Plateau environments, defined as geographical regions with elevations surpassing 2,500 m, presenting extreme conditions of low oxygen, low pressure, low temperature, dry air, and strong ultraviolet radiation, which have a significant impact on the human cardiovascular system, respiratory system, metabolic system, and immune system. Especially due to hypoxia, the heart is one of the most metabolically active organs in the body, and myocardial metabolism is highly sensitive to ischemia and hypoxia. Prolonged and severe ischemia and hypoxia can directly lead to myocardial damage or even necrosis, endangering life (Maggiorini et al., 1990). Inhabiting, working, or traveling in such high-altitude settings increases the likelihood of individuals experiencing a range of pathophysiological responses related to altitude adaptation, with Acute Mountain Sickness (AMS) being one of the most prevalent. AMS arises from an inappropriate response to hypoxic conditions, characterized predominantly by persistent headaches and often accompanied by non-specific symptoms like nausea, vomiting, general fatigue, loss of appetite, and sleep disturbances (Hackett et al., 1976). Severe cases can progress to life-threatening High Altitude Pulmonary Edema (HAPE) and/or High Altitude Cerebral Edema (HACE).
Despite the abundance of theoretical knowledge and practical experience amassed in studying high-altitude illnesses, alongside the evolution of preventive measures and treatment strategies, accurately anticipating an individual’s predisposition to acute mountain sickness (AMS) remains a significant challenge. The application of artificial intelligence (AI), machine learning (ML), and deep learning (DL) techniques holds promise in scientifically forecasting AMS development upon entry into high-altitude environments. By particularly focusing on identifying and implementing targeted interventions for high-risk populations, these advanced methods have the potential to dramatically decrease the incidence and severity of AMS across the plateau population. However, to harness the full potential of AI, ML, and DL in this context, establishing a comprehensive and robust data repository is imperative as a foundational requirement.
This review aims to systematically and comprehensively survey the key research advancements made in recent years in the prediction of acute mountain sickness. By thoroughly examining and critically evaluating cohort study data, predictive models, biomarkers, and detection methods presented in existing literature, we also explore the potential applications of emerging technologies such as biosensing technology, genomic analysis, and artificial intelligence algorithms in AMS prediction. Our endeavor seeks to illuminate the cutting-edge dynamics of current AMS prediction methodologies, scrutinize unresolved issues and challenges, thereby providing authoritative and forward-looking reference points and practical guidance for global researchers and clinicians. Ultimately, this effort is expected to drive further advancement and development in the field of plateau medicine concerning the prevention and treatment of acute mountain sickness.
1.1 Overview of high altitude illnessesHigh altitude illnesses, as a collective term for specific pathophysiological reactions occurring in high-altitude environments, encompass both acute and chronic forms (Basnyat, 2013). The acute manifestations mainly consist of Acute Mountain Sickness (AMS), High Altitude Pulmonary Edema (HAPE), and High Altitude Cerebral Edema (HACE) (Luks et al., 2017). AMS is an adaptive disorder triggered by rapid ascent to high altitude, characterized by symptoms such as headaches, nausea, vomiting, fatigue, insomnia, and loss of appetite (Luks et al., 2017). Key determinants of AMS onset include altitude, individual sensitivity, rate of ascent, duration of stay, physical exertion levels, and adaptation to hypoxic conditions (Schneider et al., 2002; Basnyat, 2013).
Webb et al. (2009) have highlighted that the human body partially adapts to high altitude through upregulated expression of HIF-1. Due to the thin air and low oxygen partial pressure at high elevations, humans often experience hypoxia. Given the inter-individual variability in tolerance to hypoxia, distinct pathophysiological responses emerge among individuals. Studies show that those who ascend above 2,500 m, particularly above 4,000 m, obese individuals, and those with a history of migraines are at significantly increased risk for AMS (Hirata et al., 1989; Lee et al., 2002; Simancas-Racines et al., 2018).
According to symptom severity, high altitude illnesses can be classified as mild, moderate, or severe (Ogilvie, 2001; Basnyat and Murdoch, 2003), as shown in Table 1. Mild cases present with minor headaches, insomnia, and decreased appetite; moderate patients exhibit these symptoms along with frequent vomiting, marked weakness, and palpitations, which can interfere with daily life but typically resolve within 2–3 days after onset. Severe high altitude illnesses manifest with intense vomiting, chest tightness, and drowsiness, severely compromising life safety and requiring urgent intervention; HAPE and HACE represent typical complications, involving pulmonary edema and cerebral edema respectively, which if left untreated, can lead to life-threatening consequences (Chen et al., 2023).
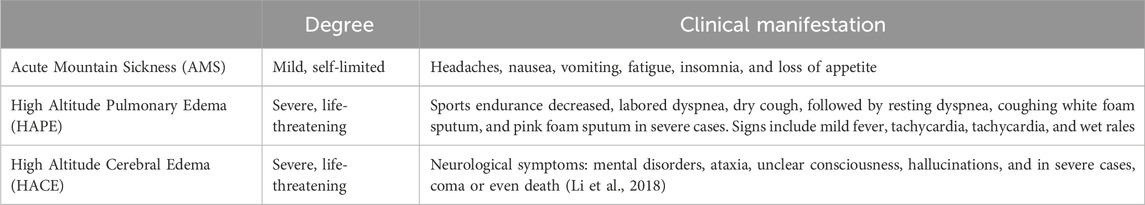
Table 1. Classification and symptoms of high altitude illnesses.
The Lake Louise Questionnaire was introduced in 1991 and was last modified in 2018. It is now the most commonly used scoring system used to assess AMS (Roach et al., 2018). It defines AMS as the presence of headaches in addition to three other symptoms, including gastrointestinal symptoms, fatigue/weakness, and dizziness/light headedness. Each symptom is appointed a point on a scale from 0 to 3, with 0 being no effect and 3 being severe. A total score of 3 or greater, with the presence of headaches, in a setting of rapid ascent to high altitude, is diagnosed as acute mountain sickness. The Lake Louise Questionnaire, although an effective assessment tool, has its limitation. It is a scoring system to standardize and diagnose AMS for use by investigators or research purposes, and it is not meant for clinical practice.
High altitude illnesses are characterized by: 1) rapid onset, with symptoms potentially emerging within 24–48 h upon reaching high altitude, as the body requires time to adapt to the hypoxic environment; 2) broad susceptibility, not restricted by factors like gender, age, or physical condition, as even healthy individuals may develop illness (Burtscher et al., 2021); 3) reversibility, with most symptoms resolving when altitude is reduced or appropriate treatment is given; and 4) significant individual variation, with substantial differences in the ability to adapt to high altitude environments among different individuals.
Understanding and appreciating these characteristics are crucial for timely, accurate, and targeted prevention and intervention of high altitude illnesses. To ensure the safety and health of individuals entering high altitude areas, effective control measures can include hypoxic preconditioning training, scientifically sound climbing plans, gradual ascending speeds, and pharmacological prophylaxis, among others (Chen et al., 2023; Toussaint et al., 2021).
Hypoxic preconditioning training involves exposure to low oxygen for a certain amount of time and intensity before hypoxic stress to generate endogenous protection and enhance the body’s tolerance to hypoxic hypoxia later on (Wang et al., 2021). Hypoxic pre-adaptation training can promote various adaptive changes in the body by simulating the conditions of high altitude and low oxygen, including increasing erythrocyte production, promoting blood vessel dilation and new blood vessel formation, optimizing metabolic mode, enhancing antioxidant and anti-inflammatory functions, adjusting gene expression, and helping the central nervous system adapt to low oxygen (Olfert et al., 2001; Baillieul et al., 2017; Viscor et al., 2018; González-Candia et al., 2022). Through the above mechanism, hypoxia pre-adaptation training can help the human body adapt to the low oxygen environment at high altitude in advance to a certain extent, reduce or avoid the occurrence of mountain diseases and related complications, and provide an effective prevention strategy for people entering high altitude areas. However, such training should be conducted under professional guidance to ensure safety and effectiveness (Girard et al., 2020).
1.2 Exploration of pathophysiological mechanisms in high altitude illnessesThe development and progression of high altitude illnesses are closely linked to the body’s adaptive responses to hypoxic environments at high altitudes, involving a series of complex pathophysiological changes, as shown in Figure 1. The following will elaborate on these critical processes:
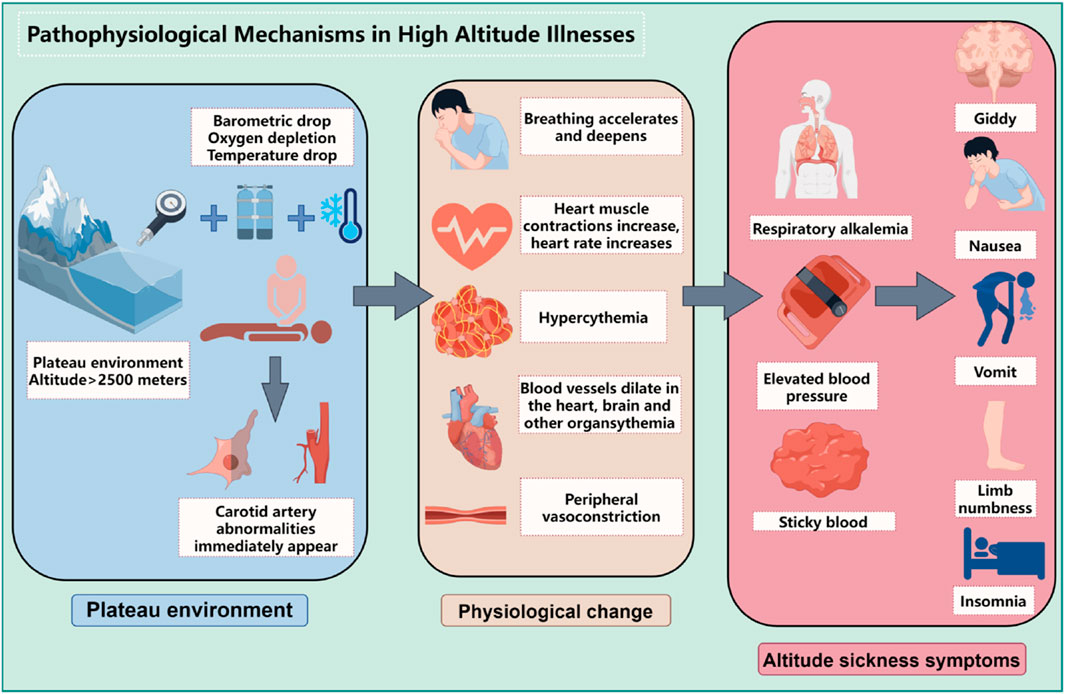
Figure 1. Pathophysiological mechanisms in high altitude illnesses (By figdraw).
1.2.1 Oxygen supply limitationAt high altitudes, with decreasing atmospheric pressure, the partial pressure of oxygen reduces correspondingly, which lessens the oxygen diffusion gradient across the alveolar-capillary interface, leading to a significant decrease in the amount of oxygen carried by the blood (lowering oxygen saturation). Insufficient oxygen supply constitutes the core pathophysiological basis for high altitude illnesses. Hypoxia-inducible factors (HIFs), particularly the HIF-1α subunit, play a pivotal role here. Under low oxygen conditions, HIF-1α stabilizes and activates, regulating the expression of thousands of genes including glycolytic enzymes, Vascular Endothelial Growth Factor (VEGF), and Erythropoietin (EPO) (Dzhalilova and Makarova, 2020). Additionally, molecules like Heat Shock Protein 70 (HSP70) and Nitric Oxide (NO) also participate in modulating an individual’s tolerance to hypoxia, as shown in Figure 2.
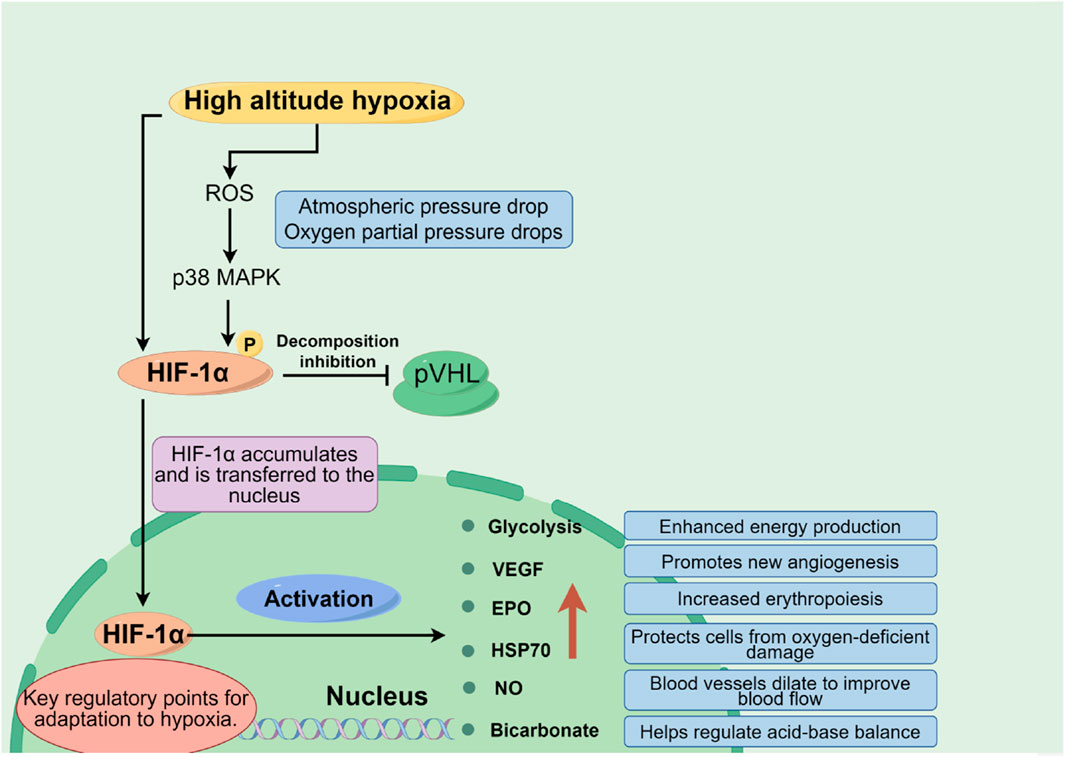
Figure 2. Oxygen supply and demand balance mechanism in plateau environment (By figdraw).
1.2.2 Compensatory physiological responses to hypoxiaIn response to the hypoxic challenge at high altitude, the body initiates several adaptive physiological responses:
Respiratory System: By increasing respiratory rate and depth to enhance oxygen uptake and carbon dioxide elimination.
Cardiovascular System: Within days of initial exposure to hypoxia, autonomic nervous system adjustments result in increased heart rate and cardiac output, optimizing oxygen delivery to tissues throughout the body, thus protecting the heart from excessive workload due to increased metabolic demands.
Blood System: Under the stimulation of chronic hypoxia, the expression of hypoxia inducible factors is activated, thereby stimulating the kidneys and liver to secrete a large amount of EPO (Du and Bai, 2015). EPO activates JAK2 and STAT-5 related signaling pathways by binding to EPO receptors in hematopoietic organs, further upregulating the expression of membrane proteins and hemoglobin, thereby inducing massive proliferation of red blood cells and increasing the number of red blood cells in the bloodstream. This can improve tissue oxygen supply to a limited extent, making the body less prone to pathological changes. As hypoxia worsens, EPO regulation becomes dysregulated, stimulating the proliferation of immature red blood cells at different stages of bone marrow development, and changing the size and shape of red blood cells to meet physiological needs, expanding the contact surface with oxygen, effectively improving tissue hypoxia. But when severe hypoxia occurs, the number of red blood cells increases, and the compensatory significance of the above changes into harmful effects, causing an increase in blood viscosity, a decrease in cell deformability, and difficulty for cells to pass through microvessels, further leading to tissue hypoxia, a vicious cycle, and ultimately the occurrence of HAPC.
Renal System: During acute hypoxia, activation of the adrenergic system leads to tachycardia; meanwhile, hypoxia-induced pulmonary vasoconstriction raises pulmonary artery pressure, and the kidneys respond by secreting erythropoietin to stimulate erythropoiesis in bone marrow, further augmenting red blood cell numbers (Richalet et al., 2023).
1.2.3 Increased blood viscosity and hemodynamic changesDue to the increased number of red blood cells and heightened hemoglobin concentrations, blood viscosity significantly rises under high-altitude conditions, presenting as increased resistance to blood flow, exacerbating the heart’s workload, and potentially affecting microcirculation and overall circulatory efficiency (Semenza, 1994; Savioli et al., 2022).
1.2.4 Tissue damage and pathological processesLong-term or severe hypoxia can trigger various tissue damages and pathological processes:
Cellular Oxidative Stress: Cellular oxidative stress is a pathophysiological state in which the balance between intracellular oxidation and antioxidant action is upset in favor of the oxidative process, resulting in the abnormal accumulation of reactive oxygen species (ROS) such as superoxide anions (O2 ⁻), hydrogen peroxide (H₂O₂), hydroxyl radicals (OH⁻), etc. This process not only affects normal cell metabolism, but also directly or indirectly damages biological macromolecules, including proteins, lipids, nucleic acids, etc., thus interfering with many aspects of cell signaling, energy production, gene expression, and cell cycle regulation (Tan et al., 2018). Under low oxygen conditions, the oxygen supply to cells is limited, and the function of the electron transport chain of the mitochondria may be impaired, leading to electron leakage and promoting ROS production. At the same time, low oxygen can also affect the antioxidant defense system, such as reducing the activity of enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx), making it more difficult for cells to clear ROS, thus exacerbating oxidative stress (Zaarour et al., 2023). Oxidative stress is not only closely related to the occurrence and development of a variety of diseases, such as cardiovascular diseases, neurodegenerative diseases, diabetes, cancer, etc., but also participates in the aging process (Arfin et al., 2021). In recent years, intervention strategies for oxidative stress have become a research hotspot. These include the use of antioxidants, regulation of the balance between intracellular ROS production and clearance, and drug development targeting specific ROS-related signaling pathways (Leyane et al., 2022). For example, certain natural compounds such as flavonoids (such as genistein) have been found to reduce the damage of cells caused by oxidative stress, which may be related to their direct removal of free radicals or regulation of the antioxidant defense system (Alorda-Clara et al., 2022).
Inflammatory Response: The inflammatory response is a complex biological defense mechanism designed to eliminate harmful irritants and promote tissue repair. The latest scientific research continues to deepen our understanding of this process. Hypoxia, a lack of oxygen supply, is one of the key factors that triggers the inflammatory response. Under hypoxia, cells induce the release of pro-inflammatory mediators through a variety of pathways, including but not limited to tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), and vasoactive mediators such as histamine and bradykinin (Malkov et al., 2021).The release of proinflammatory mediators initiates a cascade of reactions that includes the following key steps (Gusev E et al., 2021; Soares et al., 2023): vascular response; Leukocyte mobilization; Cytokine storm; Tissue repair and remodeling. Recent studies have also focused on the fine regulatory mechanisms of inflammatory response, such as the activation of NLRP3 inflammasome in sensing various danger signals (Seoane et al., 2020), and the complex role of Hypoxia-inducible factors (HIFs) in regulating inflammation and immune response (Foglia et al., 2021). Hypoxia induces the release of pro-inflammatory mediators, triggering an inflammatory cascade that results in vasodilation, increased vascular permeability, and other inflammatory manifestations.
Tissue Edema: Examples include high altitude cerebral edema (HACE) and high altitude pulmonary edema (HAPE), which are severe complications of high altitude illnesses. These occur mainly due to circulatory disturbances, increased capillary leakage, and fluid accumulation (Scoggin et al., 1977; Dehnert et al., 2005).
1.2.5 Complications and their riskSevere cases of high altitude illness can lead to potentially fatal complications:
High Altitude Pulmonary Edema (HAPE): HAPE is non cardiogenic pulmonary edema caused by acute severe hypoxia, which leads to pulmonary vein vasoconstriction, causing a sharp increase in pulmonary arterial pressure, local hyperperfusion, increased pulmonary blood flow, and imbalanced ventilation blood flow ratio. In addition, fluid leaks into the pulmonary interstitium and alveoli through pulmonary capillaries, hindering normal gas exchange (Choudhary et al., 2022).
High Altitude Cerebral Edema (HACE): HACE is considered to be the final stage of acute high-altitude reaction. In addition to worsening symptoms such as headache, dizziness, and vomiting mentioned above, ataxia, mental confusion, varying degrees of consciousness disorders, fever, and in severe cases, brain herniation can occur, endangering life (Li et al., 2023).The exact pathophysiological mechanisms underlying the development of AMS and HACE have not been fully determined. One of the main theories explaining the development of AMS is the increase in intracranial pressure. There are two important factors that cause an increase in intracranial pressure: the generation of cerebral edema and an increase in intracranial blood flow. Vascular edema (accumulation of extracellular water due to increased blood-brain barrier permeability) and cytotoxic edema (disruption of Na+/K + pump function on the cell membrane) are considered the underlying mechanisms of HACE (Bailey et al., 2009).
The pathophysiology of high altitude illnesses encompasses limited oxygen supply, hypoxia-induced physiological adaptations and compensatory responses, changes in blood rheology, and the development of related tissue damage and complications. A deep understanding of these mechanisms is essential for guiding the formulation of prevention strategies, early diagnosis, and effective treatment of high-altitude illnesses, both theoretically and practically.
2 Current status and advances in cohort studies of high altitude illness populationsCohort studies, due to their exceptional ability to unravel complex interplays between compounded exposures such as high-altitude environments, lifestyle habits, and genetic components with health outcomes, have been universally recognized as a preeminent research approach in exploring etiological mechanisms and formulating strategies for disease prevention and management (Li and Lyu, 2015). Within the realm of high altitude illness research, the creation and meticulous examination of long-term, prospective cohorts among populations chronically exposed to high altitudes allow scientists to meticulously discern the fundamental patterns of disease manifestation and progression, along with related risk factors. The data harvested from these studies act as a pivotal resource and groundwork for developing predictive models. Researchers utilize long-term, forward-looking cohort methodologies to methodically gather and interpret data concerning individuals dwelling in high-altitude settings, with the aim to uncover the incidence patterns, developmental trends, and risk factors associated with high altitude illnesses, as well as to assess the practicality and effectiveness of prophylactic and treatment strategies.
(1) Study Subjects and Scope: Currently, cohort studies on high altitude illness populations encompass a diverse range of groups, including but not limited to permanent residents living in high-altitude regions like the Tibetan Plateau, workers frequently traveling between low and high altitudes, professional and amateur athletes participating in mountaineering expeditions, and tourists visiting high-altitude destinations. The diversity of study subjects helps to comprehensively understand the differences in adaptability and susceptibility among various populations to high-altitude environments.
(2) Incidence and Severity Research: Several cohort studies show that the incidence of high altitude illnesses (such as Acute Mountain Sickness, Chronic Mountain Sickness, High Altitude Pulmonary Edema, and High Altitude Cerebral Edema) increases with rising altitude and exhibits significant individual variability (Hackett et al., 1976; Gongga et al., 2014; Wang et al., 2014). Through longitudinal follow-ups of large samples, research teams have quantified changes in the frequency and severity of high altitude illnesses at different altitudes, providing key data for risk prediction models.
(3) Exploration of Influencing Factors: Researchers have thoroughly investigated and statistically analyzed a variety of potential factors affecting the occurrence and progression of high altitude illnesses. These factors include modifiable habits such as lifestyle, physical fitness, and nutritional status, as well as non-modifiable biological traits like genetic background, age, and gender. For instance, certain gene polymorphisms have been associated with an individual’s capacity to adapt to hypoxic high-altitude environments, thereby influencing their risk for high altitude illnesses (Zhao et al., 2023).
The activation of RAAS at high altitudes, where hypobaric hypoxia leads to reduced oxygen availability, plays a pivotal role. Under such conditions, there is an increase in renin secretion from the kidneys, initiating a cascade that results in the production of angiotensin II (Ang II) and subsequent aldosterone release. Ang II, a potent vasoconstrictor, contributes to the maintenance of blood pressure but can also lead to pulmonary vasoconstriction and increased vascular permeability, predisposing individuals to HAPE. Meanwhile, aldosterone enhances sodium retention and fluid accumulation, potentially exacerbating the edematous processes observed in both HAPE and HACE (Ferrario and Mulick, 2017; Hundemer and Sood, 2021). Furthermore, recent studies have highlighted the potential therapeutic benefits of RAAS inhibitors in mitigating the severity of altitude sickness symptoms, emphasizing the system’s critical involvement in the disease progression (Duo et al., 2023; Richalet et al., 2023).
(4) Evaluation of Prevention and Intervention Measures: Based on cohort data, scientists assess various methods for preventing and treating high altitude illnesses, such as drug prophylaxis (e.g., acetazolamide and dexamethasone use), stepwise acclimatization training, physiological and psychological conditioning, and rational dietary supplementation strategies. The research findings provide a scientific basis for developing targeted and effective health protection plans for high-altitude settings.
Cohort studies on high altitude illness populations play a vital role in etiological and epidemiological research, continually enriching and deepening our understanding of physiological and pathological changes in humans under high-altitude conditions. They significantly propel the development of high-altitude medicine and are of great importance for ensuring the health of people living in high-altitude regions and improving work efficiency in these areas. Currently, both China and other parts of the world have established numerous population cohorts to further this research agenda.
2.1 Status of cohort studies in ChinaIn China, cohort studies have made significant progress in different geographical regions across the country, as shown in Table 2. For instance, the “Southwest Region Natural Population Cohort Study” project, led by Professor Xiaosong Li from the West China School of Public Health/West China Hospital Fourth Affiliated Hospital, has made outstanding contributions to establishing a natural population cohort with unique environmental features, ethnic diversity, and disease distribution characteristics in the Southwest region. Since its inception in 2017, the “Southwest Cohort” has successfully completed baseline surveys and a follow-up visit with an impressive 93% retention rate, collecting biological samples from 112,000 individuals. It has also established baseline databases, follow-up databases, and a biobank covering the natural populations across five provinces and cities in the Southwest region, marking a major breakthrough in large-scale natural population cohort research in multi-ethnic areas of Southwest China. Additionally, this study innovatively developed a high-standard technical specification system for cohorts applicable to China’s multi-ethnic regions. Through biomedical big data mining and analysis, it revealed the prevalence patterns and risk factors of prominent health issues in the Southwest region, translating scientific findings into policy, thereby providing a practical and feasible “Southwest Solution” for precision prevention and proactive health strategies, garnering international recognition for its research outcomes (Zhao et al., 2023).
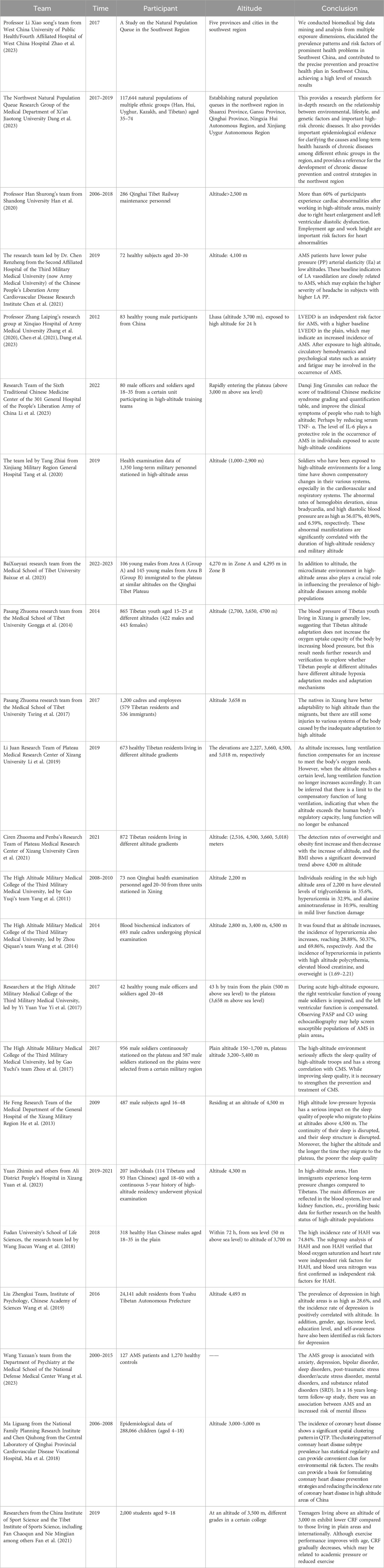
Table 2. Status of cohort studies in China.
Simultaneously, addressing the distinctive natural environment conditions and unique health status and disease characteristics among residents in the Northwest region, a Northwest Region Natural Population Cohort Study was established between 2017 and 2019 in Shaanxi, Gansu, Qinghai, Ningxia Hui Autonomous Region, and Xinjiang Uygur Autonomous Region. This study enrolled 117,644 multi-ethnic (including Han, Hui, Uyghur, Kazakh, and Tibetan) individuals aged between 35 and 74 years old, systematically collecting various exposure information at individual, environmental, and social levels and procuring a large number of peripheral blood samples. Over 900,000 biological samples of various types were stored in a standardized biobank. Currently, a combination of routine monitoring and active follow-ups is being used for long-term tracking observations. The average age of cohort members is 52.43 years, with women comprising 59.8%. There are differences in socioeconomic status and lifestyles among different ethnic groups, although they share some similarities in overall health status while displaying distinct traits. This Northwest Region Natural Population Cohort Research platform provides robust support for exploring the relationships between multiple factors such as environment, lifestyle, and genetics and major chronic diseases. It holds promise in providing key epidemiological evidence to elucidate the etiology of chronic diseases and their long-term health impacts among different ethnicities in the region. Ultimately, this could provide a scientific basis for formulating national chronic disease prevention and control strategies, not only for the Northwest region but also on a nationwide scale (Dang et al., 2023).
In the field of high-altitude illness research in China, Professor Shurong Han’s team from Shandong University conducted a retrospective cohort study focusing on maintenance workers of the Qinghai-Tibet Railway. This study enrolled 286 individuals working at high altitudes and through long-term follow-up and testing, found that during the observation period, heart morphological and functional abnormalities were present in 173 participants, with the most common being right atrial enlargement, left ventricular diastolic dysfunction, and tricuspid valve regurgitation. The findings further revealed that older workers engaged in work at extremely high altitudes were more prone to cardiac abnormalities compared to younger workers (under 20 years old) who worked at altitudes below 4,000 m. Overall, over 60% of the study subjects showed cardiac abnormalities after working at high altitude, primarily manifested as right heart enlargement and left ventricular diastolic dysfunction. Based on these results, the study emphasized the importance of employment age and workplace altitude as significant risk factors for cardiac abnormalities and recommended intensified regular cardiovascular health check-ups for workers at high altitudes (Han et al., 2020).
Another cohort study was carried out by the research team led by Dr. Renzheng Chen from the Second Affiliated Hospital of the Third Military Medical University (now Army Medical University), PLA Cardiovascular Disease Institute (Chen et al., 2021). They selected 72 subjects who ascended to the Lhasa region at an altitude of 4,100 m and used 24-h ambulatory blood pressure monitoring devices and echocardiography for detailed assessments. The study results showed that patients with Acute Mountain Sickness (AMS) had lower LA PP and Ea values, indicating a significant association between these baseline indicators of LA vascular relaxation and the occurrence of AMS, potentially explaining why subjects with higher LAPP experienced more severe headaches.
Professor Leping Zhang’s research group from Xinqiao Hospital, Army Medical University, employed a high-altitude field-based cohort study method to analyze the physiological changes in 83 young Chinese male subjects following acute exposure to high altitude (Zhang et al., 2019). The study reported that after acute high-altitude exposure, participants’ SAS (Social Support Rating Scale) scores, FSAS (Flight Anxiety Scale) scores, and heart rates significantly increased (p < 0.05), while oxygen saturation levels notably decreased (p < 0.05). Concurrently, there was a marked increase in systemic and cerebral blood flow velocities (p < 0.05), accompanied by elevated endothelin-1 and bradykinin levels (p < 0.05), as well as decreased concentrations of nitric oxide, prostaglandin E, and serotonin (p < 0.05). Based on these findings, the study concluded that left ventricular end-diastolic diameter (LVEDD) measured at sea level has some predictive value for AMS; moreover, changes in hemodynamics and psychological states such as anxiety and fatigue after high-altitude exposure may play a role in the development of AMS.
At the Sixth Medical Center of Traditional Chinese Medicine, PLA General Hospital 301, researchers led by Li Min conducted a field-based cohort study in high-altitude settings involving 80 participants to investigate the protective effects of Danshen-Qi-Jing Granules against acute mountain sickness (AMS) among rapid ascent personnel (Li et al., 2023). The treatment group was administered Danshen-Qi-Jing Granules, which primarily consist of herbs like Astragalus membranaceus, Salvia miltiorrhiza, and Polygonatum sibiricum, while the control group received Rhodiola rosea capsules. The study revealed that a continuous 14-day oral administration of the granules, initiated 7 days prior to ascending to high altitude, effectively reduced scores on the Syndrome Differentiation Quantitative Scoring Table for Traditional Chinese Medicine and improved clinical symptoms among the subjects. Additionally, the findings suggested that the granules might exert a protective role against AMS development through downregulating serum levels of TNF-α and IL-6.
At the Xinjiang Military Region General Hospital, a team led by Tang Zhi’ai conducted a comprehensive investigation into the health impacts of high-altitude environments on military personnel (Tang et al., 2020). The findings revealed clear correlations between physiological parameter alterations and both the duration of stay at high altitudes and the specific altitude levels occupied.
Researchers at the School of Medicine, Tibet University, including Bai Xueyazi, carried out an observational cohort study aimed at elucidating the variation in incidence rates of high-altitude illnesses under similar altitudes but contrasting microclimatic conditions (Baixue et al., 2023). The study observed a total of 251 migrants in two regions, A and B, on the Qinghai-Tibet Plateau, using a scoring system based on the international diagnostic criteria for chronic high-altitude illness. Results showed that Region A, characterized by significant seasonal temperature variations and harsh climate, had a significantly higher overall incidence rate of chronic high-altitude illnesses compared to the more temperate and lushly vegetated Region B. This indicated that aside from altitude, the microclimate environment of high-altitude regions also plays a pivotal role in influencing the prevalence of high-altitude illnesses among migrant populations.
A team led by Pasang Zhuoma from the same institution focused on hypoxia adaptation patterns in high-altitude areas (Gongga et al., 2014). Through studying blood pressure characteristics in 845 native Tibetan young adults living at elevations of 2,700, 3,650, and 4,700 m, they found that the prevalence of hypotension gradually increased with rising elevation, reaching 59.1%, 59.3%, and 76.9% respectively. This suggests that during long-term adaptation to high-altitude environments, the body does not rely on raising blood pressure as a means to enhance oxygen uptake. Furthermore, their survey on high-altitude maladaptation among employees and workers in Lhasa demonstrated that across respiratory, nervous, circulatory, and digestive systems, native Tibetans exhibit superior adaptive capacity than migrants. However, even among native Tibetans dwelling in high-altitude areas, there is still a notable degree of functional dysregulation resulting from incomplete adaptation (Tsring et al., 2017).
At the Plateau Medical Research Center of Tibet University, researcher Li Juan conducted a study on the relationship between pulmonary function and high-altitude adaptation among native Tibetan residents across different altitude gradients (Li et al., 2019; Fan et al., 2021). Through random sampling of 673 Tibetans from four distinct altitudinal regions—Yigong (2,227 m), Lhasa (3,600 m), Chamdo (4,500 m), and Purang County (5,018 m)—she primarily assessed their static lung ventilation function and basal physiological indicators. The results showed that Chamdo residents had significantly higher mean values for vital pulmonary function parameters such as FVC (forced vital capacity), PEF (peak expiratory flow rate), FEF25 (forced expiratory flow at 25% of FVC), and FET (forced expiratory time), suggesting that enhanced pulmonary ventilation is a key indicator of Tibetan populations' adaptation to high-altitude environments. However, when altitude increases to certain heights, such as in Purang County, this physiological regulatory capability might be suppressed, leading to a plateau or decline in pulmonary ventilation function.
A cohort study by Ciren Zhuoma and Penba’s team from the same center aimed to investigate the distribution of body mass index (BMI) across various altitudes in Tibet (Ciren et al., 2021). They selected a total of 872 native Tibetan residents living at 2,516, 3,660, 4,500, and 5,018 m as research subjects. The findings revealed that the detection rates of overweight and obesity initially rose and then fell with increasing altitude, reaching 50.4%, 56.2%, 46.7%, and 21.3%, respectively, thus uncovering for the first time the correlation between BMI and high-altitude adaptability: ascending altitude may affect BMI, resulting in relatively lower BMI among residents in high-altitude areas.
At the High Altitude Military Medical College under the Third Military Medical University, Gao Yuchi and Zhou Qiquan’s team analyzed blood biochemical indices of non-Qinghai resident examinees stationed in Xining (2,200 m). Data from 2008 to 2010 indicated that 35.6% of the population had abnormal triglyceride concentrations, 32.9% had elevated uric acid levels, and 10.9% had increased AST (aspartate transaminase) levels, indicating mild liver dysfunction (Yang et al., 2011). Subsequently, the team further validated the prevalence of hyperuricemia among male cadres residing at varying altitudes. By collecting blood biochemical data from 693 male cadre examinees living at 2,800, 3,400, and 4,500 m, they found that the incidence of hyperuricemia progressively increased with rising altitude, reaching 28.88%, 50.37%, and 69.86%, respectively. Concurrently, there was a notably high comorbidity rate (ranging from 1.69 to 2.21) between hyperuricemia and conditions like polycythemia, elevated serum creatinine levels, and overweight status. These research outcomes provide significant scientific foundations for delving into the pathogenesis of hyperuricemia in high-altitude dwellers, guiding clinical prevention and treatment measures, and improving acclimatization levels in these populations (Wang et al., 2014).
Researchers at the High Altitude Military Medical College of the Third Military Medical University, led by Yi Yuan Yue, conducted a thorough investigation into changes in cardiac function before and after rapid ascent to high altitude and their relationship with Acute Mountain Sickness (AMS) (Yi et al., 2017). They swiftly transported 42 healthy subjects from Chongqing at an elevation of 500 m to Lhasa at 3,658 m within 43 h. On the third day before and after arrival, a series of assessments were made on the subjects' heart rate, blood pressure, oxygen saturation levels, echocardiograms, and Lake Louise Score. The study revealed that following the rapid ascent to high altitude, the subjects experienced elevated pulmonary artery systolic pressure (PASP) and mean pulmonary artery pressure (MPAP), along with enhanced left ventricular contractility. The incidence rate of acute mountain sickness was recorded as 35.7%, and notably, those in the AMS group had higher PASP values compared to the non-AMS group. Additionally, there was a negative correlation observed between cardiac output and AMS scores, suggesting that individuals with higher pulmonary artery systolic pressure and lower cardiac output at low altitudes are more prone to develop AMS upon entering high-altitude environments, thereby providing new insights for identifying and screening susceptible populations for AMS. Furthermore, the research team also focused on the correlation between sleep quality and chronic high-altitude diseases (Zhou et al., 2017). A total of 1,543 participants were enrolled, including 956 stationed at altitudes ranging from 3,200 to 5,400 m for over 6 months, while the control group consisted of 587 participants stationed in plains areas with elevations between 150 and 1,700 m. Utilizing the Pittsburgh Sleep Quality Index (PSQI) and Chronic Mountain Sickness Symptoms Questionnaire (CMS), it was found that the sleep quality of the high-altitude group was significantly lower than the plains group. In both healthy subgroups and those with CMS, the PSQI total scores were substantially higher in the high-altitude group, and there was a moderate positive correlation (r = 0.2) between the PSQI total score and the CMS score. These findings underscored the significant detrimental effect of high-altitude environments on sleep quality and its strong association with the occurrence of chronic high-altitude diseases. Therefore, it is crucial to simultaneously improve sleep quality and strengthen preventive and therapeutic measures for chronic high-altitude diseases, which corroborates the conclusions drawn by He Feng’s research at the General Hospital of the Tibet Military Region (He et al., 2013).
A longitudinal cohort study by Yuan Zhi Min et al. from Ali District People’s Hospital of Tibet investigated the impact of high altitude on hematological indices in Tibetan and Han populations (Yuan et al., 2023). This retrospective analysis, a first of its kind, monitored changes in blood indices over 3 years for low-altitude Han individuals who migrated to extreme heights, contrasting them with native Tibetans. The study involved 114 Tibetans and 93 Han subjects living at high altitudes for over 2 years. Results showed that migrating Han individuals experienced increased platelet, white blood cell, and red blood cell counts, suggesting sustained hematopoietic system stress to adapt to the environment. Additionally, this group had elevated TBIL, DBIL, and triglyceride levels, indicating heightened liver metabolic burden and potentially increased cardiovascular risk under hypoxic conditions. These findings underscore unique stress responses and adaptive differences in hematological profiles and liver-kidney functions between Han immigrants and native Tibetans, thereby contributing valuable data for future research into high-altitude population health.
At Fudan University’s School of Life Sciences, the research team led by Wang Jiucun conducted an observational cohort study investigating physiological, hematological, and biochemical risk factors associated with high-altitude headache (HAH) following acute high-altitude exposure (Wang et al., 2018). The study enrolled 318 Han Chinese male participants who underwent a rapid ascent to an altitude of 3,700 m within 72 h via train travel from an elevation of 50 m, simulating a scenario of rapid ascent to high altitudes. The study findings revealed that HAH incidence reached a striking 74.84% among the subjects, emphasizing HAH as one of the most prevalent symptoms in acute mountain sickness. Notably, significant physiological changes were observed in these individuals upon transitioning from lowland to high-altitude conditions; oxygen saturation levels dropped from approximately 98% at sea level to 88% post-ascent, and hematological parameters such as red blood cell count, mean corpuscular volume, and hemoglobin concentrations, along with liver and kidney function markers like alanine transaminase and creatinine levels, all exhibited marked elevations. Through subgroup analyses comparing HAH patients with non-HAH participants, the research team substantiated that decreased oxygen saturation and altered heart rate serve as independent risk factors for the development of HAH. Moreover, they made the novel finding that elevated blood urea nitrogen (BUN) levels also constitute an independent risk factor for HAH. These discoveries contribute significantly to the understanding of the pathophysiological mechanisms underlying headache occurrence during acute high-altitude reactions and are pivotal for devising preventive and therapeutic strategies for high-altitude illnesses.
At the Institute of Psychology, Chinese Academy of Sciences, Liu Zhengkui’s team conducted a large-scale investigation into depression and its risk factors among the Tibetan population (Wang et al., 2019). The study involved 24,141 adult residents from Yushu Tibetan Autonomous Prefecture in Qinghai Province. Employing the Center for Epidemiologic Studies Depression Scale (CES-D), they discovered that the hypobaric hypoxic environment prevalent in high-altitude regions is intricately linked to individuals’ mental state, fatigue levels, and neural activity, thereby exacerbating negative emotions and contributing to significant mood disorders. Their findings revealed a striking prevalence rate of depression at 28.6% in high-altitude areas, with a positive correlation between depression incidence and altitude. Furthermore, gender, age, income level, educational attainment, and self-perception were also identified as relevant risk factors for depression.
Wang et al. (2023) from the Department of Psychiatry at the Medical College of the National Defense Medical Center explored the relationship between Acute Mountain Sickness (AMS) and the risk of psychiatric disorders in Taiwan. After conducting a 16-year follow-up study on 127 AMS patients and 1,270 controls, and analyzing the data using the Fine-Gray model, they found that AMS sufferers had a relatively higher likelihood of developing mental illnesses. Specifically, AMS was associated with increased risks of anxiety disorders, depression, bipolar disorder, sleep disorders, post-traumatic stress disorder/acute stress disorder, schizophrenia, and other substance-related disorders.
Ma et al. (2018) approached the issue from a spatial epidemiological perspective by presenting the first report on the spatial distribution and changes of congenital heart disease (CHD) among children living on the Qinghai-Tibet Plateau. They analyzed epidemiological data from 288,066 children aged 4–18 years old in this region, categorizing CHD prevalence according to sex, subtypes of CHD, ethnicity, and altitude. The study disclosed an overall CHD prevalence rate of 5.8% among children, with girls exhibiting significantly higher rates than boys; atrial septal defect was the most common subtype. Among different ethnic groups, Mongolians had the highest prevalence, followed by Tibetans, Han, and Hui, potentially reflecting distinct hypoxia adaptation genes or genetic characteristics within these populations. Notably, CHD prevalence escalated with increasing altitude, reaching its peak in Yushu Tibetan Autonomous Prefecture at 4,000 m above sea level. This research uncovered a pronounced spatial clustering pattern of CHD in the Qinghai-Tibet Plateau, emphasizing the substantial influence of environmental factors on CHD prevalence. It thus provides crucial reference information for formulating preventive strategies and reducing CHD incidence in high-altitude areas in clinical practice.
Researchers from the China Institute of Sport Science and the Tibet Institute of Sports Science, including Fan Chaoqun and Nie Mingjian among others, conducted a cross-sectional study on cardiorespiratory fitness (CRF) among adolescents living in high-altitude regions (Fan et al., 2021). They randomly sampled nearly 2,000 students aged between 9 and 18 years from a school located at an elevation of 3,500 m in Tibet, employing the 20-m shuttle run test (20mSRT) as a measure of aerobic endurance capacity. This assessment indirectly reflected cardiovascular function by quantifying completed laps and predicting peak oxygen uptake. The research findings indicated that adolescents residing in altitudes above 3,000 m displayed a generally lower CRF compared to their peers in lowland areas such as Shanghai and other international locations. While there was some improvement in physical performance with age, CRF exhibited a declining trend over time in this population. This phenomenon might be associated with increased academic pressure and reduced exercise time experienced by adolescents in the high-altitude environment.
2.2 International advances in cohort studiesDr. Charles B. Duke from the Department of Emergency Medicine at Yale School of Medicine led a team that conducted an observational cohort study aimed at investigating the association between hypertension and Acute Mountain Sickness (AMS) among trekkers in the Himalayas of Nepal. The study enrolled 672 participants who engaged in hiking activities at an altitude of 2,860 m, including 60 individuals with a history of hypertension. By recording Lake Louise Scores and monitoring blood pressure changes at altitudes of 2,860, 3,400, and 4,300 m, researchers primarily focused on AMS incidence rates. The results showed that AMS was prevalent among trekkers in Nepal, but no correlation was observed between blood pressure measured at 3,400 m and AMS incidence. Notably, a history of hypertension seemed to be related to a potentially lower risk of developing AMS (Duke et al., 2020).
Researchers at the Department of Medicine, Armed Forces Medical College in Pune, India, led by Dr. Virendra Narain, carried out a prospective longitudinal study to delve into the epidemiology and pathophysiology of thrombosis formation in lowland residents adapting to high-altitude environments (Nair et al., 2022). Initially, they screened 960 healthy male subjects at sea level, with 750 subsequently transported to regions above 15,000 feet (4,472 m). The study revealed that the high-altitude environment induced a prothrombotic state characterized by decreased natural anticoagulant activity, fibrinolysis suppression, endothelial cell activation, platelet activation, and elevated inflammatory markers. The findings demonstrated that, within this group of healthy subjects, the incidence rate of clinically significant thrombotic events (predominantly venous over arterial thromboses) was notably higher at elevations exceeding 15,000 feet (4,572 m) compared to reported values near sea level. Thus, it was concluded that altitudes above 15,000 feet (4,572 m) may serve as an independent risk factor for thrombosis formation, even among otherwise healthy individuals.
A research team led by Dr. Michel Claes, MD, from the Institute of Tropical Medicine (ITM), Belgium, conducted a prospective cohort study examining the incidence of severe altitude illness symptoms and their predictive factors among Kilimanjaro trekkers (Croughs et al., 2022). The study included 1,237 leisure hikers and 266 porters or guides. The data showed an 8.6% incidence rate of severe altitude illness symptoms among leisure hikers, whereas the proportion was only 1.5% for porters and guides. About 1.1% of leisure hikers required hospitalization due to Severe Acute Mountain Sickness (SAI). Factors such as a history of SAI, youth, failure to reach the summit, lack of clear ascent recommendations were found to be associated with a higher likelihood of experiencing severe altitude illness symptoms. In contrast, the absence of severe symptoms, the use of acetazolamide prophylaxis, ascending to higher altitudes during daylight hours, younger age, and longer climbing durations were all significantly correlated with successful summiting. The study emphasized the critical importance of avoiding further ascent when mild symptoms are present and descending immediately upon the onset of severe symptoms. Based on these findings, the study concluded that SAI incidence is alarmingly high among Kilimanjaro trekkers, emphasizing the need for travel health advisors to underscore the importance of not ascending until mild symptoms have subsided and the necessity for immediate descent when severe symptoms arise.
At the Department of Sports Science at the University of Innsbruck, Austria, Professor Martin Faulhaber and his team conducted a prospective cohort study to assess whether resting arterial oxygen saturation (SaO2) and respiratory rate could serve as effective predictors for the development of Acute Mountain Sickness (AMS) (Faulhaber et al., 2014). The research involved placing 55 subjects in an environment simulating an altitude of 4,500 m, achieved under normobaric hypoxia conditions with a fractional inspired oxygen concentration (FiO2) of 12.5%. After just 30 min of exposure to the low-oxygen environment, researchers measured cardiovascular and pulmonary function parameters, including oxygen saturation levels, blood lactate concentrations, and blood pressure in the participants. Subsequently, AMS symptoms were recorded using the Lake Louise Scoring System at intervals of 3, 6, 9, and 12 h post-exposure. Through multivariate logistic regression analysis, the study explored whether incorporating additional physiological measurements at rest could enhance predictive accuracy for AMS onset. The results demonstrated that, after only 30 min of hypoxic exposure, non-invasive measurement of resting SaO2 was both convenient and sufficiently sensitive to identify individuals susceptible to high-altitude reactions. Moreover, incorporating respiratory rate as a variable further improved the accuracy and predictive power for identifying those at risk of developing AMS. This study provides new practical tools and theoretical support for the prevention and management of acute mountain sickness.
At the Institute of Social and Preventive Medicine at the University of Zurich, Professor Susi Klimmer’s research team conducted a prospective cohort study aimed at investigating symptoms, incidence rates, and associated risk factors for Acute Mountain Sickness (AMS) among family members rapidly ascending to 3,450 m (Kriemler et al., 2014). The study included 87 children, 70 adolescents, and 155 parents, totalling 312 participants who were assessed using the Lake Louise Score (LLS) system for AMS symptom severity at two time points—8–10 h and 20–24 h post-ascent. They also monitored pain sensitivity and oxygen saturation (SO2), as well as analyzed clustering of AMS symptoms among family members. The findings revealed that on the first day following rapid ascent to 3,450 m, children had a lower prevalence of AMS compared to adolescents and adults; however, by the second day, this difference was no longer evident. This suggests that despite potential risk factors such as pain sensitivity and possible genetic influences, children can tolerate entering high-altitude regions like 3,500 m as safely as adolescents and adults, at least in the short term. This study provides valuable scientific insights into the differing abilities of people across different age groups to adapt to high-altitude environments. For details of the aforementioned studies, see Table 3.
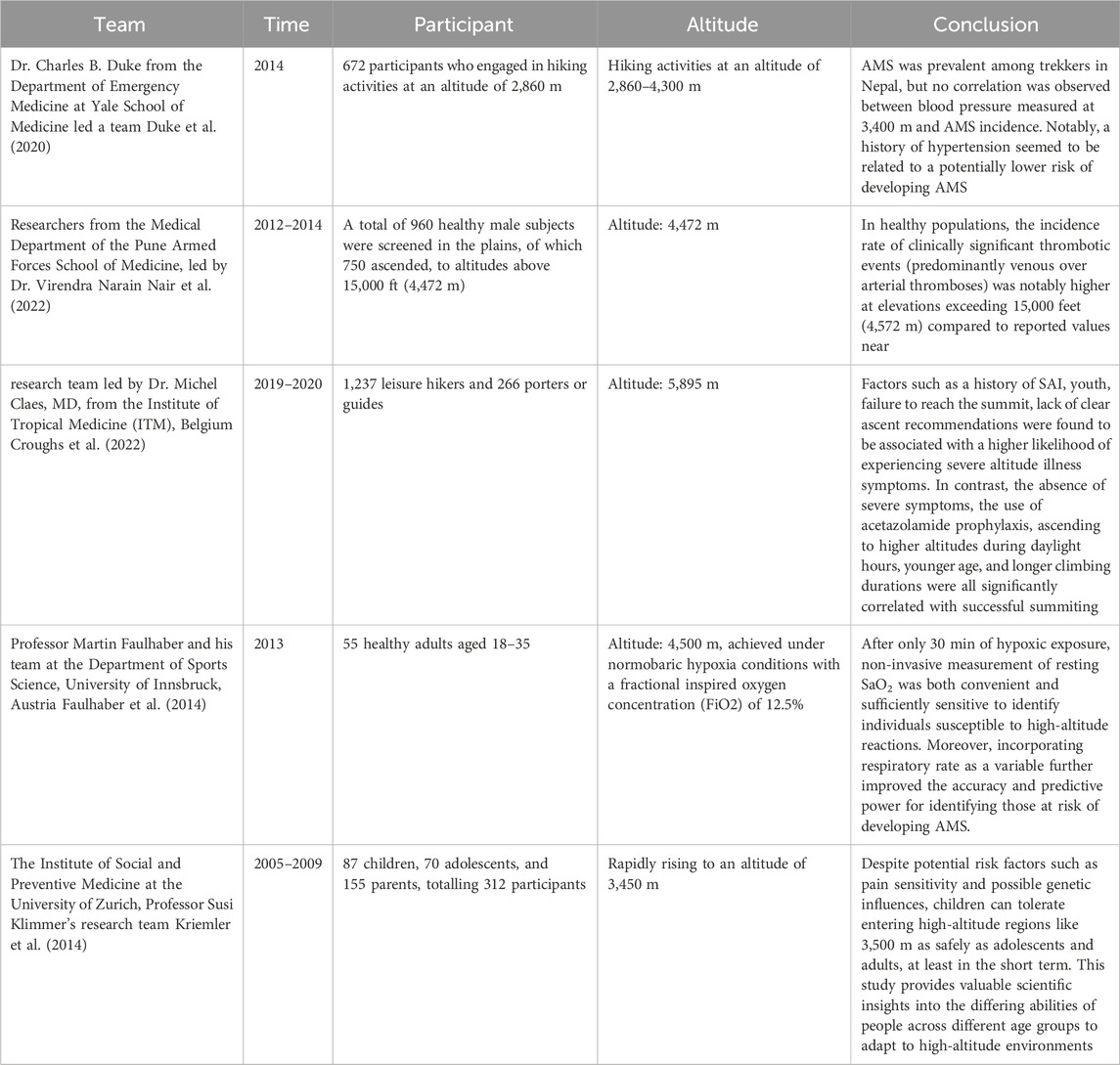
Table 3. International advances in cohort studies.
The incidence and influencing factors of high-altitude illnesses in populations have been subject to several pivotal breakthroughs through cohort studies both domestically and internationally. The research has primarily focused on three central domains:
(1) Identification of risk factors associated with the onset of high-altitude illnesses: Large-scale epidemiological surveys and in-depth data analyses have demonstrated significant associations between physiological parameters such as age, sex, body mass index, and pulmonary function, and an individual’s adaptability to high-altitude environments, as well as the incidence and severity of high-altitude illnesses.
(2) Exploration of the genetic underpinnings of high-altitude illnesses: Rigorous genetic research ha
留言 (0)