Down syndrome (DS) is the most common human chromosomal disorder, accounting for approximately 1 in 500 live births globally (Al-Biltagi, 2015). This disorder is caused by a partial or complete triplication of chromosome 21 and was first recognised by John Langdon Down (1887). Individuals with DS often present with characteristic facial features, intellectual disability (ID), hypotonia, muscle weakness, early-onset Alzheimer’s disease, increased incidence of leukemia, and heart deficits (Malt et al., 2013). While the presence and severity of these phenotypes may vary between individuals, almost all exhibit some degree of ID. The underlying cause of this is primarily the result of neurodevelopmental complications in specific brain regions. Unfortunately, there is currently no cure nor effective treatment for those with DS, leading many individuals to depend on familial or societal support for their lifetime. However, recent research has identified the Dual specificity tyrosine phosphorylation regulated kinase 1 A (DYRK1A) gene and protein as a potential therapeutic target for DS and other diseases due to its dose-dependent nature (Feki and Hibaoui, 2018; Liu et al., 2022). This gene, located at 21q22.13, plays a critical role in the development of the cognitive phenotypes associated with DS. Researchers have since been attempting to modulate the expression of the DYRK1A protein to recover one’s cognitive ability (Feki and Hibaoui, 2018). Currently available DYRK1A inhibitors such as epigallocatechin gallate (EGCG) derived from green tea or harmine, a hallucinogenic alkaloid, have shown a preliminary reduction in neurodevelopmental abnormalities across various animal models of DS and some human trials (Guedj et al., 2009; Adayev et al., 2011; Jarhad et al., 2018). However, these inhibitors are designed to non-specifically modify the protein’s function, increasing undesirable off-target effects, and rendering them unsuitable for DS intervention in humans.
In contrast, antisense oligonucleotides (ASOs) are emerging as a potential therapeutic modality for numerous genetic diseases that can be addressed by modulating gene expression. The specificity of the ASOs rely on Watson and Crick base-pairing to the target sequence and the oligomer chemistry then determines the subsequent mode of action. RNA or DNA-like oligomers, when annealed to a target RNA strand can induce degradation of the target through RNA-induced silencing complex or activation of RNase-H (Deleavey and Damha, 2012). In contrast, other oligomers with fully-modified bases and/or backbones can act as steric blockers and redirect normal protein translation, pre-mRNA splicing and polyadenylation (Iversen, 2001; Amantana et al., 2007; Liang et al., 2015; Wang et al., 2018; Scoles et al., 2019). Therefore, by altering the expression of DYRK1A at the level of RNA, we propose that ASOs could provide a highly specific and effective treatment option addressing ID and cognitive issues for those with DS, improving their overall quality of life.
This review will outline the current knowledge of DS, with a focus on its cognitive phenotypes and include a brief discussion on the debate surrounding the DS critical region (DSCR). This will be followed by a summary of DYRK1A, its role in DS and the DYRK1A inhibitors currently under investigation. Additionally, we will provide a brief analysis of ASOs with a focus on their mechanism of action and current U.S. Food and Drug Administration (FDA) approvals. The review will then conclude with an outline of some of the current challenges and strengths of developing and distributing ASOs. At present, there is a crucial need for novel therapies that can treat these individuals with high efficacy and low side effects. This review introduces the rationale behind the research intended to treat DS using ASO-based therapeutics.
2 Down syndromeThe complete trisomy of chromosome 21 is the most common cause of DS and occurs from an error in cell division during the early development of the egg or sperm. It has been observed that approximately 88% of cases are of maternal origin, with incidences increasing with higher maternal age (Gómez et al., 2000; Fitzpatrick et al., 2017). Complete trisomy most often results from meiotic nondisjunction, accounting for 90–95% of DS cases (Papavassiliou et al., 2015). This occurs when the chromosomes fail to segregate to the opposite poles during meiosis, resulting in either trisomy or monosomy (Coppedè, 2016). Mosaicism can also result in DS, which accounts for 2–4% of DS cases, and causes a partial triplication of chromosome 21. Mosaicism can occur in two ways; after fertilisation, when an early mitotic error in an embryo results in the partial triplication of chromosome 21 (Papavassiliou et al., 2015); or it can occur in a DS zygote where a mitotic error causes some cells to revert to a normal karyotype (Papavassiliou et al., 2015). The remaining 2–4% of DS cases are caused by the inheritance of a chromosomal rearrangement/translocation which results in a partial triplication of chromosome 21. This occurs when chromosome 21 attaches to another chromosome, typically binding to chromosome 14 but can also bind to 13, 15, 21 or 22 (Bornstein et al., 2010). The various mechanisms for the development of DS are depicted in Figure 1.
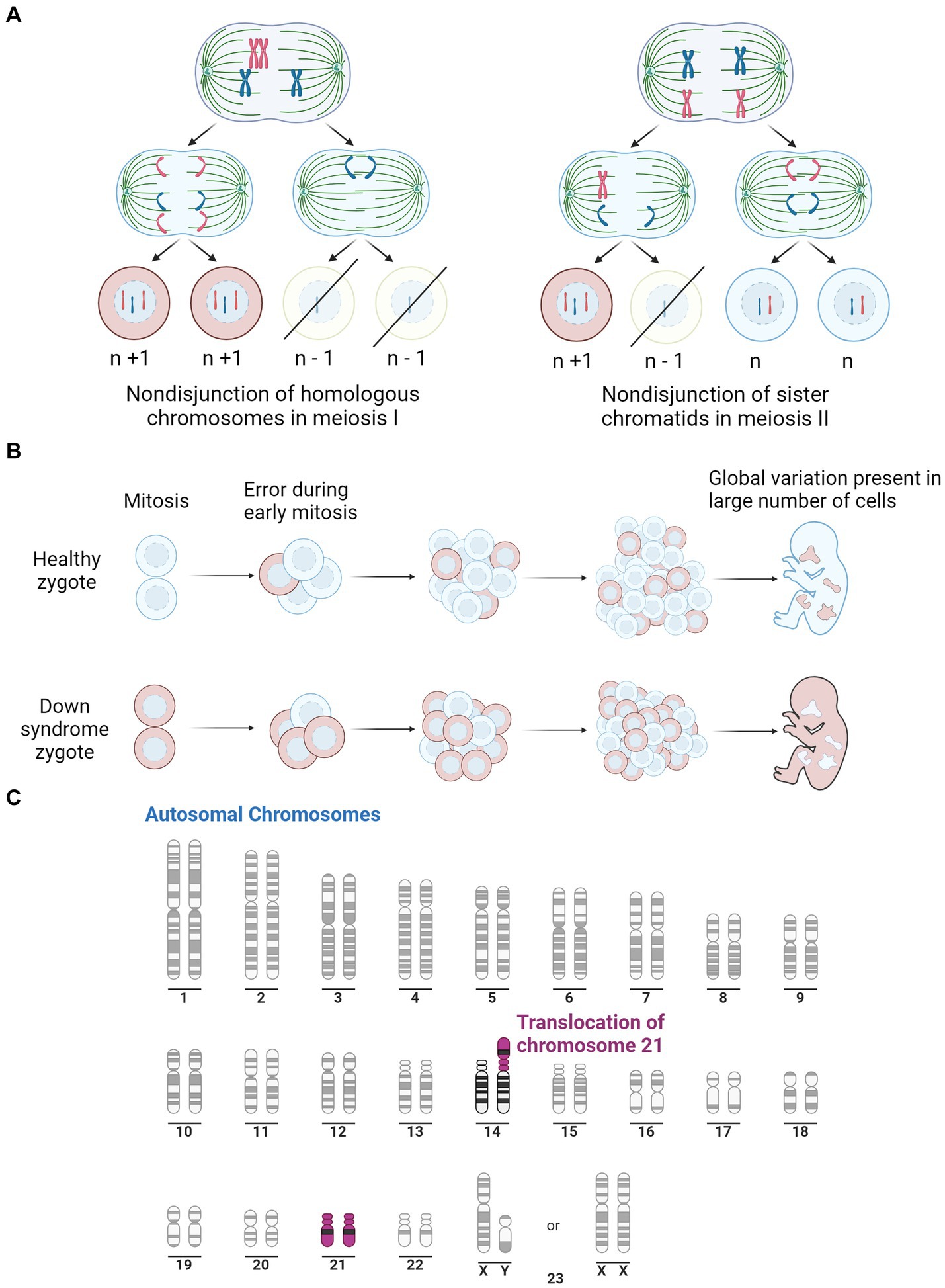
Figure 1. Illustration of the three pathways for Down syndrome development. (A) Representation of mitotic nondisjunction with the left being the most common form, resulting in a complete trisomy of chromosome 21. Where ‘n’ represents the typical number of chromosomes. (B) Is a representation of the partial trisomy of chromosome 21 through mosaicism which occurs from an early error in mitosis which results in a variation in chromosome number across the cells. Peach colour representing cells with typical number of chromosomes, light blue representing chromosomes with trisomy 21. (C) Is a representation of a second partial trisomy of chromosome 21 where chromosome 21 binds to chromosome 14, the most common site, leading to a partial trisomy in some cells. Purple represents Chromosome 21. Adapted from “Human Karyotype,” by BioRender.com (2023). Retrieved from https://app.biorender.com/biorender-templates.
2.1 PhenotypesThose affected by DS present with a host of characteristic phenotypes and health complications that impede regular functioning, outlined in Table 1. These individuals have an increased risk of heart disease, early onset Alzheimer’s disease, leukemia and testicular cancer (Hill et al., 2003; Hasle et al., 2016). This not only disturbs the individual’s quality of life, but also puts a strain on family, carers and the health care system (Hill et al., 2003). While individual phenotypes vary, certain characteristics such as ID, hypotonia, craniofacial dysmorphology and the histopathology of Alzheimer’s disease, are present to some degree in all cases of DS. Understanding the key cognitive components of DS is crucial in comprehending the unique challenges of developing effective interventions that can improve their overall wellbeing.
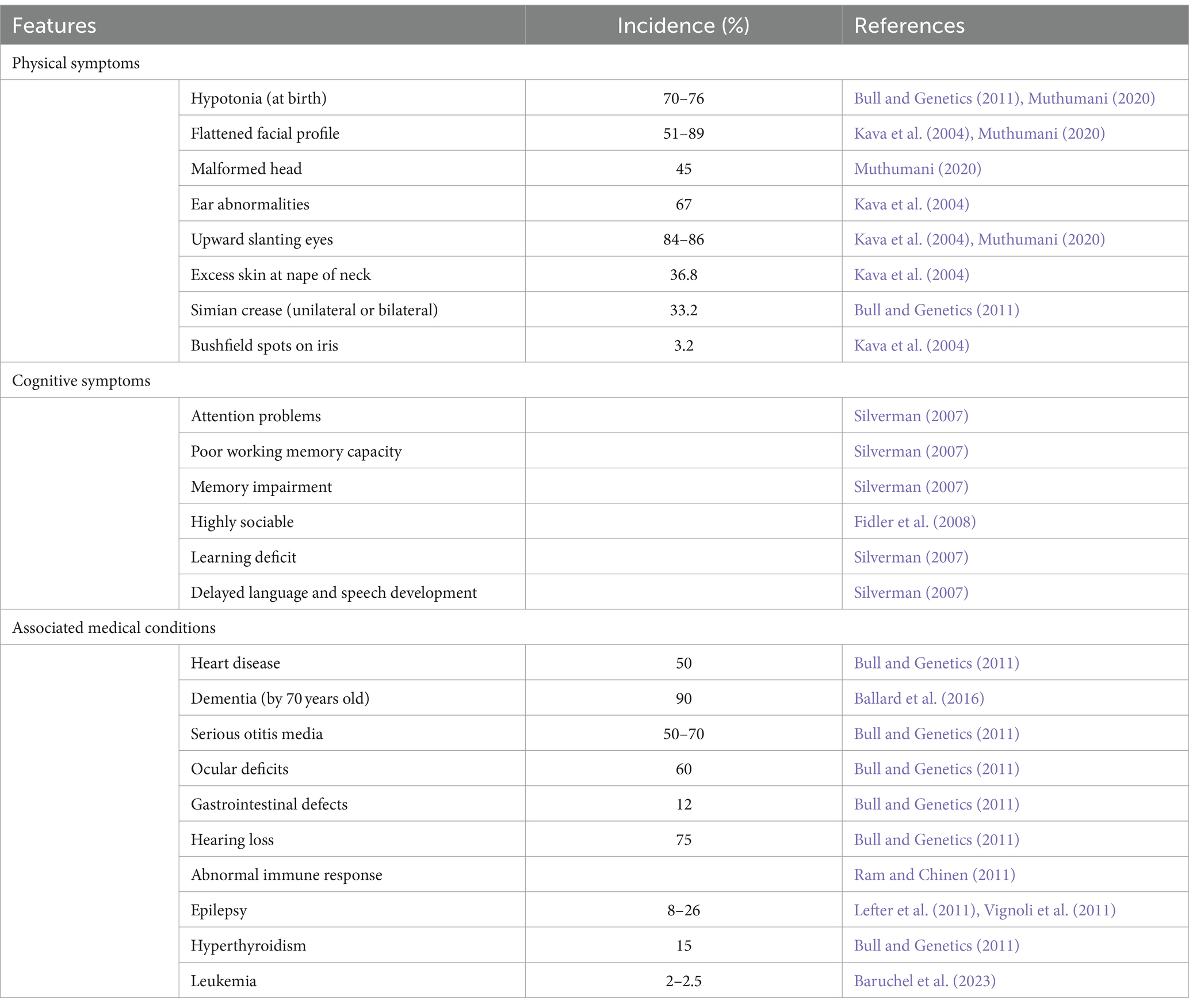
Table 1. Outline of the common features observed in Down syndrome and incidence where applicable.
2.2 CognitionIndividuals with DS account for approximately 10–20% of the intellectually disabled population, with some form of impairment present in nearly all cases of DS (Torr et al., 2010). This is the result of overexpressed genes altering neural development and functioning. Individuals with DS generally have an intelligence quotient (IQ) that ranges from 20 to 80. Their IQ being its highest when they are children and gradually declines as the individual ages (Carr, 1988; Anneren and Edman, 1993; Chapman and Hesketh, 2000). This is believed to be a consequence of the slow rate of neural development when compared to unaffected children (Carr, 1988; Chapman and Hesketh, 2000). By adulthood, an individual with DS is likely to have an IQ of 25 to 55, equating their mental age to be approximately 7–8 years old (Pennington et al., 2003).
The specific cognitive shortfalls associated with DS include deficits in memory, language, speech, hearing, processing speed and numerous aspects of executive function (Silverman, 2007; Lanfranchi et al., 2010). In early childhood, ID is not as prominent, with steady cognitive decline being observed from late childhood into early adulthood, with a further decline in middle to late adulthood, typically associated with early onset Alzheimer’s disease (Grieco et al., 2015). Interestingly, there is a discrepancy between cognitive tasks, with verbal-based tasks being performed worse than non-verbal-based tasks (Abbeduto et al., 2001; Couzens et al., 2011; Channell et al., 2014). Language skills until age 5 are generally within the expected developmental range, however, steadily decline into adulthood (Guralnick, 2002). These language shortfalls have been hypothesised to be a secondary consequence of the deficit in verbal working memory more specifically, in the phonological loop located in the temporal lobe (Chapman, 2006; Vicari and Carlesimo, 2006). Additionally, those with DS have been shown to exhibit numerous deficits in executive function, including impairments of attention, inhibition, processing speed, multi-tasking, self-monitoring, working memory and organisation (Grieco et al., 2015). These executive function skills are processed in the frontal lobes and have a secondary impact on one’s learning ability. Furthermore, learning and memory deficits are believed to result from errors in the encoding and retrieval of memories, which are processed in the temporal lobe and the hippocampus (Carlesimo et al., 1997). While there is some debate in the literature over the underlying mechanisms that result in these cognitive deficits, there is largely an agreement that the individual deficits in each cognitive area are largely of primary origin, with some top-down cascade also influencing cognitive functioning (Grieco et al., 2015). This conclusion has been supported by studies that identify alterations in brain structure in similar brain regions.
2.3 Alterations in brain structureDeficits in cognition are almost always the result of abnormal brain structure, with global cortical volume loss often being correlated with cognitive decline (Jack et al., 2008; Soria-Pastor et al., 2008; Calabrese et al., 2011; Mcdonald et al., 2012). Consistently, the brains of individuals with DS are smaller when compared to healthy individuals, with a study on children finding a 13.3% reduction in total brain volume (Rachidi and Lopes, 2011; Śmigielska-Kuzia et al., 2011). This has been observed to increase to 20% in adults with DS (Kemper, 1991). Interestingly, this global reduction in brain volume has not been observed in trisomic mouse models (Contestabile et al., 2010). Some suggest that these findings indicate that volume is not directly related to cognitive impairment. However, we postulate that this may be a result of the difference in neuronal complexities between mice and humans, resulting in more severe and widespread disability in humans due to the complexity of our neuronal development when compared to that of mice. Global cortical volume loss often results from a catastrophic cascade of errors that leads to drastically reduced neuronal cell numbers. With that said, early DS mouse models exhibited increased ventricle size and brachycephaly (Guedj et al., 2012). These phenotypes typically indicate some form of cortical atrophy, which may be attributed to improper neurodevelopment or neuronal cell death that has also been observed (Arbones et al., 2019).
Across the literature, the primary regions affected in mice are the cerebellum and the hippocampus. Some mouse models have detected a cerebellar volume loss of up to 12% and in models that do not exhibit a volume loss, a lower density of neuronal cells is still observed (Baxter et al., 2000; Olson et al., 2004a). A well-characterised mouse model for DS is the Ts65Dn mouse which is trisomic for approximately two-thirds of the genes orthologous to human chromosome 21 (Hsa21). Interestingly, in Ts65Dn mice, there is no alteration in hippocampal volume at 7 months of age and no alteration in neuronal density at one month of age (Lorenzi and Reeves, 2006; Olson et al., 2007). However, at 16–17 months old, neuronal density has been found to be significantly lower in the cornu ammonis (CA) 1 region of the hippocampus and synapse density has also been found to be reduced in the dentate gyrus, CA1 and CA3 when compared to diploid mice (Kurt et al., 2004; Lorenzi and Reeves, 2006). This is similar to what is observed in humans, where these regions have been reported to have a reduced volume of the brainstem, frontal, temporal and parietal lobes. It is suggested that this is indicative of a decreased formation of new neurons during development and increased atrophy in adult life. An overview of the common neuronal features of DS in humans can be found in Figure 2. Additionally, in this figure we have indicated similar phenotypes observed in the murine models previously mentioned.
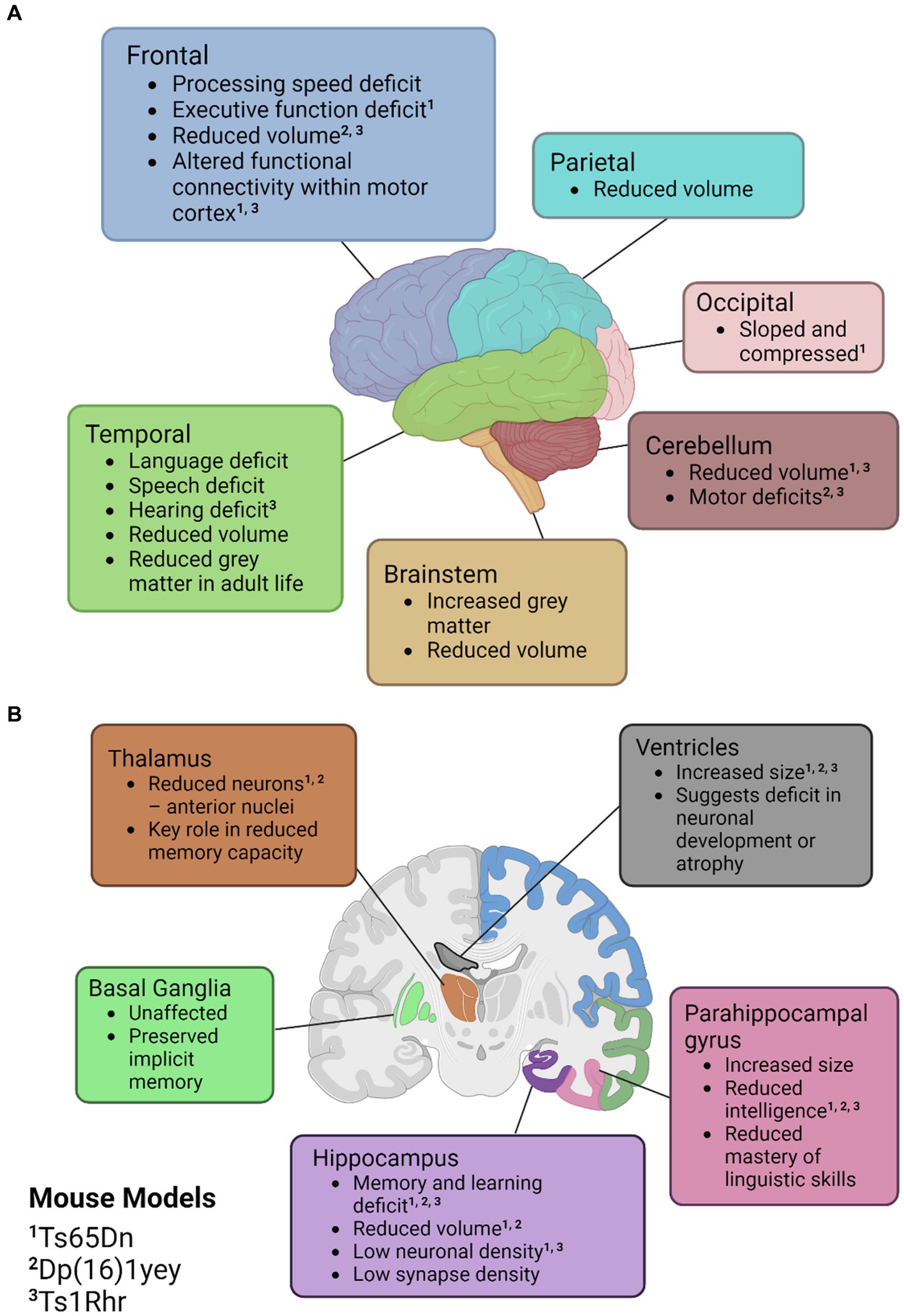
Figure 2. Illustrations of the neuroanatomical and cognitive features of Down syndrome. (A) Cortical mapping of affected regions. (B) Coronal section revealing the subcortical structures influenced by Down syndrome. Mouse models with known similar phenotypes have been identified with superscripts; ‘1’ for Ts65Dn, ‘2’ for Dp(16)1yey, and ‘3’ Ts1Rhr. Created with BioRender.com.
Studies on humans with DS have noted similar abnormalities to the murine models listed in comparable brain regions. Evidence of improper cortical development from DS-affected brains have been observed at the cellular level with abnormal dendrite formation, impaired neurogenesis, lower neurotransmitter counts, and reduced synaptic proteins (Vacca et al., 2019). Notably, dendritic abnormalities in the hippocampus and the dentate gyrus appear to be the most pervasive, with murine models exhibiting reduced synaptic density, altered dendritic arbours, and altered dendritic spines (Contestabile et al., 2010). Numerous other alterations in neurotransmitter systems, cellular mechanisms, and degradation due to age-associated dementia are outlined in the literature. However, these are outside of the scope of this review, but have been reviewed by Arbones et al. (2019) and Vacca et al. (2019). The previously mentioned alterations in brain morphology are common hallmarks of many forms of ID and provide the morphological basis for the poor cognition observed in DS individuals. Consistent neurobiological abnormalities had sparked interest in the possibility of a critical region of chromosome 21 that may be present in all cases of DS, providing a rationale for the main phenotypes and potential therapeutic target.
2.4 Down syndrome critical regionAs there exists both a complete and partial trisomy of chromosome 21 in DS populations, the concept of a DSCR has been debated amongst the literature. Korenberg et al. (1990) hypothesised a DSCR that extended from the start of q21.2 to the end of chromosome 21. This was heavily based on earlier literature that pinpointed a crucial area on the distal end of chromosome 21. This was later refined by Delabar et al. (1993) using genotype–phenotype analysis of 10 people with partial trisomy 21 to the region D21S55 (~37.8 Mb) to MX1 (~41.7 Mb). They hypothesised that this region is responsible for 19 of the 33 phenotypes they assessed. Another paper by Korenberg et al. (1994) emphasised the importance of genes outside the D21S55 region and, through their own molecular and phenotypic analysis, defined a region with a proximal boundary of D21S17 (~35.9 Mb) and a distal boundary at MX1. All DCSRs mentioned can be seen in Figure 3 (Korenberg et al., 1990; Delabar et al., 1993; Korenberg et al., 1994; Yamamoto et al., 2011; Schnabel et al., 2018).
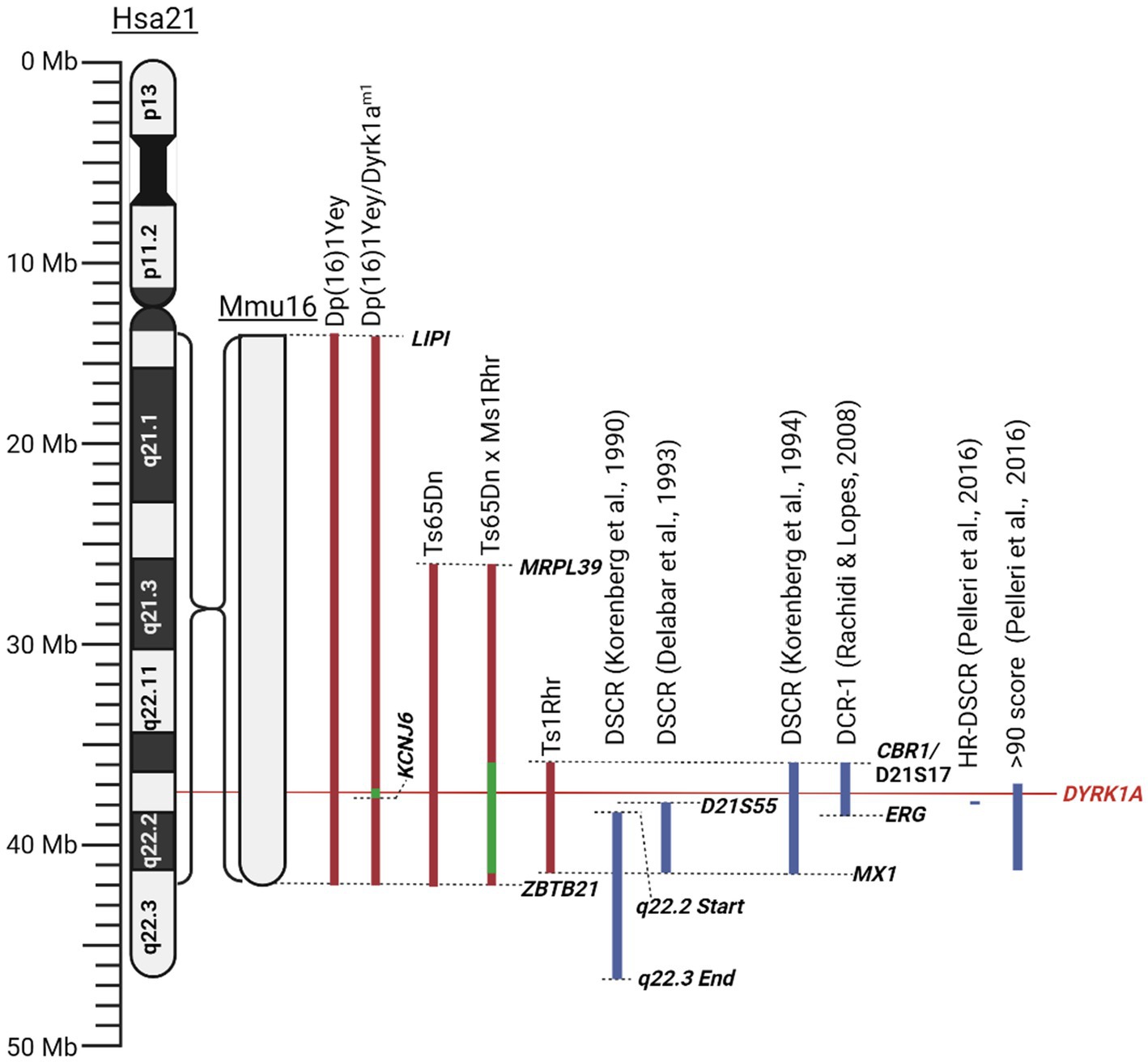
Figure 3. Schematic of mouse mutants aneuploid segments and Down syndrome critical regions described in this literature review. Human chromosome 21 (Hsa21) and the orthologous region of mouse chromosome 16 (Mmu16) are shown. Dashed lines identify locations of specific genes or chromosomal landmarks. All mouse models are aligned according to Hsa21, and the Mmu16 chromosome segment is scaled according to Hsa21. Red indicates trisomic sections, green indicates haploid sections, and blue indicates hypothesised Down syndrome critical regions. Created with BioRender.com.
Since its conception, the idea of a DSCR has been heavily debated, with some reviews and more recent literature finding their own definitions of the region, such as in the review by Rachidi and Lopes (2008). This led to the question of whether it is valid to conclude that such a region exists at all. Recent research has failed to support the prevalence of a DSCR, as per the original Korenberg et al. (1990) hypothesis. Two critical studies on mice had shown that the triplication of the DSCR alone does not result in the characteristic facial or neurological DS phenotypes (Olson et al., 2004b, 2007). The physical phenotypes observed when comparing DS mice (Ts65Dn) and triplicated DSCR-only mice (Ts1Rhr) presented an opposite phenotypic pattern, where Ts65Dn mice were smaller while Ts1Rhr mice were larger (Olson et al., 2004b). Additionally, neurological characteristics like cerebellar volume, hippocampal size and function were either not as severe or protected in Ts1Rhr mice, suggesting that the DSCR proposed in the original hypothesis does not fully recapitulate the main DS phenotypes (Olson et al., 2007). However, some caution is advised when drawing conclusions based on this study as the orthologous region of Hsa21 in murine models is located across three separate chromosomes, potentially adding some variability. Additionally, animal models may require multiple copies to replicate the human phenotype, such as in experiments involving SOD1 replicating motor neuron disease (Ferraiuolo et al., 2007; Nardo et al., 2016). Future studies should be conducted with more copies of the DSCR to see if phenotypes differ. Additionally, the researchers bred trisomic Ts65Dn mice with monosomic DSCR Ms1Rhr mice, producing offspring that were trisomic for chromosome 21 with a normalised analogous DSCR (Olson et al., 2007). These DSCR-normalised mice were found to perform similarly in the Morris water maze when compared to a control euploid mouse (Olson et al., 2007). These researchers agree that the DSCR hypothesis has been disproved, however, they concluded that the genes within the DSCR are necessary but not sufficient to produce a learning deficit in DS (Olson et al., 2007). Most importantly, this suggested that a return to normal gene expression in DSCR genes may induce an improvement in cognitive functioning. Contradicting these studies, other researchers concluded that the DSCR is sufficient to produce the DS phenotype, coming to this conclusion as Ts1Rhr mice were found to significantly differ on 20 of 48 characteristics when compared to control ‘2 N’ mice (Belichenko et al., 2009). These included altered long-term potentiation effects, dendritic spine enlargement and density in the fascia dentata, among others (Belichenko et al., 2009). While they concluded that the triplicated region present in Ts1Rhr mice is sufficient to reproduce the DS phenotype, they also mention that differing combinations of single or multiple gene dosage effects may give rise to different phenotypes. This is very similar to a more recent ‘gene dosage effect’ hypothesis proposed by Moreau et al. (2021). This hypothesis suggests that DS results from an imbalance in gene dosage, leading to the overexpression of specific causative genes, which can alter interactions between other genes in the genome. Unlike the DSCR hypothesis, which attributes the DS phenotypes solely to the genes within a specific region. The gene-dosage effect hypothesis considers a broader range of genetic interactions across the entire genome that are responsible for the DS phenotypes. We consider this to be more plausible than the original DSCR hypothesis. However, the gene-dosage hypothesis requires more sophisticated methods to identify the most relevant genes involved in DS.
Pelleri et al. (2016) conducted a thorough review which detailed the score for association with DS of numerous individuals with partial trisomy 21 to identify a highly restricted DSCR (HR-DSCR). This region is defined by a triplication of genes present in all DS cases and absent in all non-DS cases. They identified the HR-DSCR region as genes with a prevalence score over 97. However, it contained genes only homologous to the chimpanzee genome that have not been thoroughly researched. We suggest that it would be more useful to direct future exploration toward genes just outside this HR-DSCR that are homologous to more common disease models. The small region with a score over 90 includes nearly the entire q22.13 segment (Pelleri et al., 2016). A high score in this study indicates an increased probability of association with DS. This region includes seven protein coding genes: DYRK1A, DSCR3, TTC3, PIGP, RIPPLY3, KCNJ15, KCNJ6, and DSCR4. Of these the former 5 are expressed in the adult brain and are potential targets for DS treatment. Given the wealth of literature and its potential implications for various diseases, our review will focus on the gene DYRK1A. From the Pelleri et al. (2016) study, this gene has a prevalence score of 91 out of 100 for its association with DS. Additionally, DYRK1A has been consistently overexpressed in DS human and mouse models and has been found to play a vital role in neural function, processing and development (Guimera et al., 1999; Dowjat et al., 2007). Interestingly, a recovery in the developmental cognitive deficit was reported after a partial rescue of DYRK1A in DS mice. Researchers utilised mice with a gene trap vector inserted in intron 4 resulting in disruption of the 321 amino acid kinase domain resulting in a haploinsufficiency of DYRK1A and was referred to as Dyrk1am1 (Jiang et al., 2015). They bred this mouse with their DS mouse model, Dp (16)1, to generate a DS mouse with a normalised DYRK1A expression, Dp (16)1/Dyrk1am1 (Jiang et al., 2015). These mice exhibited performance improvements in T-maze and contextual fear-conditioning tests when compared to Dp (16)1 mice (Jiang et al., 2015). Thereby supporting the potential causative role of DYRK1A in the cognitive phenotype and potential for recovery if the DYRK1A gene is normalised. All the chromosomal segments for the murine models are presented in Figure 3.
The conclusion of much of the literature appears to be that a strictly defined DSCR does not exist, and while there are certain genes that appear necessary to produce the phenotypes of DS, these are not sufficient when viewed in isolation. Therefore, we also agree that the restrictive DSCR approach is not adequate to explain the phenotypic outcomes of DS. Importantly, the recovery of the widely researched gene DYRK1A has shown to alleviate the severity of cognitive phenotypes in DS models, making the regulation of these genes a promising potential therapeutic strategy.
3 DYRK1ADYRK1A has been found to play a vital role in the regulation and functioning of the processes involved in neurodevelopment (Olson et al., 2004b, 2007). The first evidence was studied in Drosophila mini brain (mnb) mutants which exhibited altered neural proliferation and smaller brain size (Tejedor et al., 1995). Mini brain (discovered in insects) is an orthologous gene to the vertebrate DYRK1A; hence forth in this review, mnb/DYRK1A will only be referred to as DYRK1A (Tejedor and Hämmerle, 2011). Among others in the DYRK family, DYRK1A is activated via tyrosine autophosphorylation in the activation loop but phosphorylates its substrates on serine and threonine residues only (Lochhead et al., 2005). However, DYRK1A is unique in that it is the most ubiquitously expressed when compared to other DYRK members. These other DYRK’s are often more restricted and often highly expressed in the testes and muscle (Becker et al., 1998; Leder et al., 1999; Zhang et al., 2005; Sacher et al., 2007). Multiple DYRK1A transcripts exist through alternative splicing and untranslated region changes Figure 4A, which in turn encodes two main DYRK1A protein isoforms Figure 4B. It should be noted that there are other shorter isoforms that are reported via internal splicing events, however, there is currently no evidence of a functional difference between these. Examining the protein isoforms 1 and 2, DYRK1A exhibits a conserved N-terminal motif that stabilises the kinase domain during protein maturation (Soundararajan et al., 2013). This N-terminal is shared amongst the other members of the DYRK family and is commonly referred to as the DYRK homology box (DH). DYRK1A exhibits a two nuclear localisation signals (NLS) one next to the DH and the other within the kinase domain. The main DYRK1A isoforms also exhibit PEST, polyhistidine (His) and serine-threonine rich (Ser/Thr) motifs.
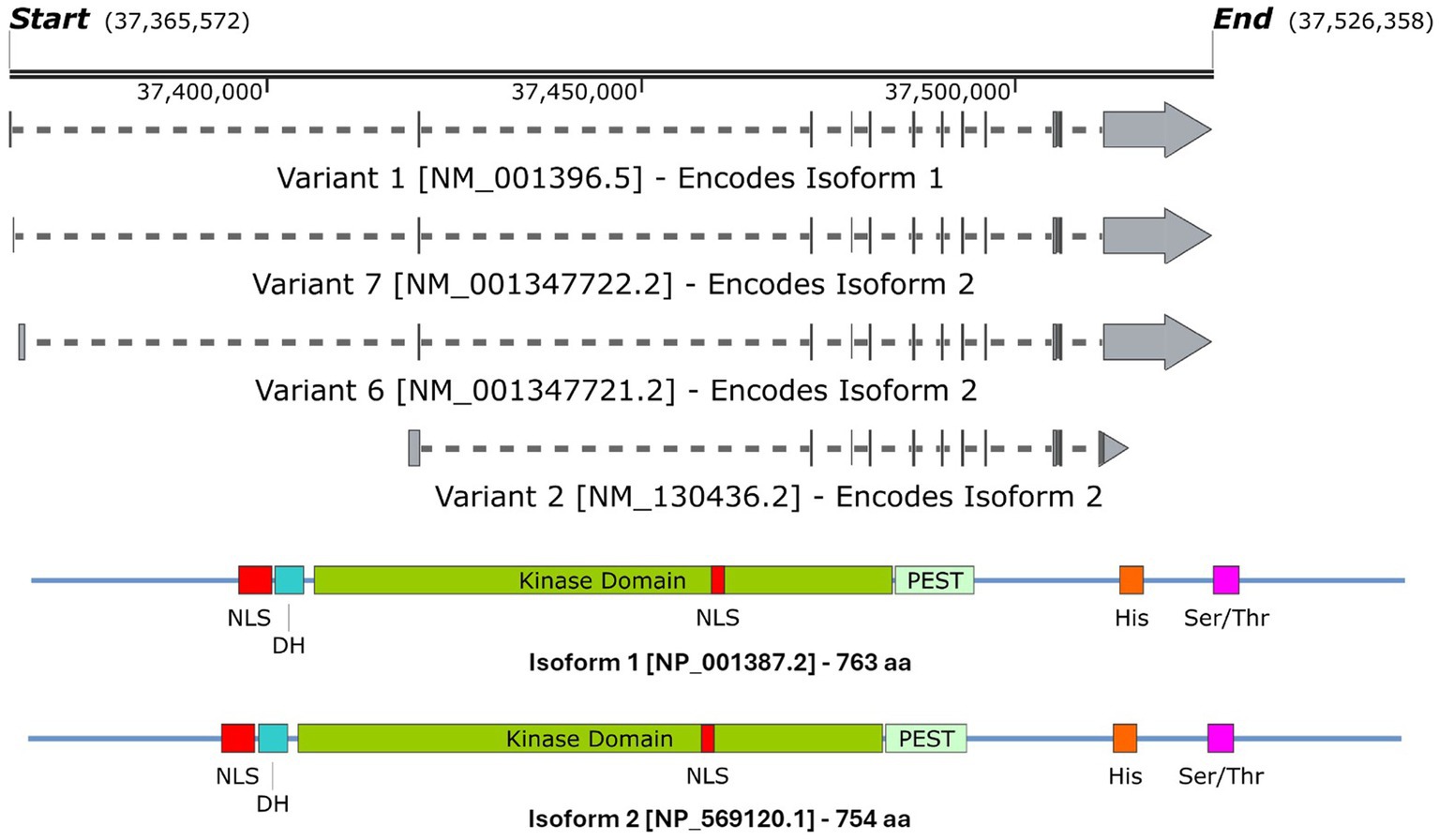
Figure 4. A schematic representation of (A) the DYRK1A mRNA transcripts that encode (B) isoforms 1 & 2 DYRK1A proteins. Both isoforms share the same features and domains but differ in amino acid coordinates. The amino acid positions of each domain/feature are specified for isoform 1. These include: two nuclear localisation signals (p117–135 & p380–386, NLS); a DYRK homology box (p137–152, DH); a PEST-rich region (p482–525, PEST); a polyhistidine stretch (p607–619, His); and a serine and threonine rich region (p659–672, Ser/Thr). Created with SnapGene software (www.snapgene.com).
DYRK1A has been found to exhibit numerous effects on key aspects of the central nervous system (CNS) such as synaptic plasticity and neuronal differentiation (Arbones et al., 2019; Atas-Ozcan et al., 2021; Ananthapadmanabhan et al., 2023). Additionally, it plays a broader role in cellular development, function and repair. This includes vital functions in cell cycle progression, splicing, chromatin transcription, cell signalling, exocytosis, endocytosis and apoptosis. Recent research highlights DYRK1A’s role in modifying RNF169, which is crucial for DNA repair following damage (Guard et al., 2019). The protein interactions that DYRK1A alters to produce these effects are outlined in Figure 5. Additionally, the effect on neurodevelopment was further observed by a study on haploinsufficient DYRK1A+/− mice that had smaller brains and fewer neurons when compared to wild-type littermates (Fotaki et al., 2002). Additional studies have noted that altered DYRK1A expression influenced neural numbers, neurogenesis, synaptogenesis, neural functions, and neurotransmission across various human and murine models (Arbones et al., 2019). Expression of DYRK1A across the lifespan in mice is relegated primarily to the CNS. It has been found to peak near birth during neuronal dendritic morphogenesis and later maintained at lower levels in adulthood (Okui et al., 1999). This provided evidence of its crucial role in neurodevelopment and maintenance and has shown that an alteration in DYRK1A expression levels can greatly affect the individual’s neural functioning.
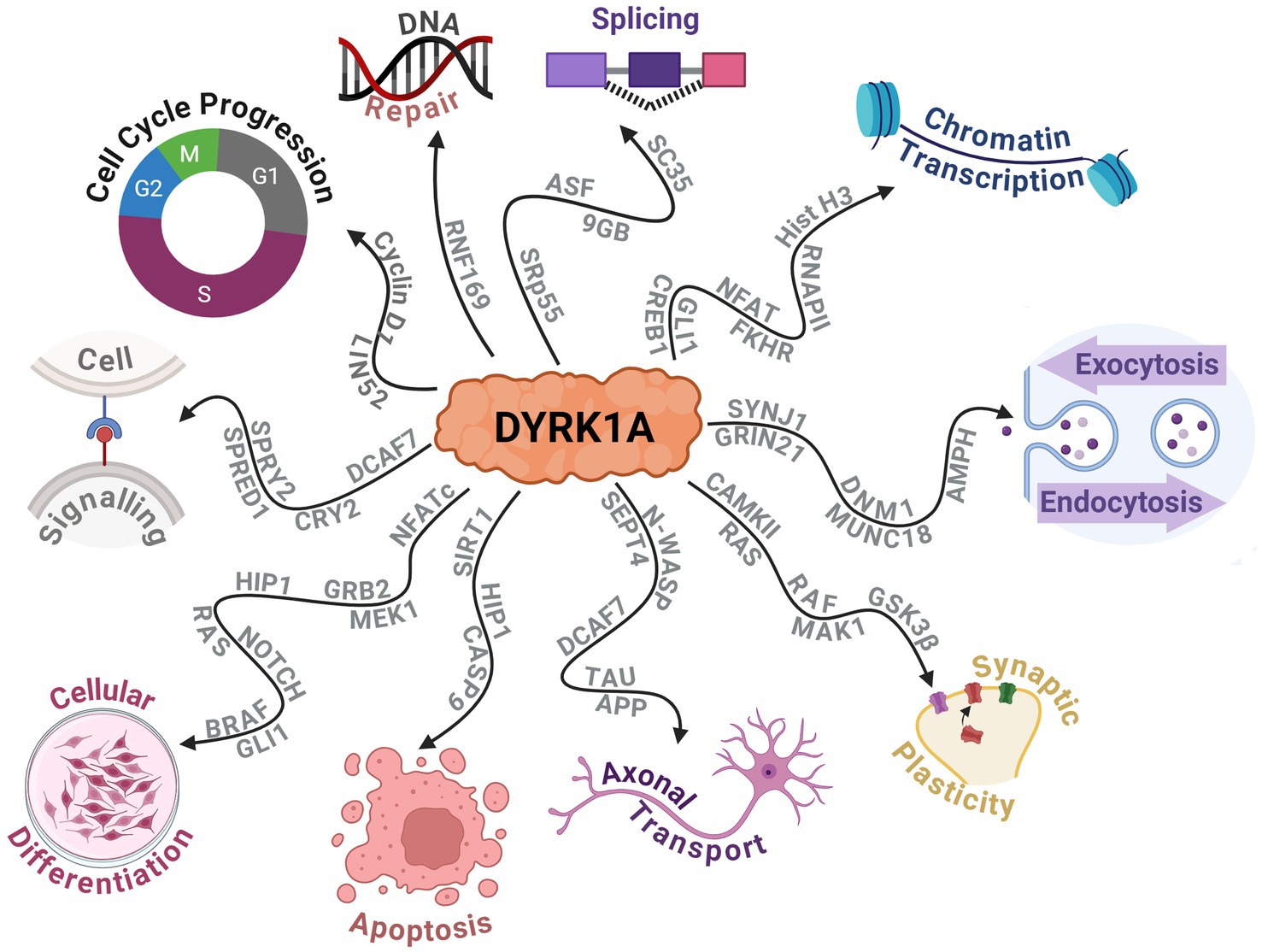
Figure 5. Schematic displaying the protein–protein interactions of DYRK1A and the widespread downstream molecular functions that are affected. Created with BioRender.com.
3.1 Dose sensitivityIn humans, chromosome 21 contains over 300 genes, with only one-third found to be dose-sensitive and are hypothesised to be the primary genes associated with the DS phenotype (Yahya-Graison et al., 2007; Becker et al., 2014). DYRK1A is one of these dose-sensitive protein-coding genes that, if over or under-expressed, can affect essential cellular development and functional roles depending on the pattern of expression. Duchon and Herault (2016) postulated that the formation of active DYRK1A protein complexes may be the cause of the dose sensitivity (Duchon and Herault, 2016). Whereby the dosage sensitive protein forms a tripartite complex with two partners (Veitia et al., 2008; Duchon and Herault, 2016). This hypothesis was corroborated by co-immunoprecipitation studies that identified DYRK1A complexes that formed with cytoskeleton filamentous actin, neurofilaments and tubulin (Kaczmarski et al., 2014). However, the symptoms observed when DYRK1A is under-expressed differ from those when it is over-expressed, contrary to what this tripartite model may suggest (Arque et al., 2013; Raveau et al., 2018). Additionally, numerous DS studies and transgenic models overexpressing DYRK1A have found that DYRK1A protein activity is increased, contradicting this model. These findings suggest that even if a tripartite system exists, it likely does not act alone (Ryoo et al., 2007; Wegiel et al., 2011; Kim et al., 2016). Therefore, the DYRK1A system is likely to be more complex, with other mechanisms influencing symptomology. Further research may elucidate this underlying mechanism, as this is speculation at the time of writing.
3.1.1 Under-expression in DYRK1A syndromeHeterozygous disruption or mutations causing a loss of function can result in a rare partial monosomy of chromosome 21 known as DYRK1A Related Intellectual Disability Syndrome (DYRK1A Syndrome), also referred to as Autosomal Dominant Mental Retardation 7. These individuals often present with numerous developmental delays, ID, dysmorphic facial features, autism spectrum disorder, microcephaly, and a high frequency of epileptic seizures (Courcet et al., 2012; Bronicki et al., 2015; Ruaud et al., 2015; Van Bon et al., 2016). A critical study by Matsumoto et al. (1997) narrowed the loci of the microcephaly and intrauterine growth retardation to a segment of 21.q22.2 that’s 1.2 Mb long that includes DYRK1A and several other genes. Further exploration into DYRK1A syndrome is outside the scope of this review. For more information, we recommend the review by van Bon et al. (2021).
3.1.2 Over-expression in down syndromeThe triplication of chromosome 21 in humans results in an over-expression of the genetic information retained within it. Lymphoblastoid cells retrieved from humans with DS exhibited an approximate 1.4-fold increase in DYRK1A expression (Yahya-Graison et al., 2007). Similarly, studies on Ts65Dn mice analysed their DYRK1A protein expression in the cortex, hippocampus and cerebellum and observed an approximate 1.6-fold increase in expression across all regions (Souchet et al., 2014). This expression pattern has been echoed in mice triplicated for DYRK1A alone, BACTgDyrk1a. However, the relative expression was found to vary depending on the brain region, with a 1.6-fold increase in the cortex, a 1.9-fold increase in the hippocampus and a 1.7-fold increase in the cerebellum (Guedj et al., 2009, 2012).
Mouse models bred to exhibit three functional copies of DYRK1A have shown some neurodevelopmental deficits and cognitive phenotypes similar to DS models. For example, TgDyrk1a mice have displayed difficulties in locomotion, negative geotaxis, and spontaneous alternation (Arque et al., 2013). Numerous studies have also reported developmental and functional changes in the murine and human brains, including suppression of cortical neurogenesis (Chakrabarti et al., 2007), increased ventricles (Schimmel et al., 2006), increased inhibitory interneurons (Pérez-Cremades et al., 2010), and altered dendrites (Dierssen and Ramakers, 2006), long term potentiation and long term depression in prefrontal cortex (Souchet et al., 2014; Thomazeau et al., 2014). Interestingly, there has been found to be an inversely correlated relationship between DYRK1A expression and neuron numbers in the neocortex, while there still exhibits a positive correlation in other brain regions (Guedj et al., 2012). This highlights the region-specific nature of DYRK1A and its importance in neuronal development.
Furthermore, Ts65Dn DS mice crossbred with heterozygous DYRK1A+/− mice produced a DS mouse model with a normalised DYRK1A expression level. The results of studies using these mice found that the long-term potentiation in the hippocampus was protected, early neurogenesis was increased, and Cyclin D1 was recovered (García-Cerro et al., 2014; Najas et al., 2015), providing evidence that DYRK1A is necessary in the development of these phenotypes. Importantly, pharmacological inhibition of DYRK1A has shown to exhibit similar deficit recovery, suggesting that normalising the gene’s expression could correct adverse phenotypes.
3.2 Current DYRK1A inhibitorsThe dose-dependent nature of DYRK1A has made it an attractive target gene and protein for therapeutic intervention, resulting in the development and discovery of numerous pharmacological therapies. Given the extensive range of DYRK1A inhibitors currently available, we will briefly outline the most notable ones, with a primary focus on those aimed at alleviating neurological deficits.
3.2.1 Epigallocatechin gallateThe first DYRK1A inhibitor in animals and humans was identified as EGCG, which is derived from green tea (Jarhad et al., 2018). It inhibits the DYRK1A protein with high potency (IC50 = 330 nM), albeit with low specificity—as it has also been found to inhibit p38-regulated/activated kinase among numerous others (Lamoral-Theys et al., 2010). This has significantly impacted the ability to establish firm conclusions regarding a causative influence on DYRK1A expression. However, it is a common supplement with a high safety profile that has still been used in studies on humans. Mice overexpressing DYRK1A were administered EGCG orally and exhibited improved structural development and cognitive abilities (Guedj et al., 2009). Additionally, it has been given to human young adults with DS and improved hippocampal functioning, particularly in visual and spatial working memory-based tasks (De La Torre et al., 2014). A phase II clinical study showed similar results, with the EGCG group performing better in some cognitive tests and adaptive behaviour up to 12 months post-treatment (De La Torre et al., 2016). Curiously, evidence suggests that EGCG does not cross the blood brain barrier (BBB) effectively, which makes these previous findings peculiar (Becker et al., 2014). It has been suggested that this may be due to the other beneficial effects of EGCG, such its antioxidant effects or its effects on other proteins (Becker et al., 2014). Additionally, recent discoveries have noted that relatively strong doses of EGCG administered to mice at early life could disrupt facial development and, in some cases, cause more severe facial dysmorphia (Starbuck et al., 2021). This could indicate that very low DYRK1A have caused these undesirable effects. However, a more likely hypothesis is that these result from the non-specific nature of EGCG.
3.2.2 HarmineHarmine is a β-carboline alkaloid initially isolated from a South American vine and was found to be a potent inhibitor of DYRK1A (IC50 = 80 nM). However, it also inhibited monoamine oxidase A (MAO-A), and other members of the DYRK family, particularly DYRK2 (IC50 = 0.9 μM), and DYRK3 (IC50 = 0.8 μM) (Bain et al., 2007). Like many other DYRK1A inhibitors, Harmine and its derivatives work via competing with ATP binding to DYRK1A, which inhibits serine/threonine phosphorylation activity (Adayev et al., 2011). Due to its potent inhibition of MAO-A, numerous derivatives have been designed to increase the selectivity to DYRK1A. However, not all have been successful and still exhibit inhibition of MAO-A to some degree (Jarhad et al., 2018).
3.2.3 Other inhibitorsA non-exhaustive list of the various DYRK1A inhibitor categories has been included in Table 2. Jarhad et al. (2018) and Liu et al. (2022) provide comprehensive reviews on these and more DYRK1A inhibitors. Currently, the field of DYRK1A inhibitor research is in a state of development. The existing inhibitors have been observed to exhibit significant off-target effects, primarily due to DYRK1A being highly homologous with numerous other kinases, particularly of the CMGC family. The off-target effects render these inhibitors unviable for clinical use and may result in inconclusive findings in research. Additionally these inhibitors display numerous issues with drug metabolism, which can include rapid degradation, low metabolic stability or low BBB permeability (Liu et al., 2022).
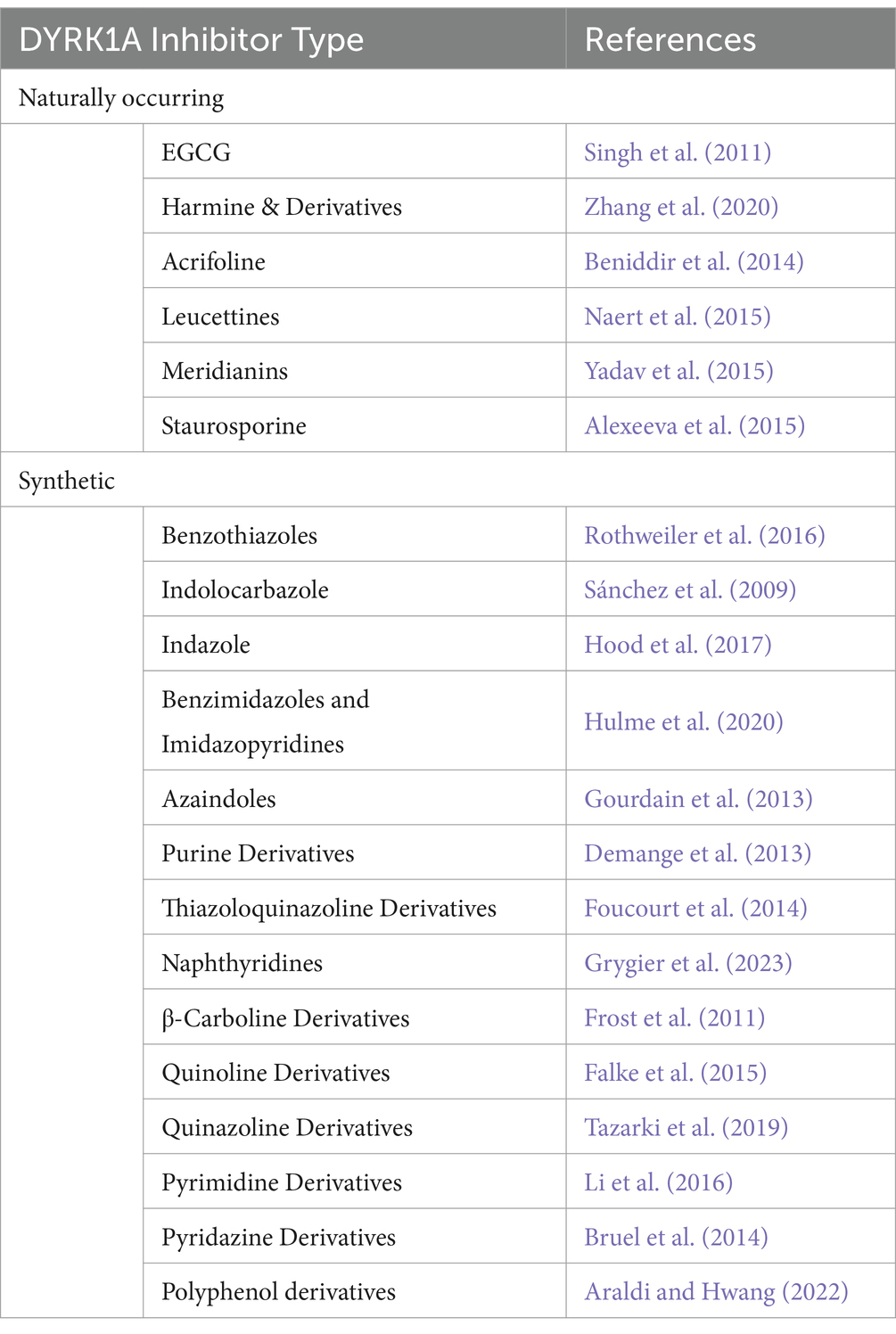
Table 2. Outline of the various DYRK1A inhibitor types outlining the number of common variants and/or structural analogues from Jarhad et al. (2018).
This is where ASOs may offer a revolutionary treatment option as they can be designed to specifically target the DYRK1A gene transcript and modulate expression of the protein. Additionally, ASOs designed to treat other CNS-based disorders have shown stability in the CNS with several having received FDA approval (Goodkey et al., 2018; Wilton-Clark and Yokota, 2021; Eser and Topaloğlu, 2022; Patterson et al., 2023; Van Roon-Mom et al., 2023). Should a DYRK1A inhibitor become available, this would not be limited to a treatment for DS as it would have benefits for cancers, Alzheimer’s disease, viral infections, heart disease, Huntington’s disease, among others (Deboever et al., 2022). Therefore, individuals and researchers would benefit immensely from the production of a highly selective and specific DYRK1A inhibitor.
4 Antisense oligonucleotidesAntisense oligonucleotides are short (~12–30 nucleotides long) synthetic nucleic acid analogues that can be used to alter gene expression via hybridisation to a complementary DNA or RNA through Watson-Crick base pairing (Crooke et al., 2021). First discovered by Zamecnik and Stephenson (1978), they noticed that ASOs inhibited viral replication in vitro. However, before the late 1980s, no effort was made toward a medicinal use for oligonucleotides. Since then, considerable effort has been made to improve upon every facet of ASO technology, aside from those required for Watson-Crick base pairing. This has amounted to many analogues being synthesised and evaluated. Critical strategies for enhancing these chemistries safety and efficiency mainly involved the modifications of the phosphodiester backbone and the 2′ position of the sugar moiety and eventually the creation of the neutrally charged, synthetic phosphorodiamidate morpholino oligomer (PMO). Several other mechanisms of manipulating gene expression include transcription blocking (Melton, 1985), polyadenylation blocking (Vickers et al., 2001), small interfering RNAs (Foster et al., 2018), translational blocking (Setten et al., 2019), and gene therapies (Anguela and High, 2019). However, exploration into these is outside the scope of this review.
4.1 Mechanism of actionThe mechanistic action of an ASO is largely dependent on its chemistry and the region of mRNA in which it is designed to anneal, which can be split into two groups: occupancy-mediated degradation and occupancy-only mechanisms, also known as steric interference. Depending upon the base modifications, phosphorothioate (PS) ASOs can be designed to exploit both groups of mechanisms, while PMOs do not support occupancy-mediated degradation.
4.1.1 Occupancy-mediated degradation (RNase-H)The earliest and most commonly applied ASO-mediated mode of action was RNase-H mediated cleavage (Crooke et al., 2021). RNase-H is essential for gene stability and most notably is used to cleave RNA primers in Okazaki fragments involved in DNA replication (Cerritelli and Crouch, 2009). Additionally, it plays a cooperative role in the prevention of R-loop accumulation which induce genome instability as a result of transcription-induced supercoiling, a hallmark of cancer cells (Broccoli et al., 2004; Santos-Pereira and Aguilera, 2015). These proteins are grouped into two distinct categories based on their substrates for enzyme cleavage. RNase-H1 can function independently of the cell cycle and cleaves the phosphodiester bonds of RNA in RNA:DNA hybrids. While RNase-H2 has strict cell-cycle requirements and plays a similar role with the addition of cleaving the single ribonucleotides embedded within DNA. RNase-H1 based cleavage is the mechanism by which many partially modified PS-ASOs operate. Once the ASO hybridises and forms a duplex with the pre-mRNA/mRNA, the RNase-H1 cleaves the RNA target, exposing the transcript to exonuclease action to accelerate degradation, resulting in the downregulation of specific gene expression (Dias and Stein, 2002). This is outlined in Figure 6A.
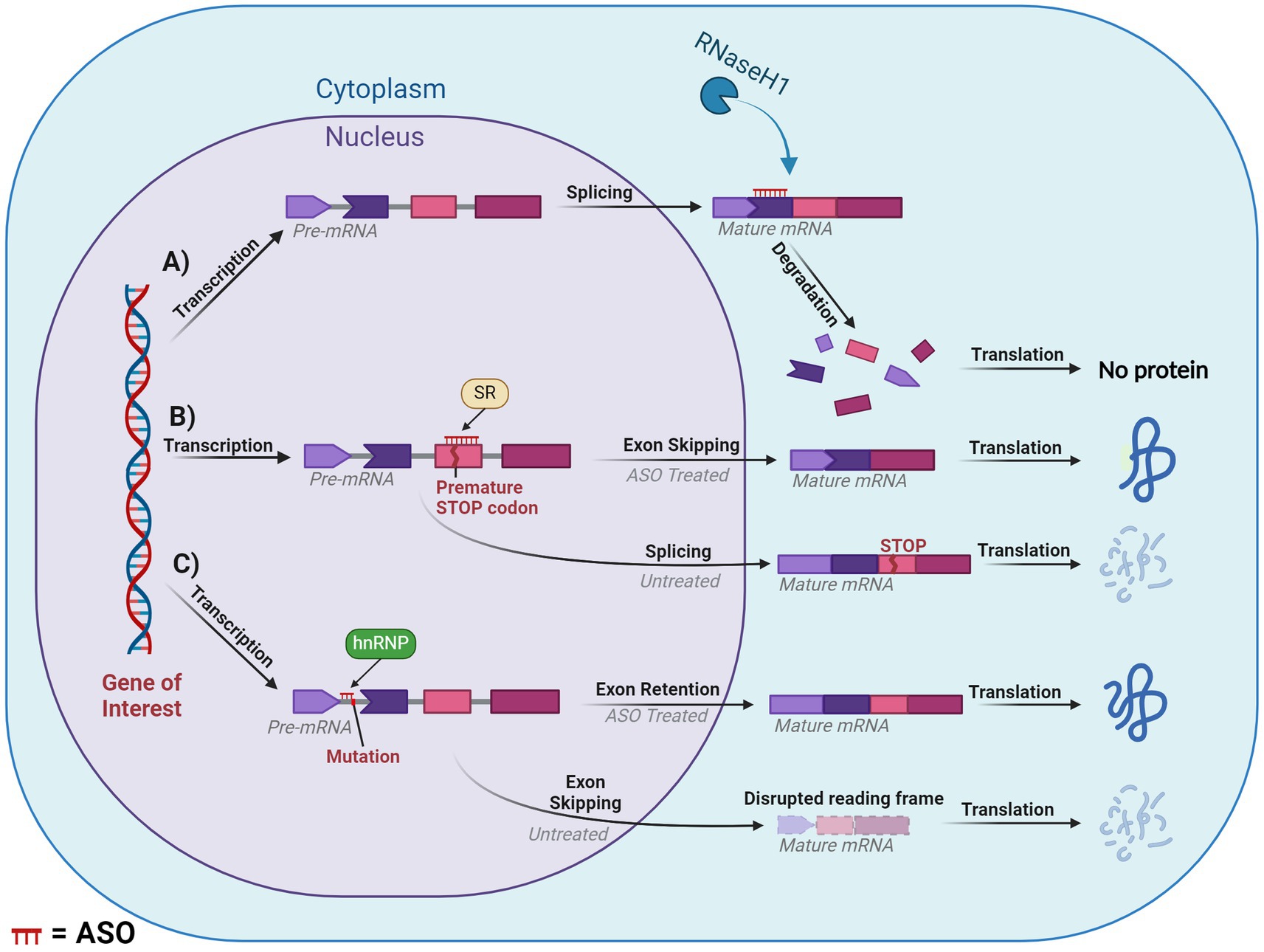
Figure 6. Diagram of splice modulation mechanism of action of antisense oligonucleotides (ASO). (A) RNase-H mediated degradation. (B) Splice-switching exon skipping. (C) Exonic retention. Chevron side indicates partial codon. SR, serine-arginine rich splicing factors; hnRNP, heterogeneous ribonucleoprotein particle. ASO is shown in red or indicated ASO in the figure. Created with BioRender.com.
4.1.2 Occupancy-only (steric hindrance)Steric hindrance offers several pathways for manipulating gene expression, these include influencing translation, splicing and polyadenylation. However, many of these methods fall outside the scope of our review. One of the greatest applications of steric hindrance in human therapeutic settings involves splice switching, which can degrade/restore reading frames and downregulate/upregulate gene and protein expression. This mechanism works through designing ASOs that anneal to complementary sequences within or flanking an exon or intron. The ASO then blocks regions critical to the delicate balance of exon:intron recognition by the spliceosome, resulting in the region being excised or retained in the mature mRNA. If the ASO targets positive splicing motifs of pre-mRNA, then this should typically induce exon skipping via inhibiting recognition of the exon by the spliceosome. Conversely, an ASO can be designed to target silencer motifs in pre-mRNA, which will result in a retention of the sequence in the mature mRNA. This mechanism essentially modifies the pre-mRNA’s usual splicing machinery via altering the recognition of the natural or cryptic splice sites by the spliceosome, (Figures 6B,C).
One such mechanism with potential to treat DS is exon skipping, which can alter the expression of the subsequent transcript dependent on the type of exon that is targeted. If an exon is targeted for excision and if that retains the reading frame, then this will result in the formation of a truncated and potentially functional protein, like that seen in the treatment for Duchenne muscular dystrophy and ATXN3 (Mcclorey et al., 2006; Moore et al., 2017; Mcintosh et al., 2019). In contrast, targeting an exon that, if excised, induced a disruption in the reading frame would result in a non-functional protein that would be degraded via nonsense-mediated decay. This approach is similar to the treatment for multiple sclerosis, which targets ITGA4 (Aung-Htut et al., 2019). Consequences of a disrupted reading frame can be seen in Figure 6C. Additionally, mechanisms such as isoform switching or translation blocking could be utilised, however, these are outside the scope of the current review.
4.2 FDA approved ASOsAs of April 2023, there have been a total of 13 antisense oligonucleotide therapies approved by the FDA, outlined in Table 3. These have received approval to treat previously untreatable genetic-based diseases like spinal muscular atrophy, Duchenne muscular dystrophy and familial amyloid polyneuropathy (Shadid et al., 2021). In this review we will not spend the time to explore the various FDA approved ASOs as this is outside the scope.
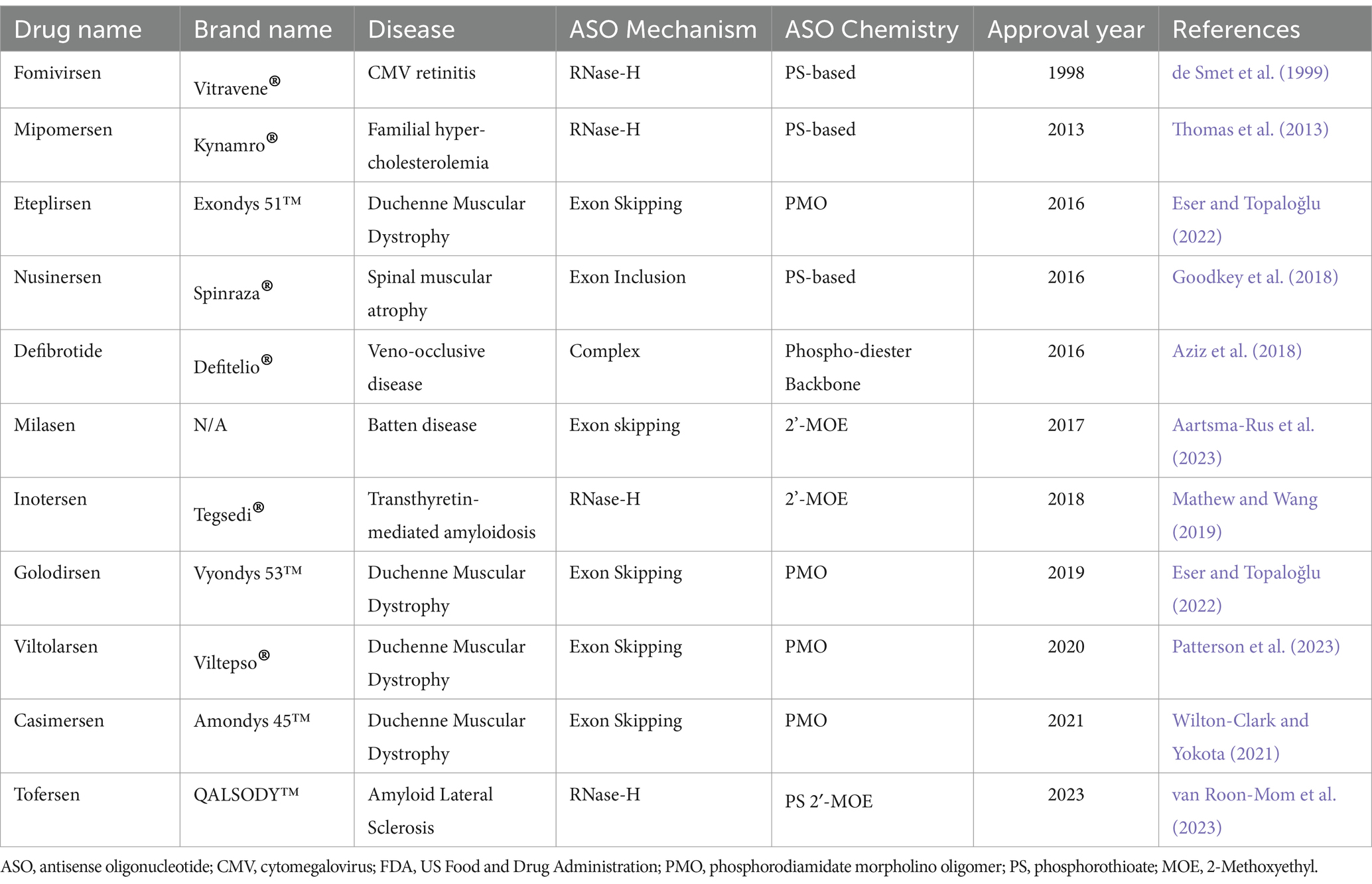
Table 3. Summary of currently available ASO therapies that achieved FDA approval.
4.3 Benefits of ASOs and challenges of CNS deliveryDeveloping treatments for neurological conditions remain some of the most challenging but these conditions have become a major focus for researchers in the field of oligonucleotide therapy. Antisense oligonucleotide researchers and clinicians treating neurological disorders are faced with the ongoing challenges of CNS drug development. Currently, there are no effective modalities of reaching the CNS without invasive methods. For example, spinal muscular atrophy treatment has seen success using ASOs, however drug delivery involves an invasive injection into the spinal canal.
To provide some context, spinal muscular atrophy is the most common genetic cause of death in infants and is an inherited neurological disorder that leads to the atrophy of the alpha motor neurons (D'amico et al., 2011). This causes a degeneration of the bulbar and spinal muscles, in addition to respiratory muscles, which later result in respiratory failure. The 2′-Methoxyethyl (MOE) PS ASO Nusinersen was approved by the FDA in 2016. This ASO increased the amount of SMN protein via inhibition of the negative splicing factors of intron 7 in the SMN2 pre-mRNA, t
留言 (0)