WBV therapy involves exposing individuals to mechanical vibrations through a specialized platform (1). WBV exploits an organism’s vibrational sense, a widespread phenomenon in the animal kingdom (2–4). This innate capacity to detect vibrations is believed to have evolutionary significance, enhancing an organism’s connection with its environment (5, 6). Humans are susceptible to mechanical stimulations (often encountered in an oscillatory form), ranging in frequency from ~1 Hz up to at least 100 kHz (7).
Mechanoreceptors in human skin process these vibrations, relaying signals to the brain through the spinal cord. In addition to those in the skin, Piezo1 and Piezo2 proteins are mechanosensitive ion channels located in the internal tissues of mammals. These channels trigger mechanotransduction, leading to the production of various hormonal and non-hormonal signaling molecules that affect cellular and metabolic physiology (8). This relaying influences physiological processes across the whole body.
While high-frequency (>100 Hz) and high-intensity (>10 mm) vibrations can lead to musculoskeletal impairment, and increase the risk of developing hand-arm vibration syndrome (9, 10), low-frequency (10–50 Hz) and low-amplitude (<10 mm) vibrations have demonstrated positive effects, particularly in therapeutic applications (3).
WBV can be viewed as a form of passive exercise. In a nutshell: an active form of exercise is where a person exerts force and puts in the effort to complete a move, whereas, in passive exercise, minimal movement is required by the individual. Either someone else moves the body for them or some machines induce exercise-like effects which is what happens in the case of WBV using vibrating platforms (3). WBV, to a level, helps to achieve the beneficial effects of conventional exercises. In older adults, WBV may improve muscle strength, power, and balance compared to untrained individuals (11–13). In many cases, WBV is used as an additional regime to the basic exercising routines as it has been shown to augment the effects of resistance training and other forms of physical exercises. It has been demonstrated that WBV exerts a positive effect on upper-limb performance in combination with exercise (14). Evidence also suggests that WBV may be a good alternative to stretching as a warm-up as it boosts strength exercises in the older population and also seems to enhance cyclist performance (15, 16). Furthermore, WBV may be a useful co-adjuvant in conventional rehabilitation therapy to improve postural stability and achieve better physical, functional, and emotional outcomes in individuals undergoing vestibular rehabilitation (17). This is further strengthened by the fact that WBV stands out as a convenient and highly accessible exercise option. WBV requires less effort, is time efficient, cost-effective, and suitable for various settings and, therefore, adaptable for home or clinical use. Particularly for individuals facing physical limitations or lacking motivation due to factors like frailty, depression, or other mental health challenges, WBV emerges as an accessible and effective means to initiate and enhance physical activity (18, 19).
While the primary focus of studies has traditionally been on elucidating the effects and applications of WBV to enhance musculoskeletal performance in athletes and individuals with musculoskeletal disorders, recent evidence highlights a noteworthy positive impact of WBV at the neurological level and brain functioning. This underscores its potential utility in addressing conditions related to brain diseases. Studies have been conducted to observe the effects of WBV on nervous system-related conditions like spinal cord injury, traumatic brain injury, stroke, anxiety, and major depressive disorder (20–23). Reviews on neurodegenerative disorders (discussed in Section 5) point toward a lack of optimized protocol. To start identifying optimized protocols for brain disorders, we believe that comprehending the molecular and cellular impacts of WBV is crucial.
One of the key players in brain disorders is the immune system; it is known to be close-knit with the nervous system (24). Both these systems have a series of communication pathways to and from each other which play a role in maintaining the overall health of the body. On the one hand, the brain sends signals to the periphery via neurotransmitters, to get the immune system fired up, and on the other hand, the immune system sends signals back to modulate brain activity. This affects, among others, body temperature, sleep, and feeding behavior. In neurodegenerative disorders like Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis, the immune system also plays a pivotal role, being both affected by and contributing to the regulation of disease progression. Cytokines such as in interleukins (IL)-IL-1B and IL6, tumor necrosis factor (TNFα), transforming growth factor (TGF)-TGFb1 and TGFα, along with immune-related factors like toll-like receptor (TLR)-TLR2 and TLR4, play key roles in this interplay. Proinflammatory and anti-inflammatory responses mediated by the immune system, along with oxidative stress, influence the delicate balance in neurodegenerative contexts (25, 26). Investigating how WBV impacts these immune responses holds promise for its therapeutic potential in immune-related conditions and its broader implications for neurodegenerative disorders. This review therefore aims to contribute to the effort to elucidate the intricate pathways influenced by WBV intervention, with a specific focus on molecular and cellular aspects linked to immune and brain functioning. We summarize what is currently known about the impact of WBV on the (brain) immune system and brain functioning, how peripheral effects influence the brain, and finally what is known about the impact on brain disorders. The literature selection criteria can be found in Supplementary Files (Methods). The molecular pathways affected by WBV are summarized in Figure 1, and Supplementary Tables 1, 2 have the molecular and cellular pathways in preclinical and clinical studies, respectively. We conclude with a brief discussion including suggesting aspects to consider while optimizing protocols based on underlying cellular and molecular mechanisms.
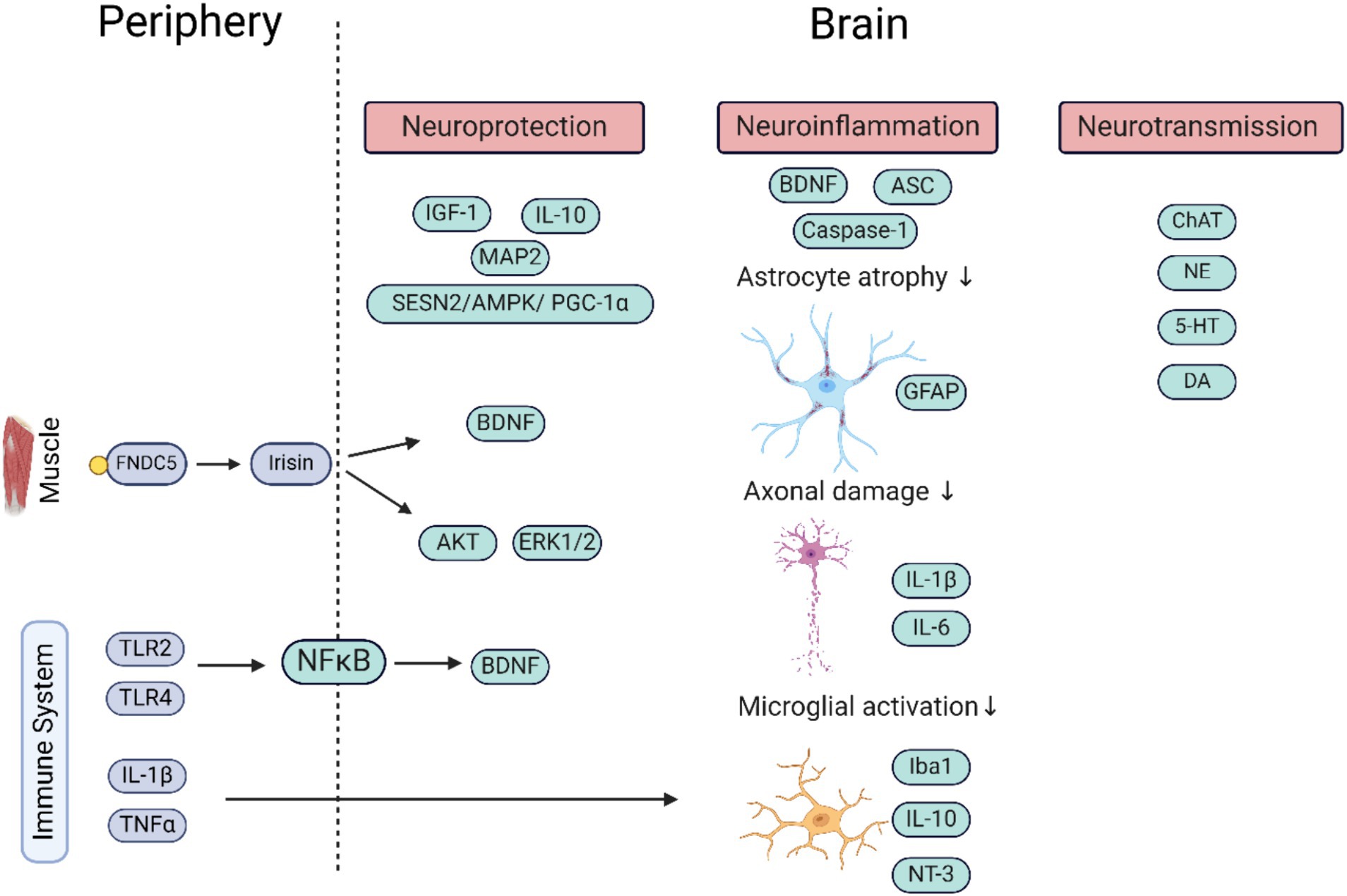
Figure 1. Schematic representation of the molecular pathways by which the periphery (factors in blue) influences the brain (factors in green) upon stimulation by WBV. More direct influences of WBV on the brain are due to only partly deciphered mechanisms. The factors secreted in the periphery (outside CNS) help regulate neuroprotection and neuroinflammation, and via stimulation of neurotransmitter system neurotransmission within neuronal networks. It shows the important role of the immune system in regulating brain functioning. The image was generated using Biorender. ASC, apoptosis-associated speck-like protein containing C-terminal caspase recruitment domain; AMPK, adenosine monophosphate-activated protein kinase; BDNF, brain derived neurotrophic factor; ChAT, choline acetyltransferase; DA, dopamine; ERK, extracellular signal-regulated kinase; FNDC5, fibronectin type III domain-containing protein 5; GFAP, glial fibrillary acidic protein; 5-HT, serotonin; IL, interleukin; IGF, insulin-like growth factor; Iba, ionized calcium-binding adapter molecule; MAP2, microtubule-associated protein 2; NFkB, nuclear factor kappa-light-chain-enhancer of activated B cells; NT-3, neurotrophin 3; NE, nor adrenaline; TLR, toll like receptor; TNF, tumor necrosis factor.
2 Immune systemThe human immune system is not only responsible for combating infection by pathogens, exposure to environmental toxins and allergens, and cellular damage but also plays a pivotal role in maintaining homeostasis of the body (27). The immune system comprises a varied population of immune cells present throughout the body instead of being localized in an organ. Below, we will discuss the literature available reporting the effects of WBV on immune functioning. First, pre-clinical data will be discussed followed by studies that combine clinical and preclinical experiments, and finally, the clinical studies will be addressed.
2.1 Preclinical studiesSimilar to exercise, WBV helps to decrease the inflammatory response as well as reverse symptoms of type II diabetes mellitus (T2DM) (28). Yu et al., have studied the effect of WBV on omental macrophages and the fecal microbiome that were isolated from mice that were subjected to 20 min of WBV per day for 4 weeks, with a frequency of 30 Hz and an amplitude of 3 mm (28). The analysis of macrophages from the abdominal adipose tissues revealed that WBV treatment resulted in a significant increase in M2 macrophage (counter-inflammatory function) count and it restored the IL-10 level in diabetic mice to the resting level in control mice. Since the differentiation of immune cells is often connected to the microbiota, the group of Yu and co-workers also performed an analysis of the microbiota of these mice in the presence and absence of WBV. The results showed that WBV remodels the alpha and beta diversity of the microbiome of the alimentary canal. Overall, these findings support the notion that WBV potentially alters the microbiota which prompts innate and mucosal immunity. This further produces anti-inflammatory responses and reverses the adverse consequences by down-regulating the hyper-inflammatory state.
Nowak et al., subjected 10 adult male Wistar rats to WBV on a vibrating platform, which generated vertical vibrations at the frequency of 50 Hz and amplitude of 2.5 mm for 5 weeks to determine the effects of WBV on hormonal and immunological parameters (29). Every session included four bouts lasting 30 s, separated by 1 min rest intervals. Following the training period, red and white blood cells, lymphocytes, monocytes, hemoglobin, granulocytes, and hematocrit, as well as IL-1b, IL-10, IL-6, and vascular endothelial growth factor levels were determined. The results showed a significant decrease in concentrations of IL-10 and a possible increase in IL-1b and IL6 in the blood serum, which are cytokines responsible for pro-inflammatory actions. However, a subsequent study done by the same group and the same WBV program showed no statistically significant changes in the complete blood cell counts or inflammatory cytokines (30). Interestingly, the only difference between the two studies was the period of WBV application. The later study showed the effect of WBV after three and 7 months of application, whereas the initial study presented the results for 5 weeks of WBV application. The disparity in the two studies shows that the total duration of WBV may be an important factor to take into consideration while studying the effects of WBV.
2.2 Combined preclinical and clinical studiesThe study of Song et al., further strengthens the notion of WBV affecting the immune system through the change in the microbiota of the alimentary canal (31). The experimental setup of this study consisted of both mice and human volunteers who were subjected to 30 min of vibration every day for 30 days. Analysis of immunological markers revealed a significant increase of CD-4 and CD25 positive lymphocytes and CD4 and CD25 positive Treg cells in the spleen of the WBV-subjected mice. These immunological results suggest that WBV alters regulatory T-cell differentiation in mice. Furthermore, Song et al., also showed that WBV affects the intestinal microbiome of both humans and mice. WBV significantly reduced the alpha diversity in mice and increased the beta diversity of both mice and human fecal microbiota. Moreover, correlation analysis revealed significant changes in bacteria variation that are linked to regulatory T-cell differentiation in mice and physical characteristics in humans. This study thus also suggests that WBV has potential interventional effects on microbiota and immune-related diseases. The malleability of T cells in response to the gut microbiome can be used as a tool for editing immune response by utilizing microbiota-mediated pathways (32). Therefore, WBV-induced changes in microbiota, immune state, and inflammation of the body are research areas with clear potential and hence warrant further investigation.
2.3 Clinical studiesWBV has been tested as a treatment approach for osteoarthritis (OA) in several clinical studies. Systematic reviews have indicated the beneficial effects of WBV on pain, knee extensor muscle strength, and physical function in individuals with knee OA (33, 34). Even though OA has been considered to be a form of joint inflammation, there are now studies that indicate the implication of a systemic inflammatory response presented by T cells and the presence of inflammatory markers in peripheral blood, including inflammatory cytokines and antibodies (35, 36).
Since the immune system plays a causative and consequential role in OA, Tossige-Gomes et al., studied the effect of WBV, in addition to squat training, on the T-cell proliferative response of elderly subjects with knee OA (37). The patients were subjected to vertical synchronous vibration with a frequency ranging from 35 to 40 Hz, an amplitude of 4 mm, and an acceleration ranging from 2.78 to 3.26 g. The results showed that WBV decreases the proliferation of TCD4+ cells in patients with OA of the knee, suggesting that the addition of WBV to training might modulate T-cell-mediated immunity in this population, thereby minimizing the disease progression in elderly OA patients.
Chronic inflammation, a hallmark of aging, contributes to various age-related diseases. A recent study investigated how WBV impacts the inflammatory status in older subjects, focusing on TLRs 2 and 4 (38). The study included older individuals (average age 70 years) without significant health conditions. Participants underwent WBV sessions (30 Hz, 2 mm amplitude) for 12 weeks followed by blood sample collection before and after the intervention. TLR2 and TLR4 expression levels were measured, along with inflammatory marker IL-10, and physical performance (e.g., gait speed) was assessed. WBV led to a significant reduction in IL-10 levels, indicating an anti-inflammatory effect. TLR2 and TLR4 expression decreased after WBV, suggesting modulation of immune responses. These changes correlated with improved physical performance, emphasizing the holistic impact of WBV. Mechanistically, WBV likely influences TLR signaling pathways, and downregulation of TLR2 and TLR4 may contribute to the observed anti-inflammatory effects. The enhanced physical function may result from reduced inflammation. Lower TLR2 and TLR4 cell surface expression is frequently associated with the anti-inflammatory situation induced by a physically active lifestyle (39).
Also, the impact of WBV on circulating stem/progenitor cells (CPC) and cytokine levels has been studied. Healthy male participants engaged in three activities randomly: standing platform vibration, repetitive leg squat exercise, or a combination of both (40). The vibrations were subjected at 35 Hz with an amplitude of 4 mm. Blood samples taken before and after each activity revealed significant increases in CPC levels with exercise alone and vibration alone, particularly in younger subjects. Combined activity notably boosted angiogenic CPCs in younger participants. Vibration alone increased non-angiogenic CPCs in younger subjects, while exercise alone showed similar effects in older individuals. Additionally, WBV led to a significant increase in anti-inflammatory cytokine IL-10 and a decrease in inflammatory IL-6 levels. Notably TNFα and vascular endothelial growth factor levels increased with vibration alone, suggesting pro-angiogenic effects. The findings suggest WBV’s potential positive effects on vascular health and inflammation.
In contrast to a relatively large number of studies that showed the positive effect of WBV on the immune system, a few studies did not observe an effect of WBV on the immune response. For example, in one of these studies, the effect of WBV in combination with resistance exercise on salivary cortisol and salivary IgA was determined (18, 41). Nine adults were subjected to two bouts of resistance exercise with and without WBV of 30 Hz for approximately 18 min, spaced at a 7-day interval. No significant increase in salivary cortisol and IgA levels was found. However, further studies are required in this area with a higher sample size and focusing on the chronic effect of WBV rather than an acute effect. Another reason why WBV did not show a strong effect on the IgA secretion in this study, could be because of the use of a WBV platform that oscillates in the vertical direction only. It was reported that at frequencies of 25 and 30 Hz, the side-alternating platform produces twice the vertical acceleration in comparison to the vertical oscillation platform (42).
Taken together, WBV seems to play a role in modulating the immune response. By altering the gut microbiome in both humans and mice, WBV alters T-cell differentiation and induces a shift in M2 macrophages. Apart from regulation through the gut microbiome, WBV also alters proinflammatory markers. Next, we will discuss the impact of WBV on brain functioning.
3 Effects of WBV on the brainEven though the initial focus of WBV treatment was to enhance muscular functioning, as the WBV applications advanced, its potential effects on the brain have become clear from various studies. The effects of WBV on brain functioning have been studied in healthy as well as patients and rodents including models for different neurological disorders or aspects of such disorders. First, the preclinical studies done with primarily mice and rats will be summarized, followed by clinical studies.
3.1 Brain functioning 3.1.1 PreclinicalMultiple studies have shown that WBV also exerts effects on brain functioning. For example, exposure to WBV (30 Hz, 5 or 30 min per day for 5 weeks) has a positive effect on novel object recognition and motor performance in a motor test (balance beam performance) in CD1 mice (43). In another study, positron emission tomography in C57Bl6 mice revealed that glucose uptake was not changed as a consequence of the 5-week WBV intervention with 30 Hz for 10 min per day, 5 days a week (44), however, the arousal-induced home cage activity was reduced. These results suggest that WBV intervention improves motor performance and affects brain functioning in mice. Moreover, it can be suggested that WBV is a safe intervention to improve brain functioning, although the somewhat subtle effects suggest that the protocol is as yet suboptimal.
Aging can affect a person adversely, not only physically but also mentally. Physical exercise has been shown to have positive effects on mental well-being and the cognitive abilities of the elderly. Due to old age, people are often not able to exercise enough to get positive effects on functions such as neurological memory, anxiety, and motor performance (45). In such cases where physical exercise is not possible, WBV can be used as a form of passive exercise to achieve similar results as physical exercise.
In rodents, aging is associated with impaired memory functions (spatial and object) (46, 47), anxiety (48, 49), depression (48), and motor performance (50). Recent preclinical studies revealed that WBV for 5 weeks can affect the cognitive abilities of 18 and 30-month-old Wistar rats (20, 21). The rats were divided into two groups, vibration and pseudo-vibration groups. The vibration group was subjected to 5 weeks of mechanical vibrations at a frequency of 30 Hz and amplitude of 0.05–0.2 mm. The study with 18 months old rats used an intervention session of 10 min whereas the study with 30 months old rats used 5 min. Evidence from both these studies indicates that WBV can curtail anxiety, significantly improve the rearing behavior, and spatial memory of the rats, and also increase their motor performance. It is important to note that even brief daily sessions, lasting less than 10 min, could be enough to enhance memory functions and reduce anxiety-like behavior in advanced aging.
Another study, further discussed in Section 3.3.1, concluded that 20 min of stimulation results in a decrease in anxiety and an increase in spatial memory awareness. Five minutes of stimulation resulted in increased motor performance. Overall, it was shown that WBV can help improve both motor and cognitive functioning in 18-month-old Wistar rats. In contrast, from what has been found in old rats, anxiety-like behavior was reduced when 5 or 10 min sessions were used, but not when 20 min was used in 12-month-old female Wistar rats (51). Taken together, the data suggest that the sensitivity of the brain to WBV is age-dependent in rats.
3.1.2 ClinicalIn a recent clinical study exploring the effects of WBV, 133 young and healthy individuals (including 112 females and 21 males) with an average age of 20.5 ± 2.2 years underwent WBV treatment (52). The treatment involved exposure to mechanical vibrations at 30 Hz with an amplitude of approximately 0.5 mm for 2 min, repeated six times. The data revealed an enhancement in stroop color-word interference test scores, suggesting a positive short-term impact on executive functions, particularly on attention and inhibition, in young adults. Fuermaier et al., studied the effects of WBV on attention in 83 healthy individuals and 17 adults diagnosed with attention deficit hyperactivity disorder (ADHD) (53). Both healthy and adults with ADHD subjected to 30 Hz, 4 mm vibrations for 2 min showed small to medium effects on attention. Their results show that WBV may have potential as an alternative form of intervention to help with cognition in humans. An additional study investigated the impact of 3 min of WBV training at 30 Hz with an amplitude ranging from 0.44 to 0.6 mm on inhibitory function in healthy children (54). Their data showed a therapeutic effect associated with intelligence and age, though they did not specifically address ADHD. In healthy young adults, three bouts of two-minute side-alternating WBV (frequency 27 Hz) and three control conditions showed positive effects on cognition (55). The participants underwent two different sessions. In one session a sitting posture was used and in the other session a standing (semi-squat) posture. Their results showed that WBV significantly improved selective attention and inhibition in the sitting posture, but not in the standing posture. While significant, the effects were small.
A separate investigation examined the effects of WBV exercise on 12 healthy subjects using a frequency of 30 Hz and a 4-mm amplitude, administered for 2 min each over five sessions (56). Initially, the group had hypothesized that exposure to vibrations would decrease cognition. However, contrary to their hypothesis, they found that vertical vibrations increased motor processing speed. The outcomes indicated that WBV training, coupled with squats with a knee flexion at a 45° angle, did not significantly impact visual or verbal memory, reaction time, or impulse control, as assessed by the Immediate Post-concussion Assessment and Cognitive Test (ImPACT). However, there was a potential increase in motor processing speed following vertical vibration.
As mentioned earlier (Section 3.2.1), aging affects cognitive function and WBV can be a useful strategy to help keep an individual active. A study was conducted on elderly individuals where 17 people were randomly assigned to either an intervention group (n = 9) or a sham operation group (n = 8) (57). The intervention group underwent 4 weeks of WBV training for 1 min each, five times, 3 days per week. From weeks 5–8, a passive trampoline program of 5 min was introduced after the vibration sessions. The findings of this study showed that the eight-week program, combining stochastic resonance WBV and exergame-dance training, induced benefits in both physical and cognitive performance among older adults residing in care homes. Another study, which has been discussed above in the preclinical Section 3.1.1 also studied the effects of WBV on humans (44). Their protocol changed from mice to humans, in terms of the duration. Humans were treated with 30 Hz of vibration for 4 min per day for 4 days a week, for 5 weeks. They observed a positive effect in the older population in the stroop test, indicating improvements in selective attention and inhibition.
Since cognition is linked to the falls experienced in aging, Rosado et al., studied the effects of WBV in combination with a psychomotor intervention for 24 weeks (58). The vibration amplitude was always 3 mm and the frequency increased from 12.6 to 15 Hz. They tested the effects on reaction time, mobility, and dual-task performance in older adults at risk of falling. Their results showed improvements in reaction time, mobility, and dual-task performance in the group that had undergone psychomotor and WBV treatment. Notably, the influence of the interventions on reaction time, mobility, and dual-task performance was no longer apparent during the 12-week follow-up period without any additional intervention. Contrary to this, another study did not observe any improvements in fall risk, life satisfaction, and cognitive status in elderly women (59). The study consisted of 8 months of WBV intervention at 20 Hz with a 2-mm amplitude (30–35 min each, twice per week).
Two systematic reviews of 18 studies (including the ones mentioned above) revealed mixed results: some studies reported positive effects of WBV on cognition, while others found no significant impact (60, 61). Participants included both individuals with cognitive impairment and healthy subjects. The reviews concluded that WBV improved motor skills (56), reaction time (62), inhibitory function (54), processing speed, and executive functions in healthy individuals (52). The positive effects extended to patients with cognitive impairments as well. Studies showed improved attention, memory, and divergent thinking in ADHD patients (53, 63), and improved cognitive abilities in multiple sclerosis (64), dementia, and stroke patients (65, 66). The positive effects of dementia and stroke are both related to the activation of the cerebral cortex. Contrary to this, there were studies showing no change in WBV subjection in healthy and diseased subjects such as mild dementia (67). The majority of the studies indicated that WBV training enhances cognitive performance, with only a minority concluding otherwise. The disparities in results may be attributed to variations in the cognitive tests utilized, patients’ disease stages, and the protocols employed for vibration.
3.2 Neurotransmission 3.2.1 Preclinical studiesInitial studies addressing the effect of WBV on the brain were mainly focused on determining the adverse effects of WBV on monoamines. Monoamine neurotransmitters like serotonin (5-HT), norepinephrine (NE), noradrenaline (NA), and dopamine (DA) play a pivotal role in the brain to further assist and regulate cognitive functions (68). They also play an important role in regulating systems other than the brain, like the cardiovascular system, respiratory system, and gastrointestinal system. Several studies have shown that an acute WBV exposure for 240 min stimulated the synthesis and release of several neurotransmitters and monoamines such as 5-HT (69, 70), NA (71, 72), corticosterone (70, 73), DA (69, 72), NE (72) and 5-hydroxy indole acetic acid (5-HIAA) (70). Although this thus suggests that monoamines are affected by WBV, it is important to note that all these studies were done with an extreme 4-h-long acute WBV session. The experimental design of these studies is inept as their vibration setup restricted the movement of the animal, the vibration protocols were very long and the results are unreproducible due to a lack of enough information like the age of the animals. Therefore, to get a better insight into the effects of WBV on these monoamines, and WBV’s effect in terms of therapeutics, it is essential to perform further studies that do not have such extreme durations, but rather use a few bouts of shorter periods and a reduced maximal acceleration with a properly designed setup.
Another study by Dmitriev et al., compared the effect of an acute WBV session to the effects of a 52–54 days long chronic WBV intervention with a frequency of 10 Hz, amplitude of 1 mm, and 15-min daily session duration (74). This vibration protocol enhanced the accumulation of 5-HT in different regions of the rat brain, especially in the hippocampus (acute) and the parietal complex (long-term). This study tried to explore the involvement of regional alterations in 5-HT metabolism and the responsiveness of serotoninergic structures in the development of somatosensory disorders linked to vibration exposure. These early studies together showed that WBV may help attenuate the levels of monoamines in the brain. To further test this hypothesis it would be interesting to study the effect of WBV in diseases where disbalance in these monoamines causes pathogenesis, for example in PD, AD, anxiety, seizure disorders, and mania. Notably, a recent study demonstrated that exposing rats to 80 Hz of WBV leads to the intracellular relocation of δ-opioid receptors from the cytosol to the membrane within rat cholinergic interneurons in the nucleus accumbens (75). This study primarily concentrated on the influence of WBV in mitigating dopamine-related mechanisms associated with morphine addiction. However, their findings also show the need for further investigation into the potential effects of WBV on dopaminergic neurons. Research could also hold considerable significance in the treatment of neurological conditions like PD.
As described previously, there is accumulating evidence that points toward a positive impact of WBV in rats and mice, on memory. However, the exact underlying mechanism by which WBV improves cognition and brain functioning remains largely unknown. To understand how WBV can affect the brain, it is important to consider its effect at a cellular and molecular level, for example by studying neurotransmitters and monoamines. It has been shown that the cholinergic forebrain plays a pivotal role in learning and memory performance (76). Five weeks of WBV stimulation in mice resulted in a significant increase in choline acyltransferase (ChAT), the rate-limiting enzyme for the production of acetylcholine. Since increased ChAT is strongly linked to increased cholinergic activity, this suggests that WBV treatment positively affects attention and memory through increased activity of the cholinergic system of the brain (77).
To recapitulate, studies indicate toward involvement of WBV in the attenuation of neurotransmitters like ChAT, NE, 5-HT, and DA (Figure 1). Although it is important to note that some of these studies have used extreme intervention protocols, therefore more research is needed in this field with better intervention protocols aimed at promoting health.
In a study led by Cariati and colleagues, they proposed that the impact of vibration training on cognitive processes might be age-dependent, and closely linked to synaptic plasticity (78). The study utilized mice of two different age groups, 4 months and 24 months. Synaptic plasticity, assessed through electrophysiological measures in the hippocampus, was investigated after exposing the mice to vibrations at 45 Hz for three series of 2 min and 30 s, with an equivalent recovery period in between, over 12 weeks. The outcomes showed a difference in response to the vibration in old mice compared to the young mice. Both age groups affected synaptic plasticity. Importantly, it is noteworthy to consider that the specific parameters of WBV, such as frequency and duration, might interact differently based on the age and possibly sex of the mice. This provides preclinical evidence and a good base point to continue these studies in aging humans who cannot perform physical exercise.
3.3 NeuroinflammationWhile studying brain functioning, it is important to address neuroinflammation. Neuroinflammation is defined as an inflammatory response within the brain or spinal cord that is mediated by the production of cytokines, reactive oxygen species (ROS), chemokines, and secondary messengers (79). It essentially is a response of the central nervous system to disturbed homeostasis. Neuroinflammatory responses may be helpful or harmful, as mechanisms associated with neuroinflammation are involved in normal brain development, as well as in neuropathological processes. There are complex and interacting immune, biochemical, physiological, and psychological consequences of neuroinflammatory responses (79, 80). As neuroinflammation is increasingly recognized as being involved in nearly all brain disorders (81, 82), it is of critical importance to decipher the impact of WBV on neuroinflammation.
3.3.1 Preclinical studiesMicroglia, are the resident immune cells of the central nervous system that respond to insults and injuries in the CNS (83). Activation of microglia prompts the release of pro-inflammatory factors, contributing to neuroinflammation (84), in conditions like AD and PD. In SCI, microglia are a double-edged sword, aiding healing but potentially causing harm through the release of cytotoxic elements (85). In a recent study, rats with spinal cord injury (SCI) underwent WBV with an amplitude of 1.5 mm and frequencies of 15 Hz and 30 Hz (86). The therapy started on day 7, day 14, or day 28 post-injury, each with 10 rats. WBV sessions occurred 5 days a week, featuring five trials, with a one-minute rest between each. A control group had 10 rats with SCI but no additional therapy. Assessing functional recovery and immunohistochemical markers ionized calcium-binding adapter molecule 1 (Iba1) and neurotrophin-3 (NT-3) revealed that WBV initiated at day 14 led to the most significant overall recovery, with a moderate increase in Iba1 and the highest increase in NT-3. Moreover, in the case of traumatic brain injury (TBI), WBV has the potential to manage neuroinflammation by suppressing the activation of microglia (see also Section 3.5) (22, 87). Furthermore, Oroszi et al., (discussed in detail in 3.3.1) demonstrate that WBV results in a significant decrease in microglial activation in the Cornu Ammonis 1 (CA1) and dentate gyrus subregions in aged male rats (88). Taken together, these studies indicate that WBV attenuates microglial activation which probably helps in mitigating neuroinflammation.
Neuroinflammation is also one of the prevalent pathological occurrences in ischemic stroke (89). Studies have suggested microglial polarization modification as a prospective treatment strategy for ischemic stroke (90, 91). An animal study investigated the efficacy of WBV in reducing frailty and brain damage post-ischemic stroke in reproductively senescent female rats (92). The animals underwent 30 days of WBV (Frequency of 40 Hz; Amplitude not reported) treatment performed twice daily for 15 min each session, 5 days each week. The data revealed a significant depletion of inflammatory markers and infarct volume with significant increases in BDNF and tyrosine kinase receptor subtype B (Trk-B). Following post-ischemic WBV, protein levels of caspase-1, ASC, and IL-1β in the peri-infarct area decreased by 88% (p < 0.05), 57% (p < 0.05), and 148% (p < 0.05), respectively, compared to the control condition. Caspase-1 activates pro-inflammatory cytokines like IL-1β, contributing to neuroinflammation (93). ASC facilitates inflammasome assembly, further promoting inflammation in neurodegenerative diseases (94). They also observed an improvement in functional activity after inducing stroke via transient middle cerebral artery occlusion (tMCAO). This was done in middle-aged female rats that were treated with WBV as compared to the no-WBV group. These results suggest that WBV intervention may be a potential therapy to reduce post-ischemic frailty in old women after a stroke (92). Furthermore, the same research group also showed that in rat stroke models, WBV can protect against cognitive decline after undergoing a tMCAO (95) (more details in Section 4).
One example of a psychological disorder caused due to neuroinflammation is major depressive disorder (MDD) (96). Multiple studies in the context of MDD have indicated that exercise can deploy neuroprotection by enhancing synaptic plasticity, inhibiting apoptosis of neurons, ameliorating inflammation, and boosting the secretion of neurotrophic factors (97–99). Since lack of motivation to exercise and psychosomatic lethargy are intrinsic symptoms of MDD, establishing an exercise routine becomes challenging for the patients. In these cases, WBV can be used as a passive form of exercise that takes little time per day, especially since it also has been shown to beneficially alter depressive status in adolescents with depression (100). A preclinical study on the effects of 8 weeks of WBV intervention on neuronal loss, synaptic protein expression, and neurotrophic factors level in a rat model of chronic restraint stress-induced depression demonstrated that WBV significantly enhanced neuroprotection and recovery of degenerated neurons (101). The rat model was subjected to a vibration frequency of 30 Hz with an amplitude of 4.5 mm, 30 min per day, 6 days a week for 8 weeks, and behavioral and biochemical tests were performed. The authors hypothesized that the mechanism underlying the neuroprotection involves inhibiting the degeneration of neurons; inhibiting reactive microgliosis and astrocyte atrophy; protecting synapses, strengthening neural connections, as well as restoring impaired memory; reducing dendritic and axonal damage via microtubule-associated protein 2 (MAP2) and protecting damaged neurons from further damage; and enhancing the expression of trophic factors.
Oroszi et al., examined the dose-dependent effect of a 5-week-long chronic WBV intervention on anxiety-related behavior, memory, and motor functions, as well as a marker of (neuro)inflammation (88). Eighteen-month-old Wistar rats were stimulated by WBV for 5 or 20 min per day along with a control group with pseudo-WBV. After 5 weeks of WBV intervention, the anxiety-like behavior and motor performance were tested, which was then followed by brain analyses via immunohistological assays to determine hippocampal neuroinflammation. Both 5 and 20 min resulted in a significant decrease in microglial activation in the CA1 and dentate gyrus subregions.
In mice with myocardial infarction (MI), increased oxidative stress and inflammation can lead to harmful changes in the brain, causing nerve damage and neurodegenerative diseases (102). Sestrin 2 (SESN2) has been shown to alleviate oxidative stress and improve cognitive and cardiovascular health in aging and disease contexts (103). Overexpression of SESN2 activates the AMPK and PGC-1α pathway, enhancing cardiac function in aged mice and reducing cerebral ischemia/reperfusion injury in rats (104, 105). Feng et al., studied the effects of WBV on prefrontal lobe injury and dysfunction in mice with myocardial infarction (106). They focussed specifically on the SESN2/AMPK/PGC-1α signaling pathway. They revealed that WBV shows promising outcomes in alleviating prefrontal lobe injury and dysfunction. Specifically, the activation of the SESN2/AMPK/PGC-1α signaling pathway which mitigates oxidative stress and inflammation was identified as a key mechanism underlying this therapeutic effect.
In PD models, SESN2 upregulation triggers a reduction in neurotoxicity (107) and an autophagic response, preventing α-synuclein expression, apoptotic caspase-3 activation, and cytotoxicity in dopaminergic cells (108). In AD models, SESN2 induction counters amyloid beta (Aβ)-induced toxicity by promoting autophagy. These findings suggest that SESN2 may serve as a prognostic marker and therapeutic target in neurodegenerative diseases (109). Hence, exploring the impact of whole-body vibration (WBV) on SESN2 in the context of PD and AD could be intriguing.
In summary, WBV shows promise in alleviating neuroinflammation by reducing microglial activation in SCI and ischemic stroke models. It holds therapeutic potential for managing conditions such as MDD and anxiety. Additionally, it attenuates oxidative stress and neuroinflammation through the SESN2/AMPK/PGC-1α signaling pathway. The molecular pathways involved in neuroinflammation are summarized in Figure 1. However, despite promising preclinical evidence, the lack of clinical studies on neuroinflammation in human brain tissues limits our understanding of WBV’s efficacy in the clinics.
3.4 NeuroprotectionNeurotrophins or neurotrophic factors (NF) are proteins that are responsible for the survival, development, and function of neurons in both the central and peripheral nervous systems (110). Given their extensive influences on neurons, NFs are a good candidate for treating neurodegenerative and other neurological disorders (111). Some of these neurotrophins have been studied in the context of the effects of WBV, which will be discussed in this section.
3.4.1 Preclinical studiesInsulin-like growth factor-1 (IGF-1) is a growth factor that is also classified as an NF. IGF-1 can cross the blood–brain barrier and stimulate protein synthesis in neurons, glia, oligodendrocytes, and Schwann cells, and favor neuronal survival while inhibiting apoptosis (112). Wu et al. found that WBV slows atherosclerosis progression in mice by regulating IGF1 (113). Mice underwent 12 weeks of WBV at 15 Hz for 30 min. It was shown that WBV significantly reduced atherosclerotic plaque area and exhibited decreased serum IGF-1 and lower expressions of IL-6, IGF-1R, and p-IGF-1R protein in the aorta. Interestingly, serum IGF-1 peaked 30 min post-WBV for durations of 10, 30, 60, and 120 min. This suggests that appropriately timed WBV may impede atherosclerosis progression, associated with acute serum IGF-1 elevation and sustained lower aortic IGF-1 and IL-6 levels. Furthermore, Li et al., showed that in mice WBV also shows promise in promoting hypertrophy through mechanisms involving signaling pathways related to muscle growth such as IGF-1/IGF-1R–PI3K/Akt signaling (114). Moreover, the study by Peng et al., discussed above (Section 3.3.1) showed significantly increased levels of BDNF and IGF-1 in the hippocampus upon WBV training (101). This was not only associated with a protective effect on nerves and synapses but also resulted in an improvement in depression-like behavior in rats. Together, it can be concluded that WBV might be affecting the brain functioning via IGF. However, it has to be noted that only one of these studies analyzed whether the WBV-induced increase of IGF affects neural cells and/or brain functioning. Further investigating the neuronal effects of WBV-induced IGF might help to understand the role of WBV in neuroprotection, plasticity, and regeneration.
A study compared the effects of active exercise (treadmill running) and passive exercise (WBV) post-surgery (115). Rats underwent abdominal surgery, followed by active or passive exercise for 14 days. WBV protocol consisted of 30 Hz, 0.05–0.2 mm. The duration of WBV was 10 min on the first-day post-surgery and twice a day for 10 min from the second day onwards. The two vibration treatments were 6 h apart. Both active and passive exercise improved cognitive flexibility and memory was not affected by either treatment. Muscle strength increased upon active exercise but was unchanged by WBV. After the sacrifice, neuroinflammation was studied by observing microglial activation and neurogenesis by doublecortin (DCX) staining. Results showed no impact on surgery-induced inflammation but the treatment induced neurogenesis in the hippocampal region after both kinds of exercises. It is noteworthy that the expression of BDNF and IGF induces neurogenesis via DCX (116, 117). The results from this study may suggest that exploring these three factors may give better insight into the neurogenesis effects of WBV by studying.
The major NF brain-derived neurotrophic factor (BDNF) plays various important roles in the functioning of the brain. It is essential for brain development due to its involvement in differentiation, migration, and neuronal survival, and it exerts a role in dendritic development and in regulating synapse genesis and plasticity (118–121). Consequently, BDNF is fundamental for hippocampal functioning and learning (120–123).
In post-ischemic mice, following 4 weeks of vibration therapy, increased levels of BDNF were observed (92). MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) lesion mice, a model to study PD that were subjected to WBV, showed increased levels of BDNF and consequently a protective effect of dopaminergic neurons (124). In addition, Peng et al., have also shown that WBV might be protecting the neurons via alleviating BDNF levels in the hippocampus of MDD rodents (101). Similarly, young mice showed increased BDNF expression in their cerebellum and hippocampus after 3 months of vibration exposure, 3 days a week (125). Furthermore, they showed that WBV also can increase FNDC5 expression, which in turn further increases BDNF and thereby results in better musculoskeletal functions via myostatin and collagen I. Interestingly, FNDC5 expression upon exercise is directly linked to the functioning of irisin, which will be discussed in detail in Section 4.
3.4.2 Clinical studiesThere is inconsistency amongst the limited clinical studies that have been done on testing the effect of WBV on IGF. There are clinical studies that have seen no significant effects on serum IGF levels in human subjects after WBV subjection (126–131), while a study in elderly individuals shows that WBV affects the levels of IGF-1 (132). Participants experienced two interventions with a minimum 2-week gap: vibration and control (no vibration). In both interventions, individuals stood on a vibration plate with a slight knee flexion, undergoing five 1-min sessions separated by 1-min rest periods. For the vibration intervention, the plate vibrated at a frequency of 30 Hz with a 4-mm amplitude. This group observed an acute increase in the circulating levels of IGF-1 and cortisol in elderly individuals to a greater extent than an exercise protocol conducted without vibration.
Concerning BDNF, it has been shown that a single session of vibration with a frequency of 35–40 Hz, amplitude of 4 mm, and acceleration gravity ranging from 2.78 to 3.26 g exerts no effects of WBV on BDNF levels in women with fibromyalgia (133). In contrast, the same researchers reported in 2021 that WBV treatment for 6 weeks can increase the expression of BDNF in women with fibromyalgia (134). Additionally, it leads to improved lower limb muscle strength, aerobic capacity, clinical symptoms, and QOL after being subjected to vibration with the same vibration settings as the previous study. The only difference between the two studies was the duration of WBV intervention. The later study showed positive effects on BDNF levels after 6 weeks of WBV, whereas the 2018 study was based on a single exposure to WBV. This indicates that a longer – chronic intervention might be beneficial over a single-acute session of WBV.
Studies on elderly women with knee osteoarthritis suggest that the observed improved lower limb muscle performance after WBV might be mediated by an increase in BDNF levels in the serum (135). Their experimental setup consisted of 12 weeks of WBV intervention with a frequency of 35–40 Hz, amplitude of 4 mm, and acceleration that ranged from 2.78 to 3.26 g. In contrast, other studies have reported no apparent effect of WBV on BDNF levels in depression, spinal cord injury, and even in young, healthy women (131, 136, 137). Wunram et al., used a training program for individuals with depression that encompassed six different types of standardized exercises, with each exercise lasting 2 min at a frequency of 20 Hz and an amplitude of 2 cm, conducted over 6 weeks (131). They failed to see an effect of WBV on BDNF levels with this WBV protocol. However, as acknowledged by the authors, this study has some limitations, including a relatively small sample size, the absence of randomized controls, and the omission of psychosocial factors from consideration.
In a study that focussed on patients with spinal cord injuries, the vibration platform operated at 35 Hz with a 2 mm vertical displacement. During the training, participants engaged in fifteen 1-min bouts of vibration interspersed with 1-min rest intervals (136). As all participants had chronic spinal cord injuries, it remains uncertain whether a single bout of WBV would induce significant acute increases in BDNF in individuals with chronic injuries. Additionally, a single exercise session might not have provided an adequate stimulus to elicit an acute neurotrophic response. Furthermore, even in the case of young and healthy women who experienced vibrations with amplitudes of 2 and 4 mm and frequencies ranging from 20 to 60 Hz (137). These women participated in individually supervised sessions three times a week for 3 months. No significant effects were observed as an effect of WBV.
In conclusion, WBV can contribute to neuroprotection through neurotrophins like IGF-1, BDNF, and IL-10 (Figure 1). WBV increases FNDC5, a precursor protein of irisin which further increases BDNF (discussed in more detail in Section 4). Therefore, by investigating how WBV impacts neurotrophin expression and function, we may uncover novel strategies for enhancing brain health and promoting neuroprotection, which could pave the way for personalized approaches to maintaining cognitive well-being.
Previously, we have structured our sections into preclinical and clinical delineations. However, henceforth, we will refrain from such categorization for the forthcoming sections, as they lack relevance within these frameworks.
4 Irisin, a possible link between the effect of WBV in the periphery to the brain?Irisin is a relatively newly discovered exercise-induced myokine, which is involved in the regulation of several bodily processes such as glucose homeostasis, reduction of systemic inflammation, and modulation of energy metabolism through the browning of white adipose tissue (122). It is secreted by muscles in response to exercise (138) or WBV in humans (139, 140).
To understand how WBV may affect the brain through irisin production, it is important to know how irisin is produced. It is known that exercise induces an increase in calcium signaling which activates the transcriptional co-activator PPAR-γ co-activator-1 α (PGC1-α), which subsequently augments the expression of type 1 membrane protein, FNDC5 which is then cleaved to irisin (141). The impact of irisin on cognition is to a large extent elicited by the induction of BDNF expression. Post-exercise FNDC5 mRNA is upregulated in the hippocampus, which coincides with BDNF expression (122).
Since BDNF is essential for various brain functions and is implicated in exercise-induced cognitive benefits (142), parallel upregulation of FNDC5 and BDNF mRNAs in hippocampal neurons following exercise might suggest that irisin has a role in neuronal survival, activity, and cognitive functions.
A study in mice demonstrated that by regulation of the Akt and ERK1/2 pathway, irisin protects neuronal cells (PC12) from ischemic injury, suggesting that irisin may be a factor linking metabolism and cardio-cerebrovascular diseases (143). A recent study carried out on stroke rat models, showed the potential use of WBV in protecting against cognitive decline after going through a transient middle cerebral artery occlusion surgery (tMCAO) (95). Treating rats with the frequency of 40 Hz (amplitude not reported) for 15 min twice a day for 1 month after tMCAO significantly reduced the cognitive deficit in rats. It was speculated that this protective nature of WBV could be because of increased serum levels of irisin and decreased proinflammatory cytokines.
A recent review discussed the role of exercise-induced irisin in improving mental health in type 2 diabetes mellitus (144). Briefly, it has been shown that patients with diabetes mellitus experience a decline in cognitive function and memory loss (145–147). Wang et al., assessed whether irisin has a positive effect on memory and cognitive performance in a diabetic mouse model (148). They induced streptozotocin to establish a diabetic mouse model in 8-week-old male C57Bl/6 mice and assessed cortical and spatial memory through novel object recognition tasks and the Y-maze spontaneous alteration task (to determine short-term memory of 8 min). Upregulation in the levels of glial fibrillary acid protein (GFAP), a biomarker for astrocytes, reduction in synaptic protein expression, and an increase in the levels of IL-1β and IL-6 was observed. They also observed inhibition in the activation of proteins responsive to stress stimuli, like P38, STAT3 (Signal transducer and activator of transcription 3), and NFkB, in the diabetic mice. The reduction of cognitive function and memory which was observed in the diabetic mice could be avoided by irisin treatment, suggesting that an increase in irisin levels can improve and avoid the decline in cognitive function in diabetic mice.
Also, in clinical trials and studies, the effect of acute and chronic WBV training on circulating irisin levels has been evaluated (140). Young, healthy, untrained females were subjected to a 6-week program of WBV training with two sessions per week. The training regime consisted of seven different isometric exercises (different types of squats, elbow dips, and triceps exercises) in combination with a vibration frequency of 16, 19, and 21 Hz (increased during training) and an amplitude of 2.5 mm during the first 4 weeks and 5 mm during the last 2 weeks. The duration of each training session was progressively increased every 2 weeks from 11 to 18.5 min. Blood was drawn before and immediately after an acute bout of exercise at baseline and after 6 weeks of training. The resting irisin levels were not different at baseline and after 6 weeks of training, whereas an acute bout of vibration exercise significantly elevated circulating irisin levels by 9.5 and 18.1%, respectively at both 0 and 6 weeks of training. These findings indicate that acute bouts of WBV exercise can increase circulating irisin levels.
Moreover, a separate clinical study examined the influence of WBV on oxidative stress markers and irisin blood levels in women with fibromyalgia (149). WBV was subjected three times per week for 6 weeks, involving dynamic squats on a synchronized vibrating platform. The mechanical stimulation parameters included a vibration frequency of 35–40 Hz and an amplitude of 4 mm. The findings revealed that WBVT resulted in a reduction of visceral adipose tissue, an elevation in blood irisin levels, and a decrease in blood levels of the oxidative stress marker thiobarbituric acid.
Apart from its function in improving cognitive function and memory loss in diabetes, irisin has also been shown to have a potential role in treating AD (150). This study implied that the neuroprotection of irisin was mediated by blocking the release of Il-1β and IL-6 from cultures of astrocytes instead of its direct action on neurons. Their results also suggested the importance of the NFκB signaling pathway in the regulation of irisin on astrocytes exposed to Aβ.
Although there are a lot of studies indicating the positive effect of the exercise-induced increase in irisin activity on cognitive functions and neuroprotection, however, there are very few studies done to specifically study the link between WBV and irisin. Further studying the effect of WBV on irisin activities might give a better insight into standardizing WBV protocols to exploit the beneficial effects of irisin upregulation for people who cannot indulge in physical exercise.
5 Brain disordersRecently, a systematic review revealed the potentially beneficial effects of WBV for brain disorders using animal models but also stressed that WBV as a form of therapy needs further development (151). Therefore, in this segment, studies conducted on animal models or patients with various brain-related disorders will be discussed only briefly.
A common problem with aging is increased frailty which increases the risk of falls that subsequently can cause TBI (152). TBI can also be caused due to reasons that are independent of age factors like assault, motor vehicle accidents, incidents related to sports activities, or any other accidents that cause an injury to the brain. A preclinical study in mice indicates that WBV treatment can be an ideal treatment for patients suffering from TBI (22). In the case of TBI, 30 Hz of WBV for 20 days can reduce neuronal damage and improve cognitive and functional outcomes after TBI. This study shows that WBV: (1) alleviates cortical edema, (2) suppresses microglial activation, (3) inhibits GFAP expression that relates to astrocyte activation, (4) prevents the increase of IL-1β, TNFα, and IL-6 and promotes the increase of IL-10 which is an anti-inflammatory cytokine, (5) facilitates neuronal apoptosis, (6) improves exploratory behavior and general activity, (7) decrease learning and memory deficit after TBI, and (8) augment learning and memory deficit caused by TBI. Another preclinical study assessed the effects of WBV on induced brain injury (subarachnoid hemorrhage) in mice (87). Mice underwent WBV twice daily for 20 days at a frequency of 30 Hz. Their findings indicate that WBV decreases apoptosis, and moderates the heightened expression of GFAP (astrocyte marker) and Iba-1 (microglia marker). Additionally, WBV alleviated the loss of neurons i
留言 (0)