Erectile dysfunction (ED) is a common disorder with a prevalence of 46.1% in American men aged 40–70 years, 42.1%–52.5% in European men aged 40–70 years, and 47.4% in Chinese men aged 40–70 years (1). ED is not life-threatening but has an enormous impact on a person’s quality of life because satisfaction with sexuality is a strong predictor of overall life satisfaction (2). Several medical, environmental, psychological, and lifestyle factors (3, 4), including cardiovascular disease, diabetes, hyperlipidemia, hypertension, metabolic syndrome, and psychological distress, have been suggested to contribute to the development of ED.
Another common chronic disease, arthritis, is increasing in prevalence; 49% of adults in the United States are predicted to develop arthritis by 2040 (5). Several previous studies have shown that various types of arthritis, including osteoarthritis (OA) (6), rheumatoid arthritis (RA) (7), gout (8), and psoriatic arthritis (9), are associated with an increased risk of ED. This may be due to the fact that arthritis, as a chronically systemic inflammatory disease, affects a wide range of systems throughout the body, including the reproductive system (10). However, some researchers found no clear association between RA and ED after deeper exploration (11). Overall, the relationship between arthritis and ED is complex and requires intensive research.
Therefore, this study investigated the relationship between arthritis and ED using data from the National Health and Nutrition Examination Survey (NHANES) from 2001–2004.
2 Materials and methods2.1 Study populationThe data utilized in this study were procured from the National Health and Nutrition Examination Survey (NHANES), which is conducted annually by the National Center for Health Statistics (NCHS), a division of the Centers for Disease Control and Prevention, with the objective of assessing the health status and behavior of unstructured populations in the United States (12). A complex multistage probability sampling design was employed in the NHANES survey to collect data representative of the population (13). All NHANES procedures were approved by the U.S. Policy for the Protection of Human Research Subjects and were reviewed and standardized annually by the NCHS Research Ethics Review Board. All individuals who participated in the survey provided informed consent, indicating their understanding of the study’s purpose.
For the purposes of this cross-sectional analysis, datasets were selected from two NHANES survey cycles: 2001–2002 and 2003–2004, because only these two cycles had complete ED and arthritis data. A total of 21,161 individuals participated in the NHANES from 2001 to 2004; the exclusion criteria were as follows: female (n=10,860) and male patients aged less than 20 years (n=5,347), missing information on ED or arthritis (n=846), and missing covariate information (n=462). A total of 3,646 patients were ultimately included in this study. Of these, 1,012 patients had a self-reported history of ED, and 795 patients had a self-reported history of arthritis.
2.2 Data collection and definitionWe used the NHANES self-report questionnaire to diagnose ED and arthritis. All participants were asked the ED-related question, “How would you describe your ability to develop and maintain an erection sufficient for sexual intercourse?” The answers were “never,” “sometimes,” “usually,” or “almost always or nearly always.” We categorized those who answered “never” or “sometimes” as having ED. All participants were asked two questions related to arthritis. First, they were asked, “Has any doctor or other health professional ever told you that you have arthritis?” Individuals who provided a positive response were asked the follow-up question, “What type of arthritis is it?” Participants were categorized as having “no arthritis,” “rheumatoid arthritis,” “osteoarthritis,” or “other types of arthritis.”
Using multivariate-adjusted models, we identified potential confounders that may influence the association between arthritis and ED. The covariates included age, race, body mass index, weight-adjusted-waist index (WWI) (14, 15), marital status (married or living with a partner/single), poverty-to-income ratio, education level, exercise status, insurance status, alcohol consumption (whether they consumed alcohol or not), smoking status (current/former/no), hypertension, diabetes, hypercholesterolemia, cardiovascular disease, stroke, and prostate disease.
2.3 Statistical methodsComplex sample weights were used to address sample inclusion selection, oversampling, and non-response bias. Overall incidences of ED and arthritis were estimated using complex sample weights. Time trends in ED and arthritis incidence were assessed after adjusting for age and race. Categorical variables were compared using the card method. Logistic regression modeling was used to adjust for covariates. Odds ratios (ORs) were calculated for 95% confidence intervals (CIs) and p values. We performed sensitivity analyses of the results using multiple interpolations, which first imputed five sets of complete datasets by calling the mouse function. Subsequently, statistical analyses, such as univariate analysis and regression models, were performed within each dataset by calling a function which sets the environment for the statistical analysis. Finally, the results obtained from each analysis were combined using the pool function (16), a method of compensating for missing data based on five replicates, and the chained equation method in the Remote Method Invocation procedure to account for the effect of missing covariates on the results (17). Subgroup analyses were performed using weighted multivariate logistic regression (Model 4) for different strata of our cohort in which the association between arthritis and ED was analyzed. All statistical tests were two-sided, with a significance threshold of p<0.05. R version 4.3.2 (http://www.R-project.org, The R Foundation) was used for statistical analysis.
3 Results3.1 Characteristics of the study participantsBetween 2001 and 2004, 21,161 participants were interviewed. Of these, 4,954 were men aged over 20 years. A total of 4,108 (83.0%) participants answered the survey questions on ED and arthritis. After adjusting for missing covariate values, 3,646 participants were included in the cohort (Figure 1).
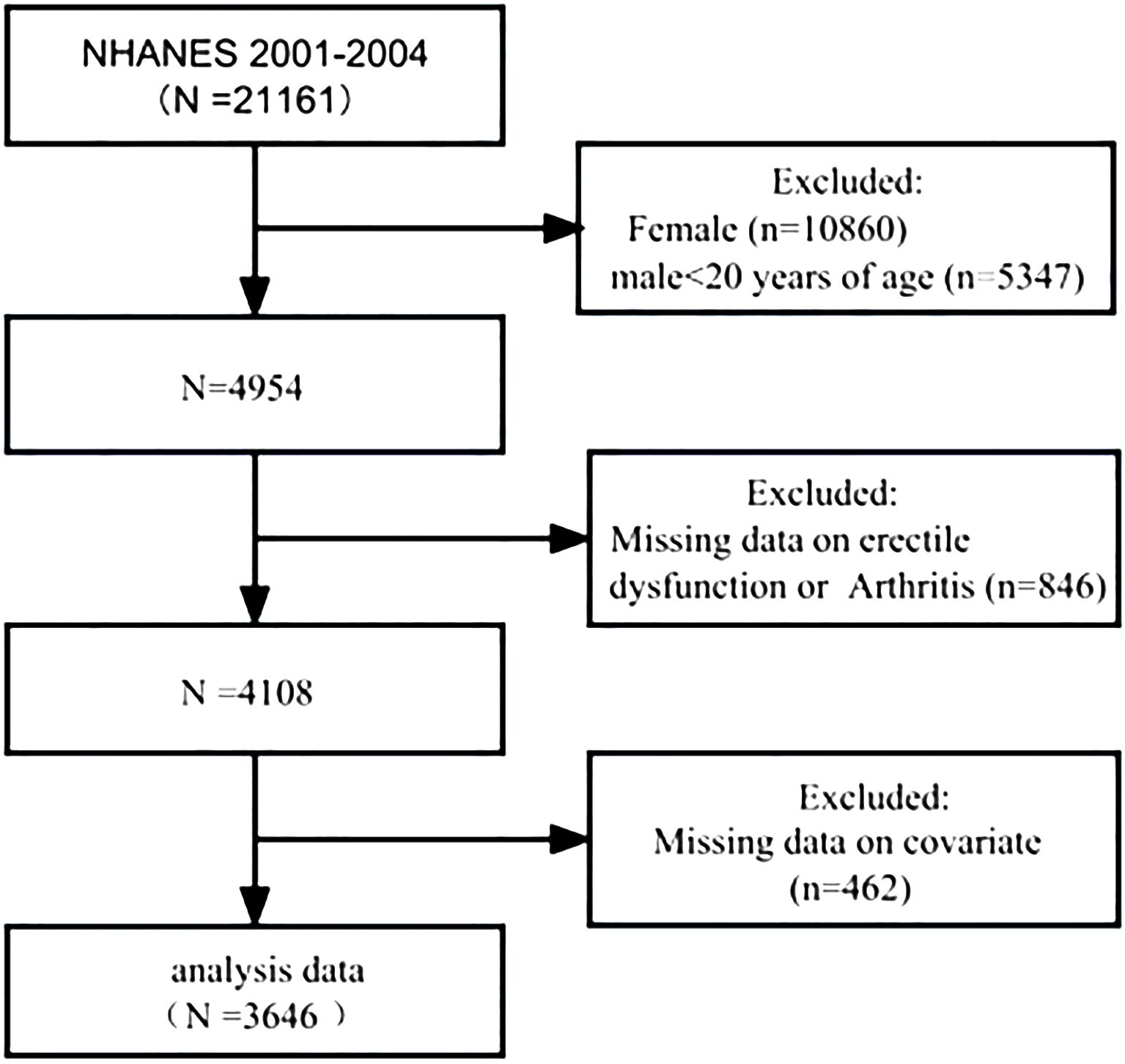
Figure 1 Schematic flow diagram of the inclusion and exclusion criteria for our study cohort.
Compared with participants without ED, those with ED had higher rates of all types of arthritis (p<0.001) and were more likely to be older, less educated, married or living with a sexual partner, socioeconomically disadvantaged, physically inactive, insured, not alcohol consumers, former smokers, not hypertensive, and unhealthy in terms of diabetes mellitus, dyslipidemia, obesity, cardiovascular disease, and prostate disease. No significant race differences were observed between the ED and non-ED groups (Table 1).
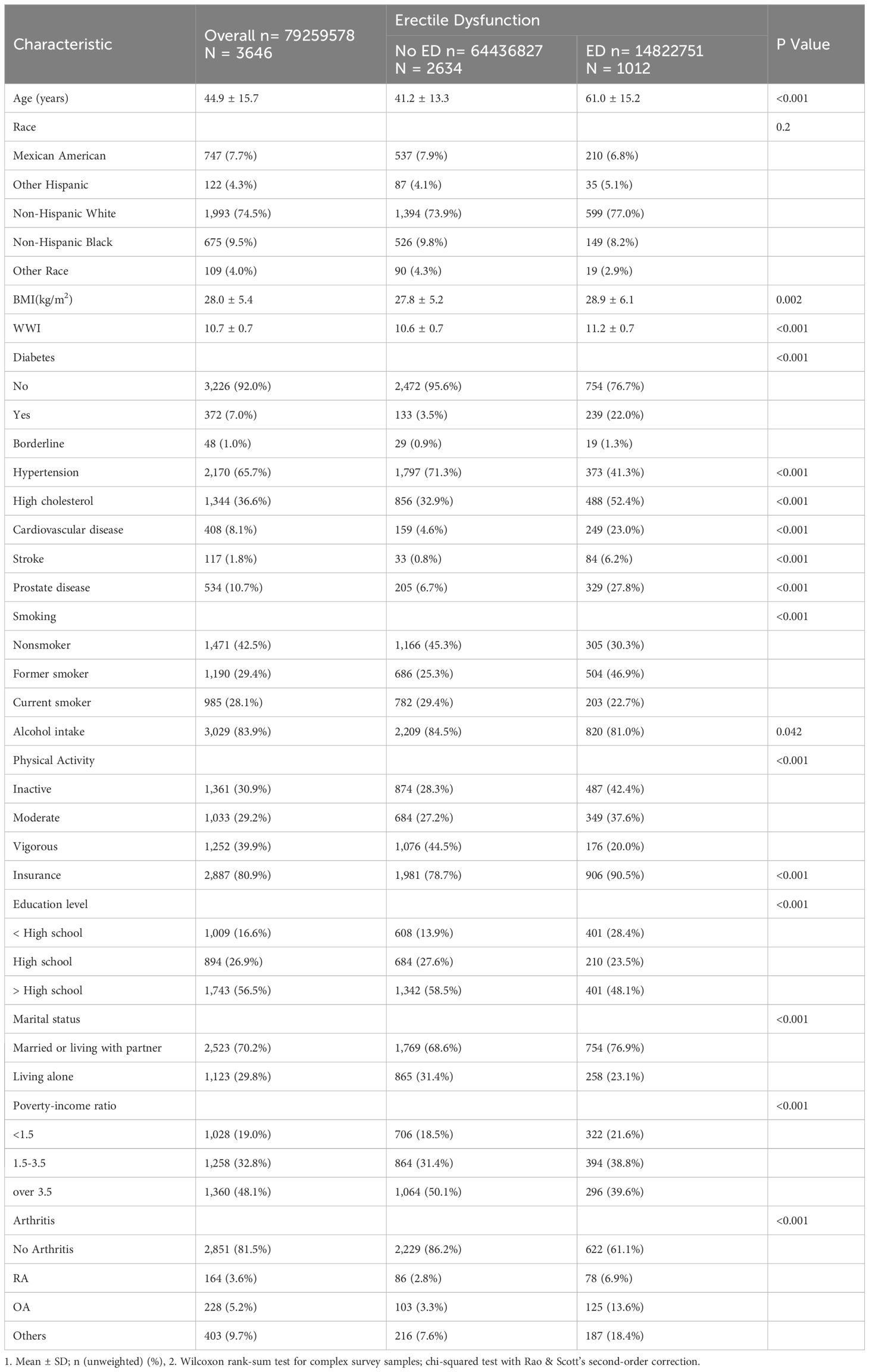
Table 1 Baseline characteristics of Erectile Dysfunction group versus the non-Erectile Dysfunction group.
Participants with arthritis had a greater rate of having ED than did those without arthritis (p<0.001) and were older, less educated, predominantly non-Hispanic white (especially those with OA), married or living with a sexual partner, socioeconomically disadvantaged, physically inactive, insured, not alcohol consumers, ex-smokers, non-hypertensive, and had diabetes mellitus, dyslipidemia, obesity, and unhealthy in terms of cardiovascular or prostate disease. There were no significant differences in alcohol consumption between the groups with and without arthritis (Table 2).
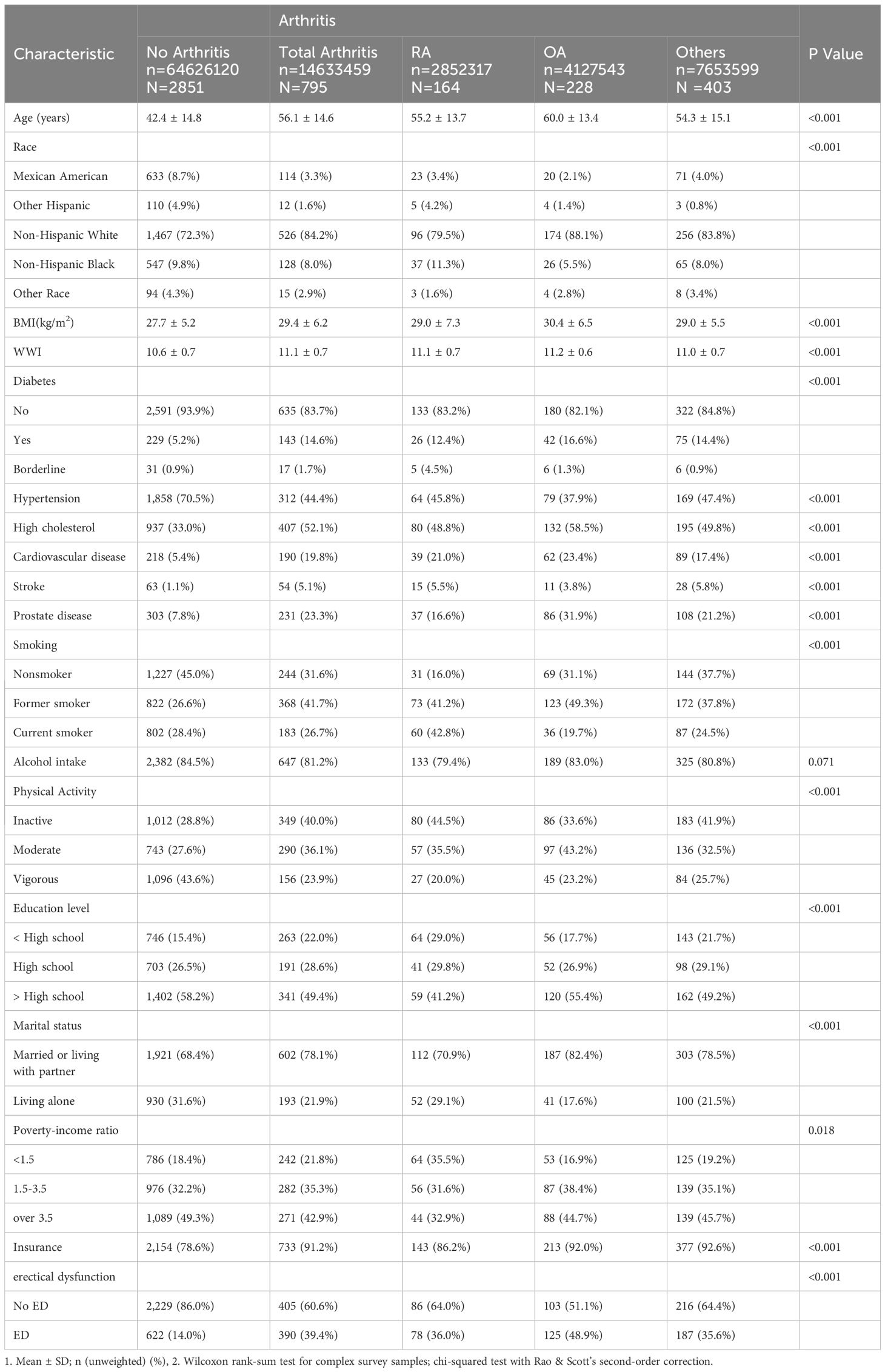
Table 2 Baseline characteristics of arthritis group versus the non-arthritis group.
3.2 Association between arthritis and EDWeighted logistic regression analysis revealed a significant positive association between arthritis and ED in all models. According to the fully adjusted model (Model 4), compared with patients without arthritis, patients with arthritis had a 42% greater risk of ED (OR=1.42; 95% CI: 1.00–1.96; p= 0.006); sensitivity analyses showed similar results in the fully adjusted model (OR=1.34; 95% CI: 1.04–1.74; p= 0.009) (Table 3). Among the different types of arthritis, OA was significantly associated with ED (OR = 1.11; 95% CI: 1.03–1.20; p = 0.017). Sensitivity analyses revealed similar results in the fully adjusted models (OR=1.10; 95% CI: 1.03–1.19); however, RA (OR=1.03, 95% CI: 0.93–1.13; p= 0.5) and other types of arthritis (OR=1.04; 95% CI: 0.98–1.11; p= 0.2) were not significantly associated with ED (Table 3).
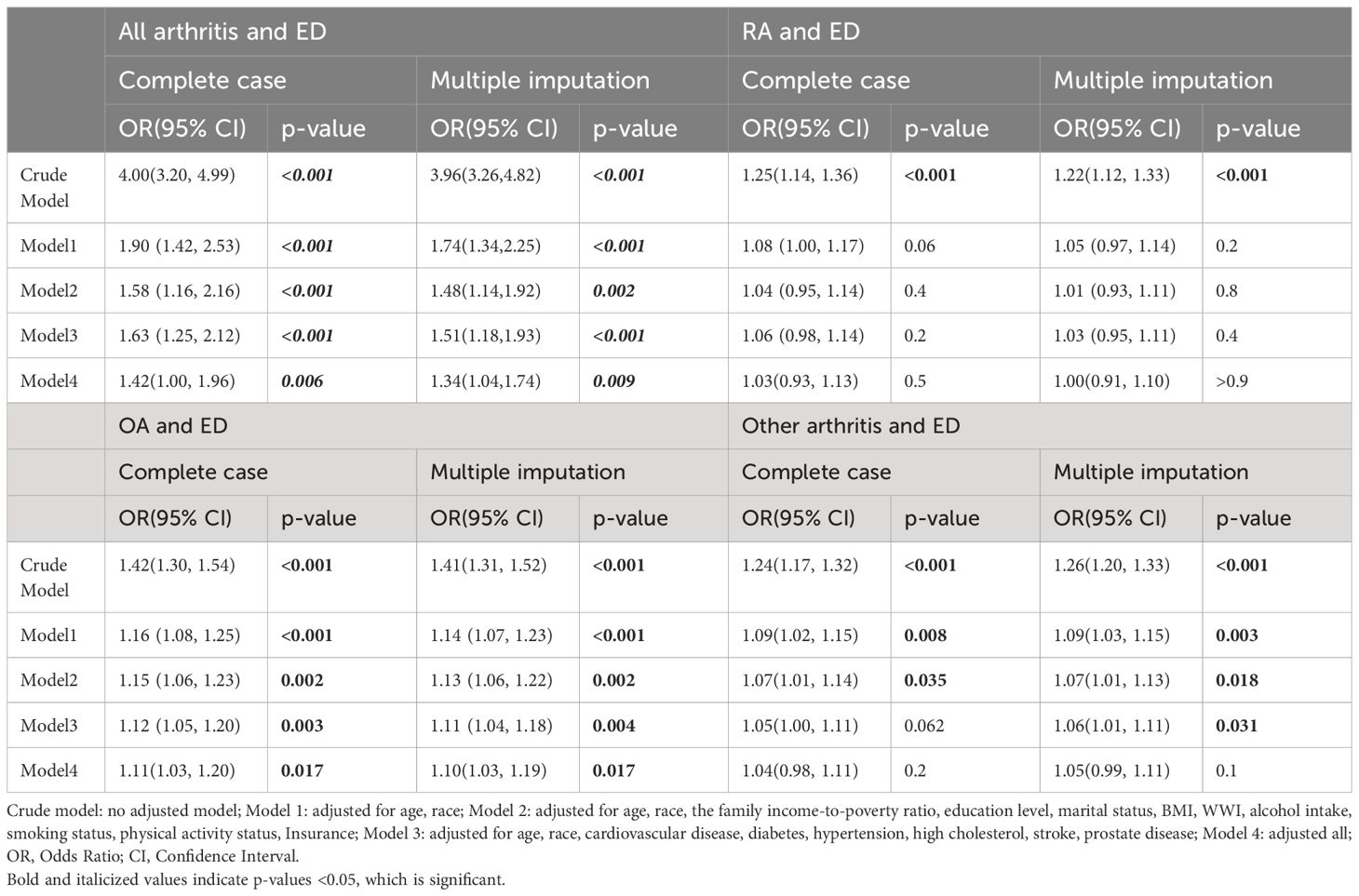
Table 3 Association between Arthritis and ED.
3.3 Subgroup analysis of the incidence of arthritis and EDSubgroup analyses revealed a consistent positive correlation between the incidence of arthritis and ED across the subgroups, indicating the robustness of this correlation. Significance was maintained in the following subgroups: age <60 years, White, and no alcohol consumption (p<0.05) (Figure 2).
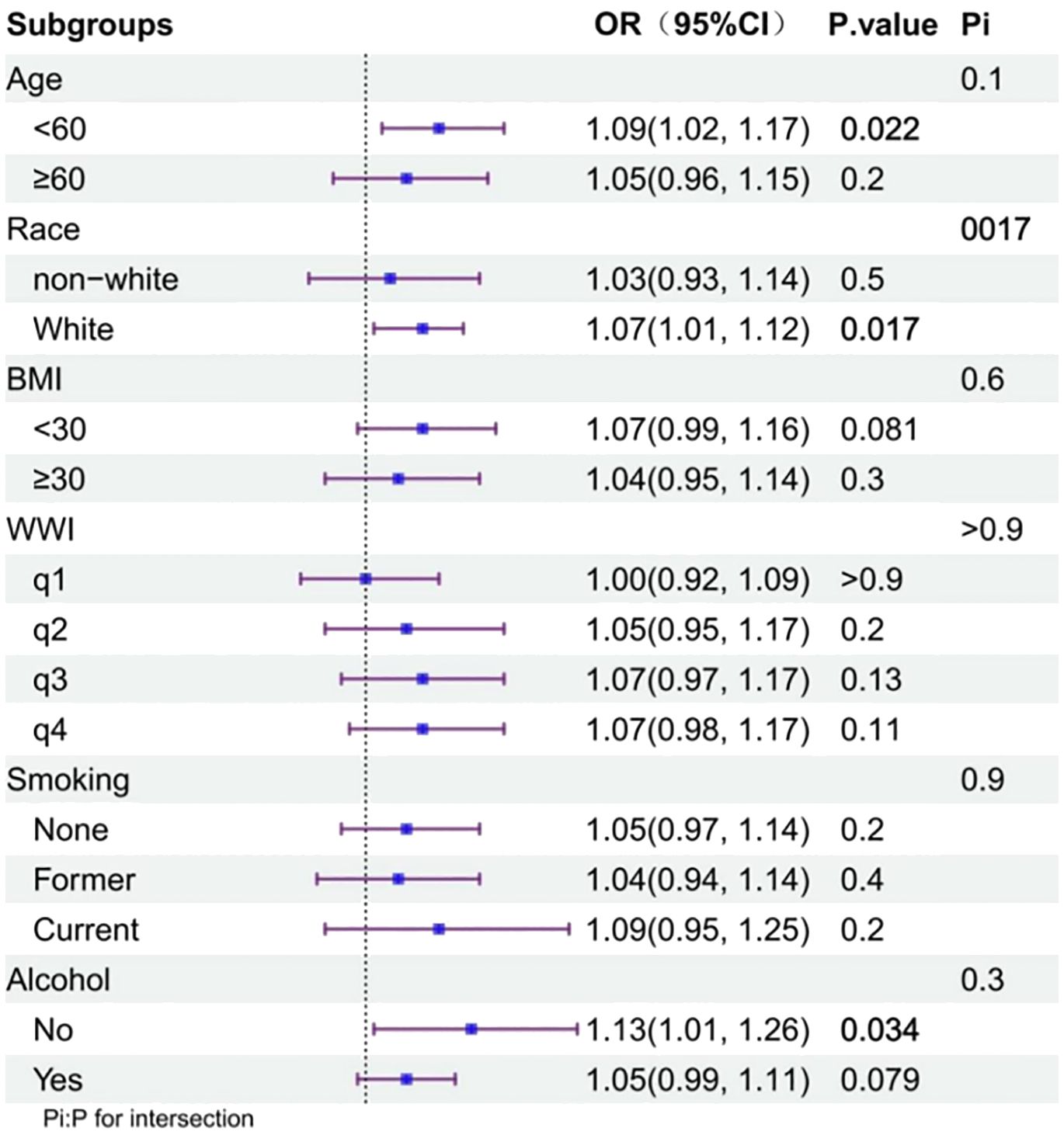
Figure 2 Subgroup analysis of the association between arthritis and ED status.
4 DiscussionIn a pooled analysis of a nationally representative sample of 3,646 men aged >20 years in the United States, we observed a positive association between arthritis and ED incidence. This association persisted, even after adjusting for confounders. Based on the large sample size and reasonable quality control, our analysis is reliable. Previous studies have reported a higher incidence of ED in patients with inflammatory arthropathies (including RA and psoriatic arthritis) (18), ankylosing spondylitis (19), and gout (20), compared with the general population without arthritis. Similarly, in this study, 39.4% of patients with arthritis developed ED, which was much greater than the 14.0% among those without arthritis. After adjusting for confounders among the different types of arthritis, we observed that the effect of arthritis on ED incidence remained significant in patients with OA, whereas there was no significant association between RA or other types of arthritis and ED. According to our interaction analysis, White race played a role in modifying the association between arthritis and ED, with White individuals showing stronger associations, compared with other races, which may be related to the greater probability of ED disclosure in the White population (21).
According to the age-stratified analyses, there was a strong correlation between ED and the incidence of arthritis. However, the correlation between ED and arthritis found in the present study did not increase with age; rather, it was more significant in participants aged less than 60 years. Although the prevalence of arthritis in older adults is relatively high at present, it is predicted that adults aged 18–65 years will account for the majority of arthritis cases (67%) by 2040 (5); therefore, the relationship between arthritis and ED is not entirely due to aging. Moreover, previous scholars have noted that lack of exercise (22) and abnormal WWIs can lead to both arthritis and ED (14, 15). However, in the present study, after adjusting for the amount of exercise and WWI, the association between arthritis and ED remained significant. Thus, they were not the main common causes of either disorder.
The biological mechanism of inflammation may be closely related to ED in men with arthritis. It has been shown that tumor necrosis factor (TNF)-α, interleukin (IL)-1β are significantly increased in patients with OA, RA, ankylosing spondylitis, and psoriatic arthritis (18, 23). TNF-α plays a specific role in inflammation-associated ED by increasing arterial reactive oxygen species and decreasing nitric oxide levels (24), leading to deterioration of endothelium-dependent vasodilatory function in different vascular regions. Nishimatsu found that injection of the pro-inflammatory cytokine-, TNF-α-, and IL-1β-producing senescent cells in mice can impair erectile response by triggering endothelial dysfunction and nerve damage (25). Additionally, as an essential treatment for various types of arthritis, anti-TNF-α treatments play an important role in preventing the development of cavernous body dysfunction (26, 27). Zuo et al. also showed that an inflammatory state-induced decrease in endothelial nitric oxide synthase and nitric oxide levels may contribute to ED development in rats (28).
Further, Wade noted that men with moderate or severe pain had more erectile difficulties than did those without pain (29). In addition, the use of nonsteroidal anti-inflammatory drugs (NSAIDs), basic medications used in the treatment of various types of arthritis, has been shown to increase the risk of ED (30, 31). In a large clinical study, a 22% and 38% increase in the prevalence of any ED and severe ED, respectively, was observed in a group of individuals who used NSAIDs (32). Similarly, a prospective observational study in Finland (33) found a relatively increased risk of ED in men with arthritis, and men with or without arthritis that used NSAIDs had a higher risk of ED than did men who did not use NSAIDs and did not have arthritis. In addition, anti-inflammatory pain and diclofenac have been observed to inhibit the erectile process by decreasing nitric oxide levels in rats (34). Another study indicated that celecoxib might induce ED by affecting the source of reactive oxygen species, thus modulating nitric oxide breakdown (35, 36). In addition, other types of analgesics, such as opioid analgesics (37), also increase the risk of ED. Therefore, pain and analgesics may serve as a bridge between arthritis and ED.
In addition, it has been shown that the specific expression of cyclooxygenase-2 (COX-2), which plays an important role in the inflammatory microenvironment, promotes inflammatory factor-induced imbalances in cartilage proteoglycan metabolism, leading to irreversible advancement of arthritis (38). Fan et al. showed that COX-2 mRNA and protein levels were significantly higher in the synoviocytes of patients with OA and RA than in healthy tissues and were significantly higher in patients with OA than in those with RA (p < 0.05) (39). Interestingly, COX-2 protein levels are significantly elevated in the synovial fluid of patients with OA but not in those with RA (39). Moreover, it has been noted that the inflammatory state of autoimmune arthritis is associated with a bio-clock (40) that is cyclical, whereas OA usually exhibits a persistent inflammatory state. As a result, the level of inflammation in OA is higher for a longer duration than in RA, and the frequency and amount of analgesics used are increased (41). These factors may be important in the close relationship between OA and ED.
This study has several strengths. First, the survey is based on a large sample size and complex survey design, providing a comprehensive overview of the US population. Second, we adjusted for as many covariates as possible, such as general information, income, health behaviors, and comorbidities, which made our results more robust. Ultimately, the extensive data set permitted the implementation of subgroup analyses while maintaining an acceptable level of statistical power, further validating the stability of our results. However, this study has some limitations. First, because this was a cross-sectional study, causal inferences regarding arthritis and ED are not feasible. Second, the diagnoses of ED and arthritis were based on self-reported questionnaires, which may have led to misestimation of the actual number of patients with ED and arthritis. Previous studies have tested the validity of a single self-report questionnaire (42, 43). Third, our analyses were based only on the US population; therefore, the applicability of our conclusions to other populations remains to be determined. Fourth, some covariates were excluded from the analyses, such as mental illness-related cofounders (depression and anxiety), because data were not available for all participants. Finally, observational studies are susceptible to residual confounders, even after controlling for potential confounders. However, this is the first study to identify correlations between all types of arthritis and ED. We performed sensitivity analyses, and these correlations remained significant.
In conclusion, our results suggest that patients with arthritis are significantly more likely to have ED than are those without arthritis, and that arthritis is significantly associated with ED. Timely identification and treatment of ED in patients with arthritis can have a significant impact on their quality of life by avoiding the use of costly healthcare. In the future, we hope that relevant research will be conducted on populations of more races. Further research is needed to investigate the underlying molecular mechanisms to take appropriate therapeutic measures for patients with arthritis and ED.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statementThe studies involving humans were approved by THE NHANES Institutional Review Board (IRB) Protocol #98-12. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsCL: Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing – original draft. WL: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Writing – review & editing. QL: Conceptualization, Data curation, Funding acquisition, Investigation, Supervision, Writing – original draft. JL: Project administration, Resources, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the 1Science and Technology Project of Quanzhou City (2023N065S) and 2Natural Science Foundation of Fujian Province (2021J01266).
AcknowledgmentsWe are grateful to all the participants involved in the present study for their enthusiasm and commitment.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Goldstein I, Goren A, Li VW, Tang WY, Hassan TA. Epidemiology update of erectile dysfunction in eight countries with high burden. Sexual Med Rev. (2020) 8:48–58. doi: 10.1016/j.sxmr.2019.06.008
CrossRef Full Text | Google Scholar
3. Lewis RW, Fugl-Meyer KS, Corona G, Hayes RD, Laumann EO, Moreira ED Jr., et al. Definitions/epidemiology/risk factors for sexual dysfunction. J sexual Med. (2010) 7:1598–607. doi: 10.1111/j.1743-6109.2010.01778.x
CrossRef Full Text | Google Scholar
4. Sooriyamoorthy T, Leslie SW. Erectile dysfunction. In: StatPearls. StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC, Treasure Island (FL (2023). with ineligible companies. Disclosure: Stephen Leslie declares no relevant financial relationships with ineligible companies.
5. Hootman JM, Helmick CG, Barbour KE, Theis KA, Boring MA. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015-2040. Arthritis Rheumatol (Hoboken NJ). (2016) 68:1582–7. doi: 10.1002/art.39692
CrossRef Full Text | Google Scholar
7. El Miedany Y, El Gaafary M, El Aroussy N, Youssef S, Ahmed I. Sexual dysfunction in rheumatoid arthritis patients: arthritis and beyond. Clin Rheumatol. (2012) 31:601–6. doi: 10.1007/s10067-011-1891-2
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. (2020) 16:380–90. doi: 10.1038/s41584-020-0441-1
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Kurizky PS, Mota LM. Sexual dysfunction in patients with psoriasis and psoriatic arthritis–a systematic review. Rev Bras Reumatol. (2012) 52:943–8. doi: 10.1590/S0482-50042012000600011
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Cutolo M, Sulli A, Pizzorni C, Craviotto C, Straub RH. Hypothalamic-pituitary-adrenocortical and gonadal functions in rheumatoid arthritis. Ann N Y Acad Sci. (2003) 992:107–17. doi: 10.1111/j.1749-6632.2003.tb03142.x
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Wilton KM, Achenbach SJ, Davis JM 3rd, Myasoedova E, Matteson EL, Crowson CS. Erectile dysfunction and cardiovascular risk in men with rheumatoid arthritis: A population-based cohort study. J Rheumatol. (2021) 48:1641–7. doi: 10.3899/jrheum.201226
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Mao W, Wu J, Zhang Z, Xu Z, Xu B, Chen M. Neutrophil-lymphocyte ratio acts as a novel diagnostic biomarker for kidney stone prevalence and number of stones passed. Trans andrology urol. (2021) 10:77–86. doi: 10.21037/tau
CrossRef Full Text | Google Scholar
13. Mao W, Hu Q, Chen S, Chen Y, Luo M, Zhang Z, et al. Polyfluoroalkyl chemicals and the risk of kidney stones in US adults: A population-based study. Ecotoxicol Environ safety. (2021) 208:111497. doi: 10.1016/j.ecoenv.2020.111497
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Cao S, Hu X, Shao Y, Wang Y, Tang Y, Ren S, et al. Relationship between weight-adjusted-waist index and erectile dysfunction in the United State: results from NHANES 2001-2004. Front endocrinol. (2023) 14:1128076. doi: 10.3389/fendo.2023.1128076
CrossRef Full Text | Google Scholar
15. Wang X, Xie L, Yang S. Association between weight-adjusted-waist index and the prevalence of rheumatoid arthritis and osteoarthritis: a population-based study. BMC musculoskeletal Disord. (2023) 24:595. doi: 10.1186/s12891-023-06717-y
CrossRef Full Text | Google Scholar
17. Li Y, Ji L, Oravecz Z, Brick TR, Hunter MD, Chow SM. dynr.mi: An R Program for Multiple Imputation in Dynamic Modeling. World academy of science, engineering and technology. World Acad Sci Eng Technol. (2019) 13(5):302–11. doi: 10.5281/zenodo.3298841
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Perez-Garcia LF, Röder E, Goekoop RJ, Hazes JMW, Kok MR, Smeele HTW, et al. Impaired fertility in men diagnosed with inflammatory arthritis: results of a large multicentre study (iFAME-Fertility). Ann rheumati diseases. (2021) 80:1545–52. doi: 10.1136/annrheumdis-2021-220709
CrossRef Full Text | Google Scholar
19. Santana T, Skare T, Delboni VS, Simione J, Campos APB, Nisihara R. Erectile dysfunction in ankylosing spondylitis patients. Int Braz J urol. (2017) 43:730–5. doi: 10.1590/s1677-5538.ibju.2016.0378
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Abdul Sultan A, Mallen C, Hayward R, Muller S, Whittle R, Hotston M, et al. Gout and subsequent erectile dysfunction: a population-based cohort study from England. Arthritis Res Ther. (2017) 19:123. doi: 10.1186/s13075-017-1322-0
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Gaines JM, Macdonald EJ, Smith AJ, Diefenbach MA, Paduch DA. Race and ethnicity have a significant effect on the disclosure of erectile function: an analysis of NHANES response patterns. Urology. (2022) 167:138–43. doi: 10.1016/j.urology.2022.05.031
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Nishimatsu H, Suzuki E, Saito Y, Niimi A, Nomiya A, Fukuhara H, et al. Senescent cells impair erectile function through induction of endothelial dysfunction and nerve injury in mice. PLoS One. (2015) 10:e0124129. doi: 10.1371/journal.pone.0124129
PubMed Abstract | CrossRef Full Text | Google Scholar
27. Demirtaş Şahin T, Yazir Y, Utkan T, Gacar G, Halbutoğulları ZS, Gocmez SS. Depression induced by chronic stress leads to penile cavernosal dysfunction: protective effect of anti-TNF-α treatment. Can J Physiol Pharmacol. (2018) 96:933–42. doi: 10.1139/cjpp-2017-0778
PubMed Abstract | CrossRef Full Text | Google Scholar
28. Zuo Z, Jiang J, Jiang R, Chen F, Liu J, Yang H, et al. Effect of periodontitis on erectile function and its possible mechanism. J Sex Med. (2011) 8:2598–605. doi: 10.1111/j.1743-6109.2011.02361.x
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Wade K, Wu FCW, O’Neill TW, Lee DM. Association between pain and sexual health in older people: results from the English Longitudinal Study of Ageing. Pain. (2018) 159:460–8. Li, T., et al., Association between use of aspirin or non-aspirin non-steroidal anti-inflammatory drugs and erectile dysfunction: a systematic review. Medicine (Baltimore), 2018. 97(28): e11367. doi: 10.1097/MD.0000000000011367
PubMed Abstract | CrossRef Full Text | Google Scholar
30. Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: A systematic review and meta-analysis. JAMA. (2019) 321:277–87. doi: 10.1001/jama.2018.20578
PubMed Abstract | CrossRef Full Text | Google Scholar
31. Patel DP, Schenk JM, Darke A, Myers JB, Brant WO, Hotaling JM. Non-steroidal anti-inflammatory drug (NSAID) use is not associated with erectile dysfunction risk: results from the Prostate Cancer Prevention Trial. BJU Int. (2016) 117:500–6. doi: 10.1111/bju.13264
PubMed Abstract | CrossRef Full Text | Google Scholar
32. Gleason JM, Slezak JM, Jung H, Reynolds K, Van den Eeden SK, Haque R, et al. Regular nonsteroidal anti-inflammatory drug use and erectile dysfunction. J Urol. (2011) 185:1388–93. doi: 10.1016/j.juro.2010.11.092
PubMed Abstract | CrossRef Full Text | Google Scholar
33. Shiri R, Koskimäki J, Häkkinen J, Tammela TL, Auvinen A, Hakama M. Effect of nonsteroidal anti-inflammatory drug use on the incidence of erectile dysfunction. J Urol. (2006) 175:1812–5; discussion 1815-6. doi: 10.1016/S0022-5347(05)01000-1
PubMed Abstract | CrossRef Full Text | Google Scholar
38. Lee KH, Abas F, Mohamed Alitheen NB, Shaari K, Lajis NH, Israf DA, et al. Chemopreventive effects of a curcumin-like diarylpentanoid [2,6-bis(2,5-dimethoxybenzylidene)cyclohexanone] in cellular targets of rheumatoid arthritis in vitro. Int J Rheumati Dis. (2015) 18:616–27. doi: 10.1111/apl.2015.18.issue-6
CrossRef Full Text | Google Scholar
39. Fan HW, Liu GY, Zhao CF, Li XF, Yang XY. Differential expression of COX-2 in osteoarthritis and rheumatoid arthritis. Genet Mol research: GMR. (2015) 14:12872–9. doi: 10.4238/2015.October.21.7
CrossRef Full Text | Google Scholar
41. Spencer-Green G, Spencer-Green E. Nonsteroidal therapy of rheumatoid arthritis and osteoarthritis: how physicians manage treatment failures. J Rheumatol. (1998) 25:2088–93.
PubMed Abstract | Google Scholar
42. O'Donnell AB, Araujo AB, Goldstein I, McKinlay JB. The validity of a single-question self-report of erectile dysfunction. Results from the Massachusetts Male Aging Study. J Gen Internal Med. (2005) 20:515–9. doi: 10.1111/j.1525-1497.2005.0076.x
CrossRef Full Text | Google Scholar
43. Peeters GM, Alshurafa M, Schaap L, de Vet HC. Diagnostic accuracy of self-reported arthritis in the general adult population is acceptable. J Clin Epidemiol. (2015) 68:452–9. doi: 10.1016/j.jclinepi.2014.09.019
留言 (0)