177Lu-PSMA-617 radioligand therapy (RLT) has been shown to improve clinical outcomes and have a favorable safety profile in men with metastatic castration resistant prostate cancer (mCRPC) (1–5). In the phase 3 VISION study, 177Lu-PSMA-617 RLT was shown to prolong overall survival (OS) and improve quality of life measures in patients with mCRPC relative to best supportive care (3), while the Phase 2 TheraP Trial demonstrated that 177Lu-PSMA-617 resulted in a higher rate of PSA decline relative to cabazitaxel chemotherapy (4). When compared to conventional imaging, prostate-specific membrane antigen (PSMA)-based positron emission tomography (PET) has higher detection rates and greater diagnostic accuracy for patients with initial high risk, biochemically recurrent or persistent prostate cancer, and mCRPC (6–9). In this theranostic approach, PSMA PET is used for the screening of patients to demonstrate the presence of PSMA expression, which makes them eligible for PSMA RLT (10–12).
There are three FDA-approved PSMA ligands for PET imaging: 68Ga-PSMA-11 (gozetotide), 18F-DCFPyL (piflufolostat), and rhPSMA-7.3 (posluma). A series of phase III trials have evaluated the use of 68Ga-PSMA-11, 18F-DCFPyL-PET/CT, and rhPSMA-7.3 in prostate cancer patients at initial staging and biochemical recurrence (7–9, 13–15). Most consider the diagnostic utility of the three PSMA ligands to be equivalent at initial staging and biochemical recurrence. VISION and TheraP trials used 68Ga-PSMA-11 PET for their trials due to the extensive clinical experience and wide availability (3, 4). Screen failures were later shown to be associated with poorer outcomes (16).
Despite the market availability of 18F-DCFPyL, the 177Lu-PSMA-617 (vipivotide tetraxetan) package insert specifically recommends selecting patients for treatment using 68Ga-PSMA-11, as the imaging agent and the utility of other PSMA ligands to select patients remains unclear. We retrospectively evaluated patient outcomes and imaging properties of 68Ga-PSMA-11 and 18F-DCFPyL in patients undergoing PSMA RLT in order to help determine if 18F-DCFPyL is appropriate to use for patient selection.
Material and methodsStudy populationIn this study, we retrospectively screened individuals who underwent pre-treatment PET imaging with either 68Ga-PSMA-11 or 18F-DCFPyL before 177Lu-PSMA-617 RLT at our institution from October 2021 to April 2023. The selection of radiopharmaceutical was determined by availability at each imaging center. Included patients had PSMA PET performed within 6 months prior to the first cycle of 177Lu-PSMA-617 RLT. This study was approved by the institutional review board, and informed consent was waived.
PSMA PET acquisitionPatient preparation and administration of either of the PSMA ligands was done as per standard published guidelines (11). The median injected activity of 68Ga-PSMA-11 was 5.7 mCi (4.9–11.4). The median injected activity of 18F-DCFPyL was 9.8 mCi (7.0–11.7). Median uptake time was 58 min (50–102) for 68Ga-PSMA-11 and 60 min (52–101) for 18F-DCFPyL, respectively. A vertex to mid-thigh PET scan was performed using either PET/CT or PET/MRI.
Image interpretationEach PSMA PET scan was interpreted using Visage (Visage Imaging). Five regions were recorded for the presence of prostate cancer including prostate bed (T), osseous (M1b), pelvic nodes (N), extrapelvic nodes (M1a), and visceral metastases (M1c). Maximum standardized uptake values (SUVmax) were recorded for osseous metastases, extrapelvic nodes, and visceral metastases with the highest uptake. Additionally, SUV was also recorded for physiological uptake in the liver (SUVmean) and parotid glands (SUVmax).
Response to RLTSerum PSA levels served as the standard of reference for response assessment to 177Lu-PSMA-617 RLT (17–19). The maximum decline in PSA that occurred anytime during or within 12 weeks of completion of RLT was taken for PSA response analysis. Baseline serum PSAs were drawn on the day of cycle 1 of RLT treatment, and the best PSA response during RLT was assessed for each patient. A decline of 50% from baseline PSA was defined as PSA50 response.
Statistical planDescriptive statistics in the form of median (interquartile) for continuous variable and count (percentage) for the binary variables was used to describe quantitative variables from the clinical data. While comparing the baseline characteristics between the groups imaged with 68Ga-PSMA-11 and 18F-DCFPyL; Student’s t-test was used for the continuous variables and Fisher exact test was used for the discrete variables. A Student’s t-test was conducted to assess the relationship between the lesion SUV and organ uptake between the groups imaged with 68Ga-PSMA-11 and 18F-DCFPyL. For the comparison of SUV and response, the median SUVmax was used to split the population evenly. p <0.05 was considered significant. A comparison of the best overall post-treatment PSA relative to baseline PSA was made between 68Ga-PSMA-11 and 18F-DCFPyL using Student’s t-test. The maximum decline in PSA during RLT was reported for each patient using waterfall plots.
ResultsPatient characteristicsOf the 80 patients who received 177Lu-PSMA-617 therapy at our institution from June 2022 to June 2023, 47 patients received 68Ga-PSMA-11 and 33 patients received 18F-DCFPyL for pre-treatment PET imaging. The patients in both these groups were similar for age, Gleason score, and pre-therapy PSA levels, and prior treatments. Patients imaged using 68Ga-PSMA-11 had higher rates of prior radiation therapy. The two groups had a similar distribution of disease in the prostate/prostate bed, and metastatic disease to lymph nodes (N1), bone (M1a), soft tissue (M1b), and distant organs (M1c) (Table 1).
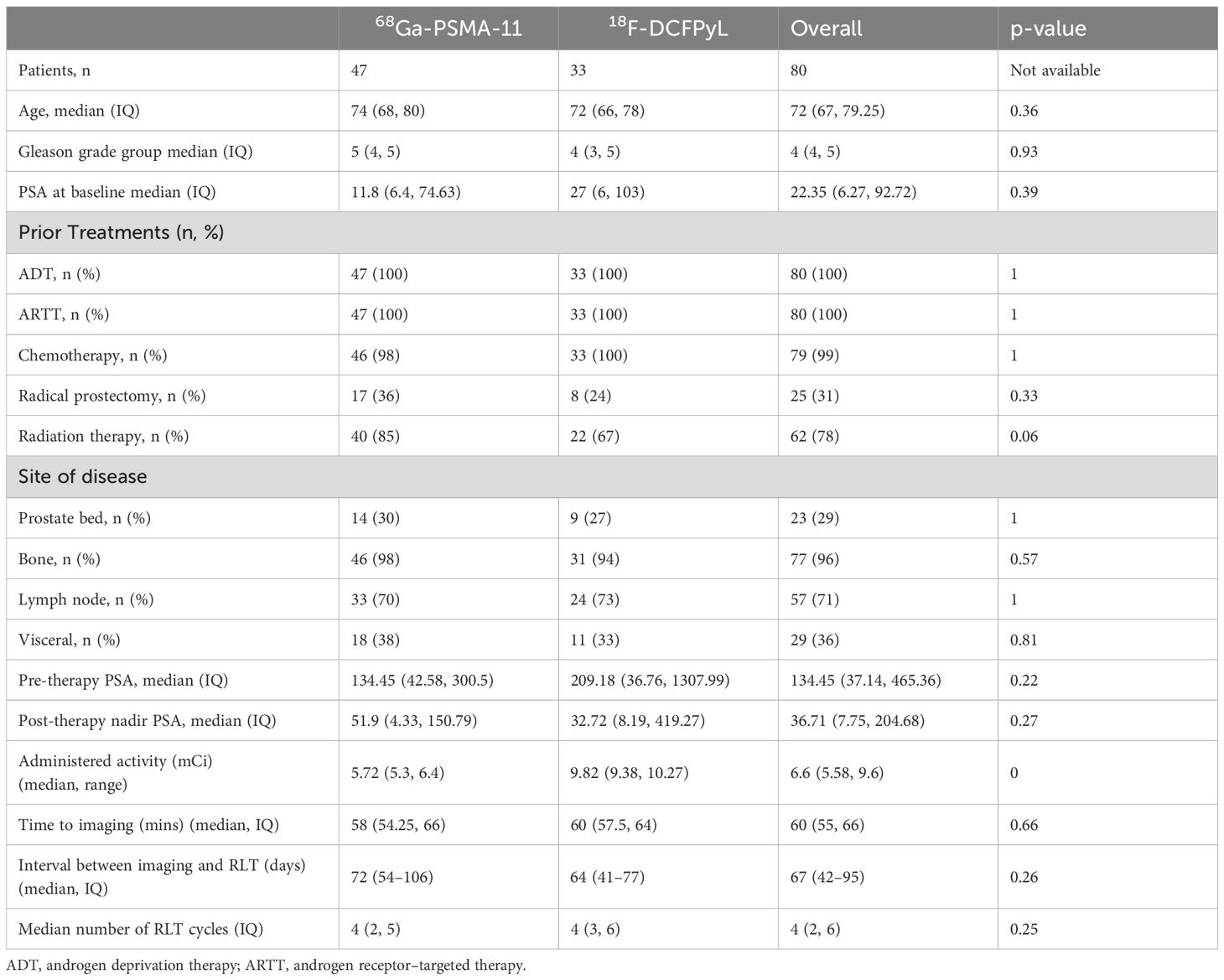
Table 1 Patient demographics.
Physiological biodistributionNo statistically significant difference was observed between the two groups for the liver SUVmean (4.2 ± 1.7 for 68Ga-PSMA-11 versus 4.2 ± 1.5 for 18F-DCFPyL, p=0.99) or the parotid SUVmax (15.2 ± 5.4 for 68Ga-PSMA-11 versus 14.5 ± 7.3 for 18F-DCFPyL, p=65; Table 2).
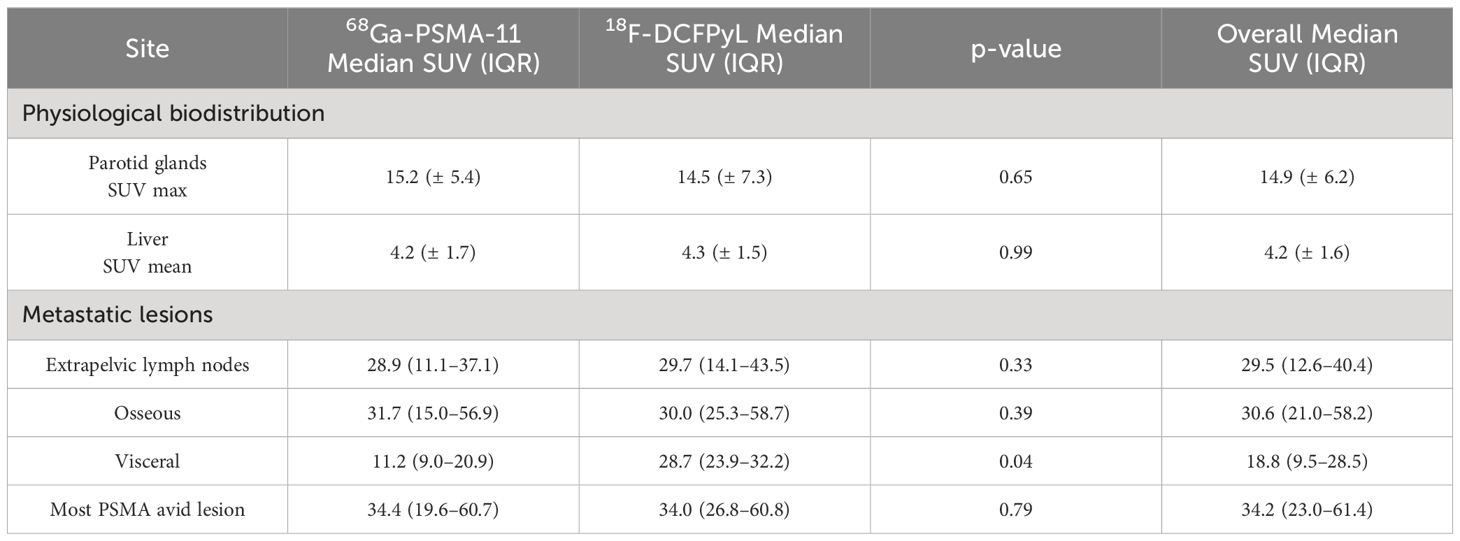
Table 2 Physiological biodistribution and metastatic lesion parameters for 68Ga-PSMA-11 and 18F-DCFPyL.
Radiopharmaceutical and PSA response analysisAmong 47 patients imaged with 68Ga-PSMA-11, 38 (80%) patients had PSA decrease relative to baseline. The average PSA response from baseline was 42%, and 20 (43%) patients had a >50% reduction in PSA (PSA50). Among 33 patients imaged with 18F-DCFPyL, 31 (93%) patients had PSA decrease relative to baseline (Figure 1). The average PSA response from baseline was 65%, and 24 (72%) patients had a PSA50 response. The PSA50 response was higher for patients imaged with 18F-DCFPyL prior to treatment compared to 68Ga-PSMA-11 (p-value = 0.03; Supplementary Table S1, Figure 2).
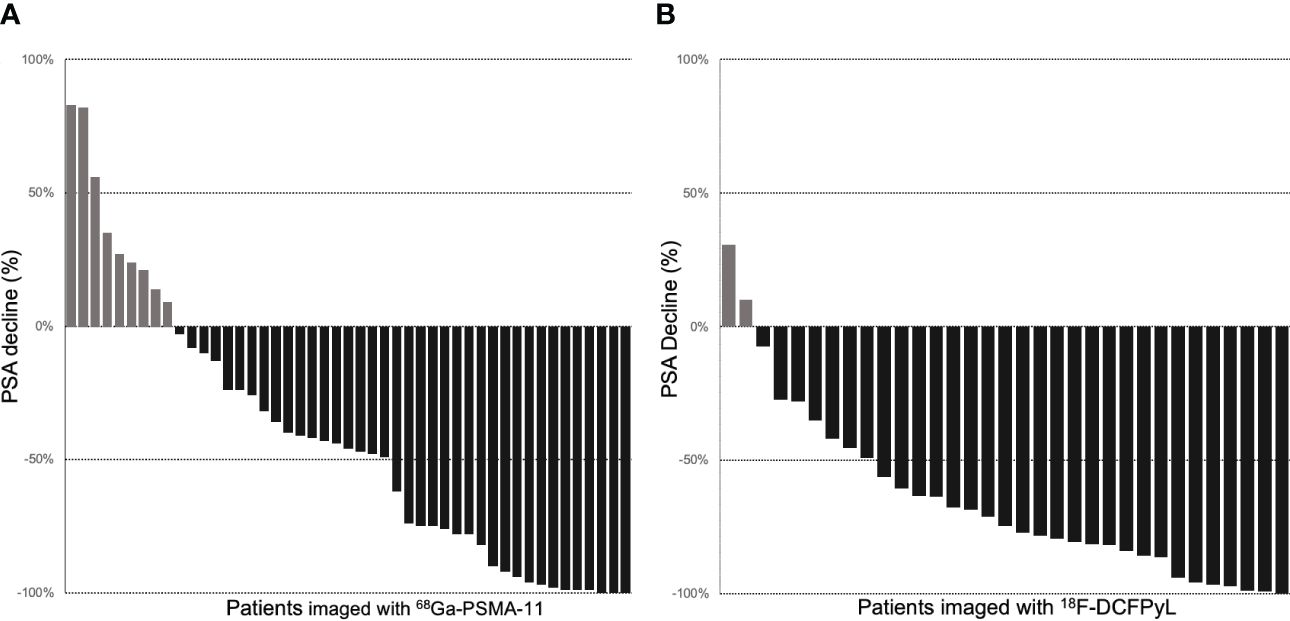
Figure 1 Waterfall plots of PSA response to RLT in patients with pretreatment PET imaging with 68Ga-PSMA-11 (A) and 18F-DCFPyL (B).
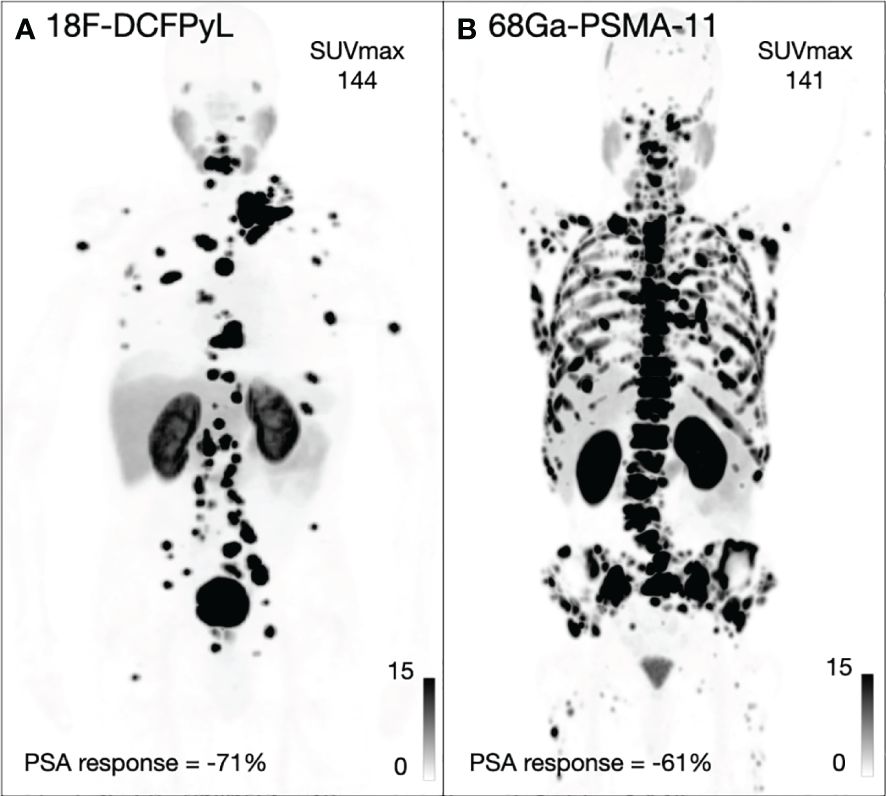
Figure 2 Two example patients imaged with 18F-DCFPyL (A) and 68Ga-PSMA-11 (B) including the SUVmax for both patients and maximum PSA response.
Semi-quantitative comparison of metastatic lesions on pretreatment PET imagingIn comparing the highest SUVmax lesion for the three metastatic sites between the 68Ga-PSMA-11 and 18F-DCFPyL groups, no statistically significant difference was observed between the extrapelvic lymph nodes (p=0.33) or osseous lesions (p=0.39), respectively. For visceral metastatic lesions, 18F-DCFPyL had a higher uptake than 68Ga-PSMA-11 (median = 11.2 (9.0–20.9) for 68Ga-PSMA-11 = versus median = 28.7 (23.9–32.2) for 18F-DCFPyL, p=0.04; Table 2).
The median SUVmax across the overall population was 34.2 and was used to divide patients into two groups: those with high uptake (SUVmax > 34.2) and those with low uptake (SUVmax < 34.2). There was a trend to a higher PSA response in patients imaged with 18F-DCFPyL compared to 68Ga-PSMA-11, which was not statistically significant (Supplementary Table S2).
DiscussionThis is the first report of outcomes in patients treated with PSMA RLT, who were selected with 18F-DCFPyL. We demonstrated that the biodistribution between the two agents was identical and showed that the PSA50 response in patients selected with 18F-DCFPyL was higher than in patients selected for treatment with 68Ga-PSMA-11. Although prior work has focused on the diagnostic utility of 18F-DCFPyL, our results demonstrate that it is appropriate to use it for PSMA RLT screening.
The PSA response to RLT was higher in patients who underwent pretreatment PET imaging with 18F-DCFPyL than those with 68Ga-PSMA-11. This was unexpected, and it is unclear from our small patient numbers if this finding is generalizable. Overall, our results indicate that patients selected with 18F-DCFPyL appear to benefit at least equally to PSMA RLT, which is consistent with guidelines that indicate that either agent can be used for patient selection (11, 12).
As has been previously reported, the biodistributions were similar between the two agents (20, 21). The SUVmax of metastatic lesions are comparable for extrapelvic lymph nodes and osseous sites. While 18F-DCFPyL outperforms 68Ga-PSMA-11 in having higher uptake in visceral lesions, this is limited by the number of lesions included, and intra-patient comparison is needed to confirm that there is in fact higher uptake in visceral lesions.
This work has focused on the difference between 18F-DCFPyL and 68Ga-PSMA-11, but with the recent approval of rhPSMA-7.3, it is uncertain how our results can be extrapolated to include this newer radiopharmaceutical, especially given the partial hepatobiliary clearance seen with rhPSMA-7.3. Although rhPSMA-7.3 has been shown to have lower urinary excretion, which may lead to enhance visualization of local recurrence, the liver uptake is higher than in the other two agents. At this time, it is unclear what the threshold should be for patient selection when using rhPSMA-7.3.
Our study has several limitations. First, this was a retrospective study. This study is additionally subject to various confounders inherent to its lack of intra-patient comparison of the two imaging agents. Specifically, mCRPC exhibits significant heterogeneity, and our analysis only considers the lesion with highest avidity, which may not fully represent the disease burden. The 18F-DCFPyL group received more treatment cycles, and their visceral metastatic lesions showed higher radiotracer avidity, which would be expected to impact the PSA response rate. In the absence of head-to-head trials, comparisons of reported radiopharmaceutical performance on outcomes should be interpreted with caution due to the significant impact of differing patient populations, treatment cycles, end points, scanning protocols, scanning equipment, and readers.
ConclusionPatients imaged with 18F-DCFPyL demonstrated clinical benefit to PSMA RLT in this retrospective study. As previously shown, the physiological biodistribution and lesion uptakes at metastatic sites are comparable for both agents. Patients selected with 18F-DCFPyL had higher PSA50 responses, but comparison to 68Ga-PSMA-11 is limited given the many confounders. Although 68Ga-PSMA-11 was utilized for patient selection in clinical trials of 177Lu-PSMA-617, screening patients can be done using either of radiopharmaceuticals and should not be limited to 68Ga-PSMA-11.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by institutional review board, University of California San Francisco. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was waived off from the participants or the participants’ legal guardians/next of kin in accordance with the institutional requirements.
Author contributionsSY: Writing – review & editing, Writing – original draft, Software, Project administration, Methodology, Investigation. SK: Writing – review & editing, Methodology, Investigation, Data curation. AT: Writing – review & editing, Project administration, Methodology, Data curation. FJ: Writing – review & editing, Validation, Software, Formal analysis. AM: Writing – review & editing, Project administration, Investigation, Data curation. RS: Writing – review & editing, Project administration, Investigation, Data curation. YW: Writing – review & editing, Validation, Supervision, Formal analysis. RJ: Writing – review & editing, Validation, Supervision, Formal analysis. CL: Writing – review & editing, Validation, Supervision, Formal analysis. VK: Writing – review & editing, Validation, Supervision, Formal analysis. TH: Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Funding acquisition, Formal analysis, Conceptualization.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by an investigator initiated trial by Lantheus. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interestTH has grant funding to the institution from Clovis Oncology, GE Healthcare, Lantheus, Janssen, the Prostate Cancer Foundation, Telix, and the National Cancer Institute R01CA235741 and R01CA212148. He received personal fees from Bayer and BlueEarth Diagnostics, Lantheus and received fees from and has an equity interest in RayzeBio and Curium.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1382582/full#supplementary-material
References1. Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [ 177 Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. (2018) 19:825–33. doi: 10.1016/S1470-2045(18)30198-0
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Heck MM, Tauber R, Schwaiger S, Retz M, D'Alessandria C, Maurer T, et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with 177Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. Eur Urology. (2019) 75:920–6. doi: 10.1016/j.eururo.2018.11.016
CrossRef Full Text | Google Scholar
3. Sartor O, De Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. (2021) 385:1091–103. doi: 10.1056/NEJMoa2107322
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. (2021) 397:797–804. doi: 10.1016/S0140-6736(21)00237-3
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Fizazi K, Herrmann K, Krause BJ, Rahbar K, Chi KN, Morris MJ, et al. Health-related quality of life and pain outcomes with [177Lu]Lu-PSMA-617 plus standard of care versus standard of care in patients with metastatic castration-resistant prostate cancer (VISION): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. (2023) 24:597–610. doi: 10.1016/S1470-2045(23)00158-4
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Lawhn-Heath C, Salavati A, Behr SC, Rowe SP, Calais J, Fendler WP, et al. Prostate-specific membrane antigen PET in prostate cancer. Radiology. (2021) 299:248–60. doi: 10.1148/radiol.2021202771
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Hope TA, Eiber M, Armstrong WR, Juarez R, Murthy V, Lawhn-Heath C, et al. Diagnostic accuracy of 68 ga-PSMA-11 PET for pelvic nodal metastasis detection prior to radical prostatectomy and pelvic lymph node dissection: A multicenter prospective phase 3 imaging trial. JAMA Oncol. (2021) 7:1635. doi: 10.1001/jamaoncol.2021.3771
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, et al. Assessment of 68 ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: A prospective single-arm clinical trial. JAMA Oncol. (2019) 5:856. doi: 10.1001/jamaoncol.2019.0096
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Morris MJ, Rowe SP, Gorin MA, Saperstein L, Pouliot F, Josephson D, et al. Diagnostic performance of 18F-DCFPyL-PET/CT in men with biochemically recurrent prostate cancer: results from the CONDOR phase III, multicenter study. Clin Cancer Res. (2021) 27:3674–82. doi: 10.1158/1078-0432.CCR-20-4573
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. (2020) 395:1208–16. doi: 10.1016/S0140-6736(20)30314-7
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Fendler WP, Eiber M, Beheshti M, Bomanji J, Calais J, Ceci F, et al. PSMA PET/CT: joint EANM procedure guideline/SNMMI procedure standard for prostate cancer imaging 2.0. Eur J Nucl Med Mol Imaging. (2023) 50:1466–86. doi: 10.1007/s00259-022-06089-w
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Kratochwil C, Fendler WP, Eiber M, Hofman MS, Emmett L, Calais J, et al. Joint EANM/SNMMI procedure guideline for the use of 177Lu-labeled PSMA-targeted radioligand-therapy (177Lu-PSMA-RLT). Eur J Nucl Med Mol Imaging. (2023) 50:2830–45. doi: 10.1007/s00259-023-06255-8
PubMed Abstract | CrossRef Full Text | Google Scholar
13. Pienta KJ, Gorin MA, Rowe SP, Carroll PR, Pouliot F, Probst S, et al. A phase 2/3 prospective multicenter study of the diagnostic accuracy of prostate specific membrane antigen PET/CT with 18 F-DCFPyL in prostate cancer patients (OSPREY). J Urology. (2021) 206:52–61. doi: 10.1097/JU.0000000000001698
CrossRef Full Text | Google Scholar
14. Jani AB, Ravizzini GC, Gartrell BA, Siegel BA, Twardowski P, Saltzstein D, et al. Diagnostic performance and safety of 18 F-rhPSMA-7.3 positron emission tomography in men with suspected prostate cancer recurrence: results from a phase 3, prospective, multicenter study (SPOTLIGHT). J Urology. (2023) 210:299–311. doi: 10.1097/JU.0000000000003598
CrossRef Full Text | Google Scholar
15. Surasi DS, Eiber M, Maurer T, Preston MA, Helfand BT, Josephson D, et al. Diagnostic performance and safety of positron emission tomography with 18F-rhPSMA-7.3 in patients with newly diagnosed unfavourable intermediate- to very-high-risk prostate cancer: results from a phase 3, prospective, multicentre study (LIGHTHOUSE). Eur Urol. (2023), S0302283823029494.
16. Gafita A, Calais J, Grogan TR, Hadaschik B, Wang H, Weber M, et al. Nomograms to predict outcomes after 177Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: an international, multicentre, retrospective study. Lancet Oncol. (2021) 22:1115–25. doi: 10.1016/S1470-2045(21)00274-6
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. JCO. (2008) 26:1148–59. doi: 10.1200/JCO.2007.12.4487
CrossRef Full Text | Google Scholar
18. Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. JCO. (2016) 34:1402–18.
19. Gafita A, Heck MM, Rauscher I, Tauber R, Cala L, Franz C, et al. Early prostate-specific antigen changes and clinical outcome after 177 lu-PSMA radionuclide treatment in patients with metastatic castration-resistant prostate cancer. J Nucl Med. (2020) 61:1476–83. doi: 10.2967/jnumed.119.240242
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Ferreira G, Iravani A, Hofman MS, Hicks RJ. Intra-individual comparison of 68Ga-PSMA-11 and 18F-DCFPyL normal-organ biodistribution. Cancer Imaging. (2019) 19:23. doi: 10.1186/s40644-019-0211-y
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Heilinger J, Weindler J, Roth KS, Krapf P, Schomäcker K, Dietlein M, et al. Threshold for defining PSMA-positivity prior to 177Lu-PSMA therapy: a comparison of [68Ga]Ga-PSMA-11 and [18F]F-DCFPyL in metastatic prostate cancer. EJNMMI Res. (2023) 13:83. doi: 10.1186/s13550-023-01033-x
留言 (0)