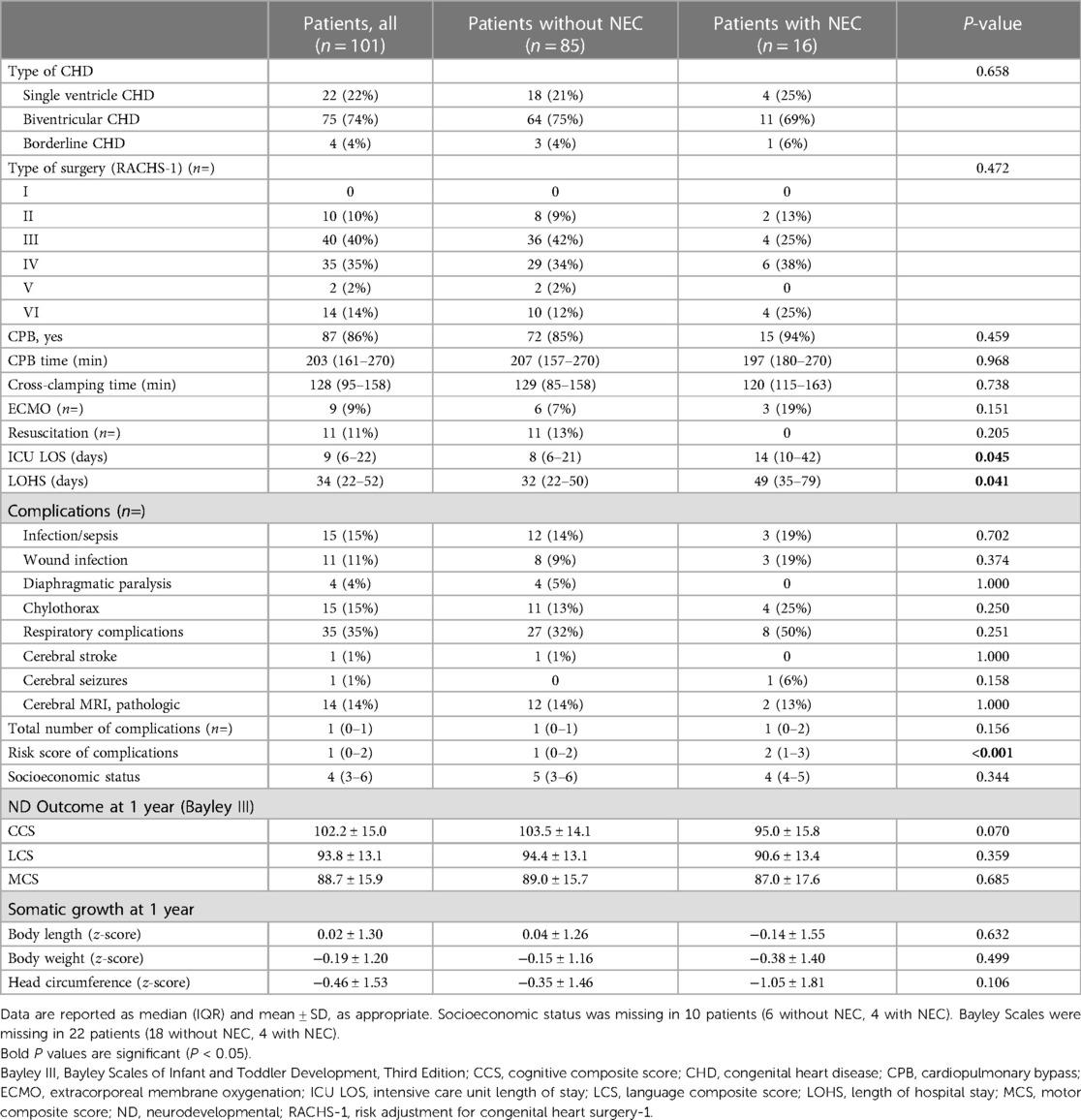
Table 1. Comparison of patients with and without NEC undergoing neonatal cardiac surgery.
Neurodevelopmental outcomeTo assess cognitive (receptive and expressive) language and (fine and gross) motor development at 1 year of age, we used the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley III), with the respective composite scores [cognitive composite score (CCS), language composite score (LCS), motor composite score (MCS)] (12).
StatisticsStatistical analyses were conducted using R (version 4.1.2, The R Software Foundation for Statistical Computing, Vienna, Austria). Univariate analysis between patients with and without NEC was conducted using an unpaired t-test for normally distributed data, Mann–Whitney U-test for non-normally distributed data, and Fisher's exact test for categorical data. The associations of NEC occurrence and risk score with ND outcome were further evaluated by applying a multivariate linear regression model, adjusting for SES and CHD categories (univentricular vs. biventricular or borderline).
EthicsThe Swiss ORCHID registry has been reviewed by the cantonal ethics committees (Req-2019-00089), and for the study analysis of the impact of NEC on ND outcome (BASEC-2022-00689).
ResultsBetween 2019 and 2021, we included 101 patients (63 female) with different types of CHD undergoing neonatal cardiac surgery within the first 6 weeks of life in the ORCHID registry. Cardiac diagnoses were CHD undergoing definitive cardiac biventricular repair (n = 75), such as D-transposition of the great arteries (TGA) with arterial switch operation or palliative stage I procedure in neonates with single ventricle CHD (n = 22), such as hypoplastic left heart syndrome (HLHS) with Norwood I procedure. In addition, patients with a borderline type of CHD (n = 4) were included. Most of the patients underwent neonatal cardiopulmonary bypass (CPB) surgery (n = 86), at a median (IQR) age of 8 (6) days, and the severity of the first surgical procedure was moderate as determined by the Risk Adjustment for Congenital Heart Surgery-1 (RACHS-1) scores 3–4 in 75% of the patients (Table 1). Median CPB time was 203 (IQR 161–270) min with a median (IQR) cross-clamping time of 128 (95–158) min at a moderate core temperature of 31.2°C ± 4.0°C. More than half of the patients (n = 53) underwent CPB surgery with selective cerebral perfusion.
After surgery, NEC occurred in 16 patients, which was the second most frequent complication after respiratory complications (n = 35) of the whole cohort (Table 1). Clinical manifestation of NEC, findings of abdominal x-ray, and abdominal ultrasound resulted in Bell’s stage II A (n = 9), stage II B (n = 6), and stage III B (n = 1), respectively (Table 2). NEC occurred after neonatal cardiac surgery at a median of 6.5 days postoperatively (IQR 4–14.75), at a postnatal age of 22 (11.5–30.75) days. Half of the patients with postoperative NEC were treated for HLHS (stage I Norwood procedure) or TGA (arterial switch). The type of CHD, the severity of cardiac surgery determined by RACHS-1 score, and the use of cardiopulmonary bypass were not associated with a higher risk for NEC. The use of postoperative ECMO or resuscitation was comparable in patients with or without NEC (Table 1). NEC was treated by intravenous antibiotics and parental nutrition for at least 5 days (n = 16), while one infant needed abdominal surgery (Table 2). This infant was treated for obstructive total anomalous pulmonary vein drainage by sutureless repair with the need for a secondary redo after 2 days. Despite intravenous antibiotics and parental nutrition, surgery became necessary including colon resection plus colostomy (Table 2).

Table 2. Diagnosis, time, and CHD type of patients with complicating NEC after neonatal cardiac surgery.
Infants with postoperative NEC had a longer ICU and total hospital stay (Table 1). Postoperative NEC was associated with increased postoperative ICU stay by more than 50% [postoperative ICU time with complicating NEC median (IQR) 14 (10–42) days vs. no NEC 8 (6–21) days, p = 0.045] and increased total hospital stay by more than 2 weeks [total hospital stay with complicating NEC 49 (35–79) days vs. no NEC 32 (22–50) days, p = 0.041] (Table 1). The overall number of other complications was not higher in the NEC population as well as comparing the patient individual number of complications between postoperative NEC and no NEC (Table 1).
The ND outcome was determined by the Bayley III at a mean (±SD) age of 11.5 ± 1.5 months. For the whole patient population, the CCS was mean (SD) 102.2 (15.0), the LCS was 93.8 (13.1), and the MCS was 88.7 (15.9).
All three scores were lower in the NEC population, but without evidence of significance (Table 1). Clinical outcome data are presented in Table 2. When adjusting the analysis for SES and CHD type (Table 3), patients with NEC had lower CCS scores than those in patients without [β = −11.2 (SE 5.6), p = 0.049]. However, results in LCS and MCS remained similar. Multiple R2 was 0.08 for the CCS, 0.08 for the MCS, and 0.07 for the LCS model.
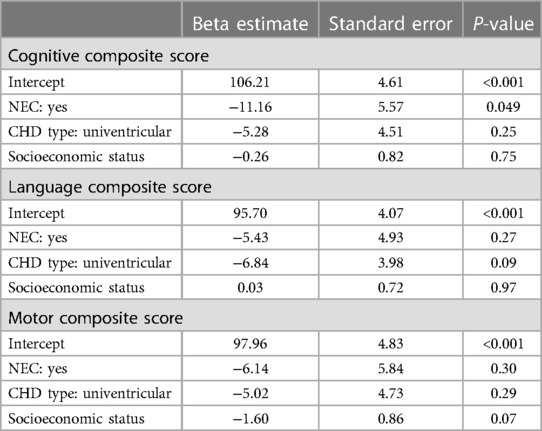
Table 3. Association of NEC, CHD type, and socioeconomic status with Bayley composite scores.
Analyzing the same models using the cumulative risk score instead of the presence of NEC only (Table 4), we found a higher risk score to be associated with both lower CCS [β = −2.8 (SE 1.3), p = 0.030] and lower MCS [β = −3.20 (SE 1.3), p = 0.016], but not with lower LCS [β = −0.7 (SE 1.1), p = 0.55]. Multiple R2 was 0.09 for the CCS, 0.14 for the MCS, and 0.05 for the LCS model.
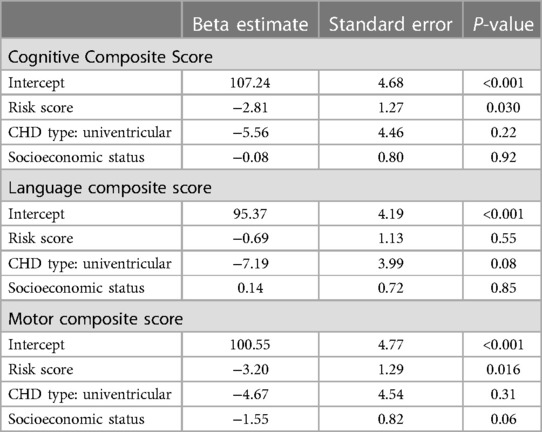
Table 4. Association of risk score, CHD type, and socioeconomic status with Bayley composite scores.
DiscussionThis is the first population-based analysis of the relationship between postoperative NEC and neurodevelopmental outcome in children with severe CHD having undergone cardiac surgery in the first 6 weeks of life (9). In our study, postoperative NEC was the second most frequent complication (after postoperative respiratory complication) during ICU treatment. As expected, the occurrence of NEC was associated with prolonged length of hospital stay, but we also found a significant association with a lower cognitive outcome at 1 year of age. However, a cumulative risk score summarizing various complications was associated with both cognitive and motor outcomes and could therefore better predict ND outcome than NEC alone. Postoperative NEC was furthermore associated with an adverse ND outcome at 1 year of age with poorer cognitive outcome when adjusted for type of CHD and SES. Therefore, preventing or early identifying and treating NEC could help to improve the long-term ND outcome.
The high incidence of postoperative NEC in 16% of our ORCHID cohort may be explained by the inclusion criteria focusing on a high-risk population leading to a high number (90%) of patients with a risk score in RACHS-1 of more than class 2 (Table 1) and by our diagnostic definition of NEC including more cases diagnosed by ultrasound compared to x-ray, which might have led to overdiagnosis. However, a comparable incidence of NEC in 19.5% of patients with severe CHD (RACHS-1 >2) was reported in a recent case–control study by Gong et al. (13). This contrasts with the overall incidence of NEC (Bell's stage >2) ranging between 2% and 11% for the general population of term-born infants (4, 14, 15).
The diagnosis of NEC was made according to the historically defined stage ≥2 based on radiography in our study, as defined by Bell et al. in the late 1980s (10, 16). Nevertheless, the findings of pneumatosis intestinalis may to some degree be examiner-dependent and have to be interpreted in the context of the clinical findings. Other imaging modalities such as ultrasound have obvious advantages regarding their bedside availability, but their diagnostic power may be overestimated due to a larger number of false-positive results (17). Nevertheless, utilizing ultrasound in suspected NEC is at least equivocal to radiography (18, 19). Recently, Doppler ultrasonography of the superior mesenteric artery has gained importance as a sensitive diagnostic (or even prognostic) tool if performed early (20). So far, modern subtyping of the different entities of NEC may provide a clearer diagnostic approach, especially for research (21).
Certainly, cases of NEC in our study reflect the broad clinical spectrum of cardiogenic NEC including different severity levels of the affected infants due to ischemic mesenteric hypoperfusion (Table 2). The diagnosis of NEC remains challenging (17). In the past, different modifications of the Bell staging system for the preterm population have been developed trying to overcome the difficulties in diagnosing NEC including gestational age, biomarkers, genetic factors, single or multi-omics approaches, and stool microbiota (17). There is a risk of overdiagnosing cardiogenic NEC due to temporary postoperative low cardiac output and mesenteric ischemia; some cases suspicious for NEC in our cohort with rather nonspecific clinical signs and a suggestive abdominal radiograph or sonography were early treated due to the risk of rapid clinical deterioration of NEC (17).
Regarding the etiology of cardiogenic NEC, patients with severe CHD are at increased risk for mesenteric hypoperfusion followed by endothelial inflammation and increased vascular permeability due to the release of cytokines, leading to intestinal epithelial barrier dysfunction, bacterial translocation, and intestinal dysbiosis. The role of intestinal dysbiosis in CHD patients has been described as a result of reduced gut perfusion and gut hypoxemia. This may lead to a reduction of healthy bacteria and an increase of proinflammatory bacteria, with increased levels of trimethylamine N-oxide affecting the liver, reduced short-chain fatty acids, and bile acids affecting myocardial contractility (22). Furthermore, in cardiogenic NEC, microcirculatory changes in CHD patients with altered hemodynamics compared to normal infants may contribute by altered cross talk of intrinsic endothelin-1 (vasoconstrictor) and nitric oxide (vasodilator), which has been shown in animal models demonstrating a maladaptive vasoconstriction to compensate for postoperative arterial hypotension and hypoxemia (23, 24). This process may be intensified after surgery due to a potential reperfusion injury, especially in higher-risk cases involving cardiopulmonary bypass surgery and hypothermia, and may be aggravated due to a more complicated postoperative course with ongoing systematic inflammation and/or low cardiac output syndrome (25). Furthermore, the use of inotropes (26), ECMO (27), and blood transfusions have been associated with an increased risk of NEC.
Before surgery, different prevention strategies including feeding guidelines for neonates have been developed for high- vs. low-risk patients (28). High-risk infants included HLHS, truncus arteriosus, single ventricle, and duct-dependent systemic blood flow. Other risk factors for cardiogenic NEC include concomitant premature birth (<37 weeks of gestation), low birth weight (<2,500 g), high RACHS-1 scores (>2), and trisomy 21. Evidence-based postoperative feeding strategies, however, are lacking. The risk of early postoperative NEC has been determined within the first 3 days after surgery (even before starting enteral feeding), as well as due to suboptimal caloric intake, catabolic stress, critical illness leading to abdominal distension, increased gastric residuals, and intestinal paralysis. Postoperative solitary human mild diet may be protective in neonates with a single ventricle type of CHD with improved short-term growth and decreased risk of NEC after cardiac surgery (29).
Although cardiogenic NEC is thought to be associated with mesenteric hypoperfusion and hypoxia in the gut, which may be attributed to low cardiac output syndrome, the impact of a concomitant reduction in cerebral perfusion causing poorer ND outcome after 1 year remains open. Cerebral hypoperfusion during the acute postoperative phase coupled with negative effects on the microbiota may lead to impaired ND outcome. Both pathophysiological mechanisms have been demonstrated to contribute to poorer ND outcome in preterm newborns. For extremely preterm infants, NEC has been described as a significant risk factor for impaired ND outcome. In these patients, gut microbiota seems to be a relevant factor for the development of the gut, immune system, and brain, described as gut–brain axis linking the gut microbiota and ND outcome (30).
Prolonged hospital stay after cardiac surgery is associated with worse ND outcome at 6 years of age (31) and serves as a surrogate marker for medical complexity. Therefore, NEC contributes as one important risk factor besides others determining a more severe postoperative clinical course leading to longer ICU stay and total hospital stay due to a higher postoperative morbidity. Nevertheless, the impact of surgery determined by the length of CPB time, cross-clamping time, and the need for postoperative ECMO did not influence the risk for postoperative NEC in our cohort (Table 1).
In the future, the management and prevention of postoperative NEC may include clear diagnostic and therapeutic strategies including balanced nutrition regimens and systematic register-based research.
Despite the prospective design of this multicentric observational registry, some limitations have to be taken into account. This includes that the perioperative management of the involved centers is not completely standardized. Furthermore, it would be very interesting to include more details regarding the perioperative management of nutrition, i.e., type, frequency, and amount of nutrition, but this is not part of the ORCHID registry and could not be standardized due to the multicentric study design. We only evaluated postoperative NEC due to the availability in the Swiss ORCHID, since preoperative NEC data are not collected. Furthermore, cerebral MRI was not analyzed systematically. The ND outcome was so far only evaluated early at 1 year of age and does not represent the longer-term outcome at school age.
ConclusionsPostoperative cardiogenic NEC is associated with longer ICU and hospital length of stay and contributes together with other complications to impaired ND outcome at 1 year of age. In the future, Swiss ORCHID may offer a nationwide research platform to better understand the impact of perioperative risk factors on the long-term ND outcome.
Data availability statementThe datasets presented in this article are not readily available due to the ethical regulations (Basec-2022-00689). Requests to access these datasets may be directed to walter.knirsch@kispi.uzh.ch.
Ethics statementThe studies involving humans were approved by the Swiss ORCHID registry, which has been reviewed by the cantonal ethics committees (Req-2019-00089), and for the study analysis of the impact of NEC on ND outcome (BASEC-2022-00689). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributionsWK: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. AS: Data curation, Formal Analysis, Methodology, Software, Writing – original draft, Writing – review & editing. VR: Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. CL’E: Data curation, Formal Analysis, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing. JN: Conceptualization, Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing. JS: Conceptualization, Data curation, Investigation, Supervision, Writing – original draft, Writing – review & editing. NS: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. BL: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. CB-T: Data curation, Investigation, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. MB: Data curation, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. KF: Writing – original draft, Writing – review & editing, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision. MG: Formal Analysis, Project administration, Supervision, Writing – original draft, Writing – review & editing. DH: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. MP: Data curation, Formal Analysis, Investigation, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. LK: Data curation, Formal Analysis, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. AP: Data curation, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. JK: Conceptualization, Data curation, Formal Analysis, Supervision, Validation, Writing – original draft, Writing – review & editing. MR: Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. SO: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The current composition of the Swiss ORCHID groupLausanne: Nicole Sekarski, Julia C. Natterer, Juliane Schneider, Christelle L`Ebraly, Rene Pretre, Amir-Reza Hosseinpour. Geneva: Maya S. Bouhabib, Angelo Polito, Cristina Borradori-Tolsa, Tornike Sologashvili. Berne: Damian Hutter, Lena Kaiser, Marc Raphael Pfluger, Martin Glöckler, Katharina Fuhrer-Kradolfer, Sebastian Grunt, Therese Fahrni, Alexander Kadner. Zürich: Walter Knirsch, Michael von Rhein, Ruth Etter, Verena Rathke, Janet F. Kelly-Geyer, Beatrice Latal, Hitendu Dave, Robert Cesnjevar. Basel: Mark Brotzmann, Aarau: Hannah Kümin, Rachel Kusche, Chur: Christa Killer, St.Gallen: Ursula Speckle, Winterthur: Regula Schmid, Bellinzona: Barbara Goeggel-Simonetti, Solothurn: Letizia von Laer, Münsterlingen: Seraina Calonder Faas, Biel: Margreet Duetz, Neuchatel: Marc Ecoffey, Fribourg: Marie Pascale Metrailler, Baden: David Wille, Luzern: Berenice Bubl.
Further collaborators in the period of the data usedAarau: Andrea Capone, Luzern: Florian Bauder, Winterthur: Ulla Jochumsen, Biel: Lena-Marie Gerecke. Technical responsibility for the database and hosting: Mark Adams (Swiss Neonet).
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article. Swiss ORCHID was supported by the Swiss Society of Pediatric Cardiology, the Anna Müller Grocholski Foundation, Corelina Foundation, Accentus Charitable Foundation. Walter Knirsch and Alexandra De Silvestro were supported by the Swiss National Foundation (SNFS 320030_184932).
AcknowledgmentsThe Swiss ORCHID is part of the SwissNeoNet, a neonatal network and follow-up group of pediatric neurologists and developmental pediatricians, as well as a multidisciplinary group of pediatric cardiologists, pediatric intensive care physicians, neonatologists, pediatric cardiac surgeons, and cardiac anesthesiologists, all contributing to the care of children with CHD.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Isani MA, Delaplain PT, Grishin A, Ford HR. Evolving understanding of neonatal necrotizing enterocolitis. Curr Opin Pediatr. (2018) 30(3):417–23. doi: 10.1097/MOP.0000000000000629
PubMed Abstract | Crossref Full Text | Google Scholar
2. Nino DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol. (2016) 13(10):590–600. doi: 10.1038/nrgastro.2016.119
PubMed Abstract | Crossref Full Text | Google Scholar
3. Lopez CM, Sampah MES, Duess JW, Ishiyama A, Ahmad R, Sodhi CP, et al. Models of necrotizing enterocolitis. Semin Perinatol. (2023) 47(1):151695. doi: 10.1016/j.semperi.2022.151695
PubMed Abstract | Crossref Full Text | Google Scholar
4. Siano E, Lauriti G, Ceccanti S, Zani A. Cardiogenic necrotizing enterocolitis: a clinically distinct entity from classical necrotizing enterocolitis. Eur J Pediatr Surg. (2019) 29(1):14–22. doi: 10.1055/s-0038-1668144
PubMed Abstract | Crossref Full Text | Google Scholar
5. Bell D, Suna J, Marathe SP, Perumal G, Betts KS, Venugopal P, et al. Feeding neonates and infants prior to surgery for congenital heart defects: systematic review and meta-analysis. Children (Basel). (2022) 9(12):1856. doi: 10.3390/children9121856
PubMed Abstract | Crossref Full Text | Google Scholar
6. Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL Jr, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. (2010) 121(1):26–33. doi: 10.1161/CIRCULATIONAHA.109.865568
PubMed Abstract | Crossref Full Text | Google Scholar
7. Ortinau CM, Mangin-Heimos K, Moen J, Alexopoulos D, Inder TE, Gholipour A, et al. Prenatal to postnatal trajectory of brain growth in complex congenital heart disease. Neuroimage Clin. (2018) 20:913–22. doi: 10.1016/j.nicl.2018.09.029
PubMed Abstract | Crossref Full Text | Google Scholar
8. Knirsch W, Mayer K, Scheer I, Tuura R, Beck I, Bauer J, et al. Cerebral MRI findings and neurodevelopmental outcome in children before Fontan procedure at 2 years of age—enlargement of liquor spaces influences outcome. Cardiol Young. (2017) 51(4):740–6. doi: 10.1093/ejcts/ezw399
Crossref Full Text | Google Scholar
9. Natterer J, Schneider J, Sekarski N, Rathke V, Adams M, Latal B, et al. ORCHID (Outcome Registry for CHIldren with severe congenital heart Disease) a Swiss, nationwide, prospective, population-based, neurodevelopmental paediatric patient registry: framework, regulations and implementation. Swiss Med Wkly. (2022) 152:w30217. doi: 10.4414/SMW.2022.w30217
PubMed Abstract | Crossref Full Text | Google Scholar
10. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. (1978) 187(1):1–7. doi: 10.1097/00000658-197801000-00001
PubMed Abstract | Crossref Full Text | Google Scholar
11. Largo RH, Pfister D, Molinari L, Kundu S, Lipp A, Duc G. Significance of prenatal, perinatal and postnatal factors in the development of AGA preterm infants at five to seven years. Dev Med Child Neurol. (1989) 31(4):440–56. doi: 10.1111/j.1469-8749.1989.tb04022.x
PubMed Abstract | Crossref Full Text | Google Scholar
12. Bayley N. The Bayley Scales of Infant and Toddler Development. 3rd ed. San Antonio, TX: Harcourt Assessment, Inc. (2006).
13. Gong X, Chen X, Wang L, Zhang M, Nappi F, Zampi JD, et al. Analysis of clinical features of neonates with congenital heart disease who develop necrotizing enterocolitis: a retrospective case-control study. Ann Transl Med. (2022) 10(16):879. doi: 10.21037/atm-22-3248
PubMed Abstract | Crossref Full Text | Google Scholar
14. Deitch AM, Moynihan K, Przybylski R, Gauvreau K, Braudis NJ, Farr B, et al. Risk factors for adverse outcomes in term infants with CHD and definitive necrotising enterocolitis. Cardiol Young. (2024) 34(1):92–100. doi: 10.1017/S104795112300121X
PubMed Abstract | Crossref Full Text | Google Scholar
15. Lau PE, Cruz SM, Ocampo EC, Nuthakki S, Style CC, Lee TC, et al. Necrotizing enterocolitis in patients with congenital heart disease: a single center experience. J Pediatr Surg. (2018) 53(5):914–7. doi: 10.1016/j.jpedsurg.2018.02.014
PubMed Abstract | Crossref Full Text | Google Scholar
18. Kallis MP, Roberts B, Aronowitz D, Shi Y, Lipskar AM, Amodio JB, et al. Utilizing ultrasound in suspected necrotizing enterocolitis with equivocal radiographic findings. BMC Pediatr. (2023) 23(1):134. doi: 10.1186/s12887-023-03932-3
PubMed Abstract | Crossref Full Text | Google Scholar
19. Lazow SP, Tracy SA, Estroff JA, Parad RB, Castro-Aragon IM, Fujii AM, et al. A role for abdominal ultrasound in discriminating suspected necrotizing enterocolitis in congenital heart disease patients. Pediatr Surg Int. (2022) 38(2):225–33. doi: 10.1007/s00383-021-05025-7
PubMed Abstract | Crossref Full Text | Google Scholar
20. Rallis D, Kapetaniou K, Machas P, Balomenou F, Giapros V, Saliakellis E. A systematic review and meta-analysis of the role of Doppler ultrasonography of the superior mesenteric artery in detecting neonates at risk of necrotizing enterocolitis. Pediatr Radiol. (2023) 53(10):1989–2003. doi: 10.1007/s00247-023-05695-6
PubMed Abstract | Crossref Full Text | Google Scholar
21. Gordon PV, Swanson JR, MacQueen BC, Christensen RD. A critical question for NEC researchers: can we create a consensus definition of NEC that facilitates research progress? Semin Perinatol. (2017) 41(1):7–14. doi: 10.1053/j.semperi.2016.09.013
PubMed Abstract | Crossref Full Text | Google Scholar
24. Gonzalez R, Urbano J, Solana MJ, Hervias M, Pita A, Perez R, et al. Microcirculatory differences in children with congenital heart disease according to cyanosis and age. Front Pediatr. (2019) 7:264. doi: 10.3389/fped.2019.00264
PubMed Abstract | Crossref Full Text | Google Scholar
25. Pathan N, Burmester M, Adamovic T, Berk M, Ng KW, Betts H, et al. Intestinal injury and endotoxemia in children undergoing surgery for congenital heart disease. Am J Resp Crit Care. (2011) 184(11):1261–9. doi: 10.1164/rccm.201104-0715OC
PubMed Abstract | Crossref Full Text | Google Scholar
26. Becker KC, Hornik CP, Cotten CM, Clark RH, Hill KD, Smith PB, et al. Necrotizing enterocolitis in infants with ductal-dependent congenital heart disease. Am J Perinatol. (2015) 32(7):633–8. doi: 10.1055/s-0034-1390349
PubMed Abstract | Crossref Full Text | Google Scholar
27. Lopez NL, Gowda C, Backes CH, Nandi D, Miller-Tate H, Fichtner S, et al. Differences in midterm outcomes in infants with hypoplastic left heart syndrome diagnosed with necrotizing enterocolitis: NPCQIC database analysis. Congenit Heart Dis. (2018) 13(4):512–8. doi: 10.1111/chd.12602
PubMed Abstract | Crossref Full Text | Google Scholar
28. Burge KY, Gunasekaran A, Makoni MM, Mir AM, Burkhart HM, Chaaban H. Clinical characteristics and potential pathogenesis of cardiac necrotizing enterocolitis in neonates with congenital heart disease: a narrative review. J Clin Med. (2022) 11(14). doi: 10.3390/jcm11143987
PubMed Abstract | Crossref Full Text | Google Scholar
29. Blanco CL, Hair A, Justice LB, Roddy D, Bonagurio K, Williams PK, et al. A randomized trial of an exclusive human milk diet in neonates with single ventricle physiology. J Pediatr. (2023) 256:105–12.e4. doi: 10.1016/j.jpeds.2022.11.043
PubMed Abstract | Crossref Full Text | Google Scholar
30. Lu J, Martin CR, Claud EC. Neurodevelopmental outcome of infants who develop necrotizing enterocolitis: the gut-brain axis. Semin Perinatol. (2023) 47(1):151694. doi: 10.1016/j.semperi.2022.151694
PubMed Abstract | Crossref Full Text | Google Scholar
31. Neukomm A, Ehrler M, Feldmann M, Chaouch A, Knirsch W, Hagmann C, et al. Perioperative course and socioeconomic status predict long-term neurodevelopment better than perioperative conventional neuroimaging in children with congenital heart disease. J Pediatr. (2022) 251:140–8.e3. doi: 10.1016/j.jpeds.2022.07.032
留言 (0)