SARS-CoV-2 (severe acute respiratory syndrome-coronavirus-2), an RNA-containing virus belonging to the Coronaviridae family, is a causative agent of COVID-19 (from CoronaVirus Disease). COVID-19 has spread throughout the world and resulted in 775,431,269 total cumulative cases and 7,047,741 total cumulative deaths reported to the World Health Organization (World Health Organization, 2024), which are not equally distributed across various states. Based on those data, the United States, Brazil, India, Russia, Mexico, United Kingdom, Peru, Italy, Germany, and France had the highest mortality due to SARS-CoV-2 infection. According to World Health Organization data, in the US, 103,436,829 and 1,186,984 cumulative numbers of COVID-19 detected cases and deaths (5 May 2024) have been noted, respectively. In contrast, in some small and/or island areas (due to their minor population), particularly on the Pitcairn, Holy See and Tokelau islands, the total cumulative cases were 4, 26 and 80, respectively. The COVID-19 outbreak has rapidly accelerated to a global pandemic, with more than 83,832,334 SARS-CoV-2 infections and 1,824,590 deaths worldwide until the end of 2020 (The American Journal of Managed Care, 2021).
The COVID-19 pandemic has challenged the life of the world population. It started in Wuhan (China), where a growing number of pneumonia cases appeared in December 2019. The main symptoms were initially high fever, persistent cough, fatigue, headache, and muscle aches, indicating bioenergetic deficiencies; furthermore, COVID-19 worsened patient mental health and sleep quality (Huang and Zhao, 2020). From the beginning of the pandemic, various models for dynamic COVID-19 transmission were discussed, however, no evidence of animal-to-human transmission of the novel virus was proposed, leading to the hypothesis that the virus spread among humans occurred through an intermediate host (Shahid et al., 2020).
Due to the complexity of mitochondrial organization and functions, a significant influence of COVID-19 on mitochondrial biogenesis should be expected. In fact, mitochondria belong to the compartments most severely affected by SARS-CoV-2 infection. Based on the current research, there is a need for an updated review where such dependencies will be exhaustively summarized. Therefore, the objective of this review is to characterize complex relationships between SARS-CoV-2 and human mitochondria at tissue, cellular, and molecular levels. Multiple impairments in mitochondrial biogenesis are discussed within system- and tissue-specific contexts. In this review, severe alterations in mitochondrial metabolism and gene expression pattern are focused. Numerous activities and interactions of SARS-CoV-2 proteins with host mitochondrial proteins are also summarized. The presence of SARS-CoV-2 subgenomic RNA (sgRNA) within mitochondria, mitogenome alterations and mitochondrial motility and dynamics in COVID-19 are exhaustively discussed. Finally, current therapeutic approaches related with the usage of various compounds alleviating COVID-19 symptoms and maintaining mitochondrial hormesis are outlined.
2 SARS-CoV-2, its genome, key proteins, and replicationCoronaviridae family consists of two subfamilies (Orthocoronavirinae and Letovirinae), with 46 virus species in total. Furthermore, Letovirinae contains only α-letovirus, and four types of coronaviruses (α-, β- γ- and δ-) belong to the Orthocoronavirinae subfamily (Leao et al., 2022; Mavrodiev et al., 2022). While γ- and δ-coronaviruses infect mainly birds, but some of them also mammals, α- and β-coronaviruses infect only mammals (Wang et al., 2022). Until now, few coronaviruses that infect human cells (HCoV) have been identified, including HCoV-HKU1, HCoV-OC43, HCoV-229E, HCoV-NL63, SARS-CoV-1, SARS-CoV-2, and Middle East Respiratory Syndrome coronavirus (MERS-CoV). They cause severe respiratory diseases that can be potentially lethal or lead to a decreased quality of life in post-acute COVID-19 syndrome. At the early onset of the pandemic, various SARS-CoV-2 subtypes were characterized by both high contagiousness and virulence (Malik et al., 2022).
SARS-CoV-2 belongs to large β-coronaviruses, which contain loosely folded single-stranded positive-polarity RNA as genomic RNA (gRNA), and the protein envelope (Figure 1). Coronavirus virions range from 118 to 140 nm in diameter, and their genomes from 25 to 32 kbs in size (Payne, 2017). The SARS-CoV-2 genome forms typical secondary and tertiary structures, containing both long arms and loops. Notably various methods, including NaI/DMS crosslinking, allowed for advanced analyses of these structures (Yang et al., 2020).
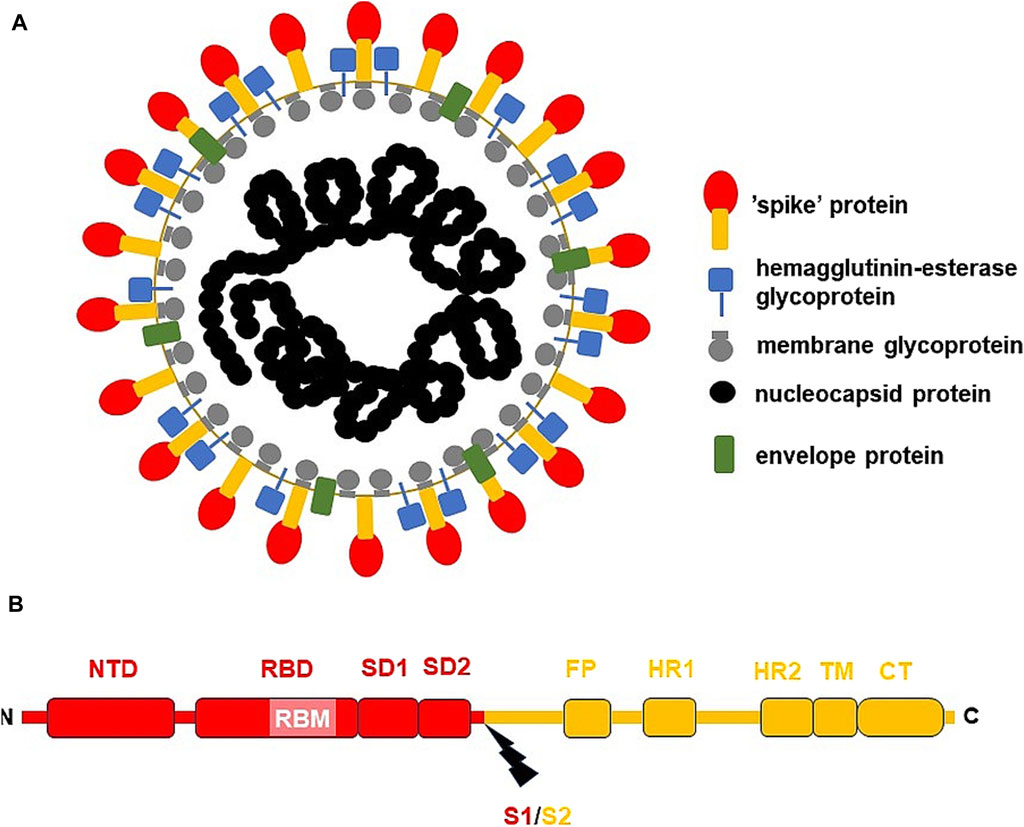
Figure 1. The general structure of SARS-CoV-2 virion and S-protein. (A) SARS-CoV-2 virion; (B) S-protein from USA/WA1 SARS-CoV-2 strain. The subunit S1 was depicted red, while subunit S2 (the membrane anchoring subunit) in orange. CT, cytoplasmic tail; FP, fusion peptide, HR1, heptad repeat 1; HR2, heptad repeat 2; NTD, N-terminal domain; RBD, receptor binding domain; RBM, receptor binding motif within RBD; S1/S2, the cleavage site (indicated by thunderbolt mark); SD1, subunit domain 1; SD2, subunit domain 2; TM, transmembrane domain.
SARS-CoV-2 virion contains an envelope composed of numerous proteins, some of which interact with the membrane of target cells (Woo et al., 2023). These proteins include “spike”- (S-), membrane- (M-) and envelope- (E−) proteins, as well as hemagglutinin esterase (HF) dimers, and the internal nucleocapsid- (N-) protein interacting with gRNA. SARS-CoV-2 genome contains multiple open reading frames (orfs) coding for both nonstructural (NSP1-NSP16), structural (e.g., E−, M-, S-, N-), and other proteins (Figures 1, 2). Among those orfs, the most prominent are orf1a and fused orf1a/1b encoding, respectively, pp1a and pp1b polyproteins for multiple non-structural proteins that form the replication-transcription complex. Products of expression of other orfs, for example, orf3a, orf6, orf7a, orf7b, orf8a, orf9b, orf9c and orf10 interact with a plethora of mitochondrial proteins (Figure 2). The main activities and interactions of all identified NSPs are shown in Table 1 and addressed in detail in Chapter 6.
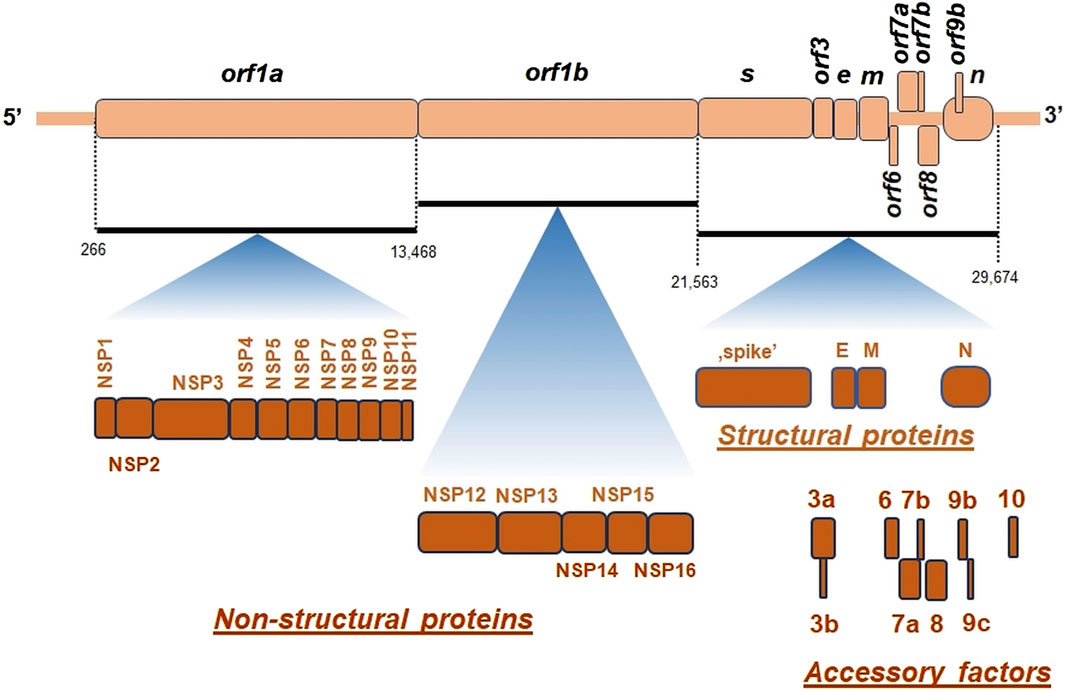
Figure 2. The general organization of SARS-CoV-2 genome. orf, open reading frame; e, gene encoding envelope protein; m, gene for membrane protein; n, gene encoding nucleocapsid protein; NSP, non-structural proteins; s, gene encoding “spike” protein. On genomic scheme, positions of coding sequences for small accessory proteins as well as for structural and non-structural proteins (below) were indicated. Key nucleotide coordinates (in nt) separating various orfs were also shown.
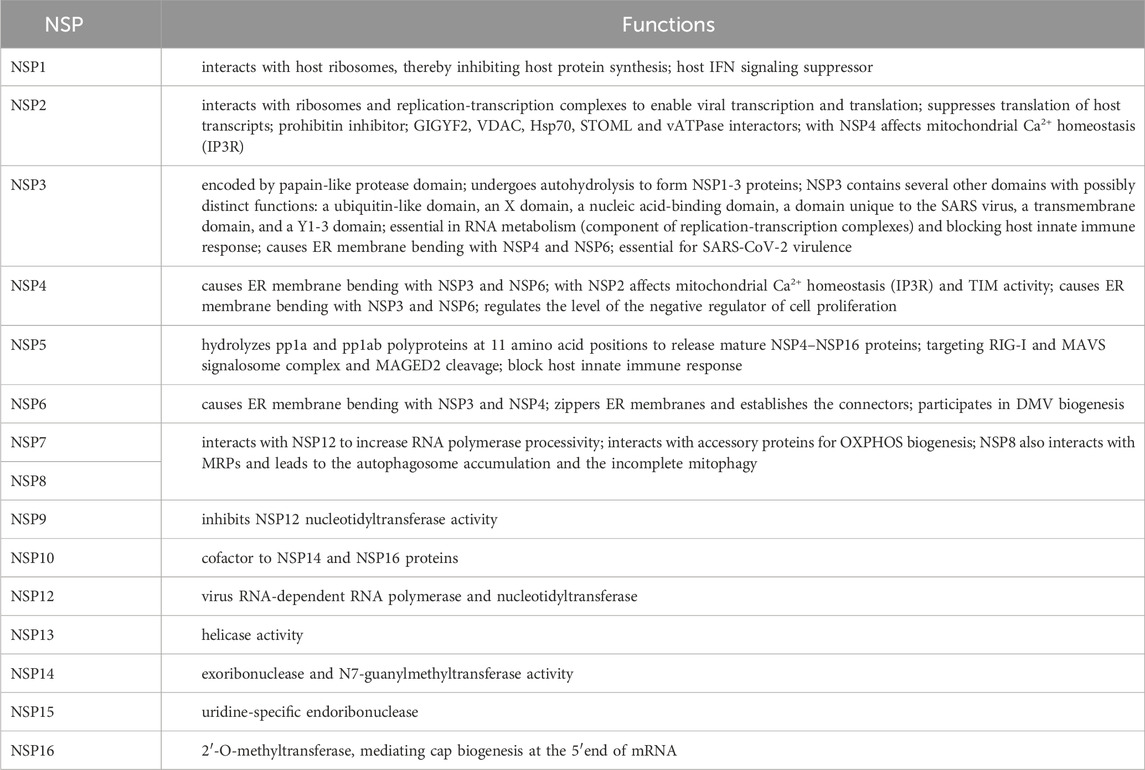
Table 1. Most important activities of SARS-CoV-2 non-structural proteins.
There are at least few additional open reading frames between the structural genes of the β-coronaviruses, encoding additional proteins (Hu et al., 2021). SARS-CoV-2 genome does not contain orf3b gene, which differentiates it from SARS-CoV-1 (Singh et al., 2020a). Interestingly, orf10 is also uniquely expressed in SARS-CoV-2 and encodes a protein that can suppress the interferon (IFN) response (Figure 2) (Li et al., 2022).
Once the virus gRNA enters the cell, orf1a and orf1b genes are immediately expressed and a replication-transcription complex is formed. Viral gRNA is released from endosomes to the cytoplasm, where the replication cycle starts (Shanmugaraj et al., 2020). gRNA synthesis occurs within DMVs and the vesicle-tubular network of coiled membranes (CMs), known as “virus replication organelles.” Replication-transcription complexes begin to produce gRNA progeny molecules and mRNA that encode several structural proteins. In general, the expression of the SARS-CoV-2 proteins in infected cells depends mainly on the translation of gRNA and sgRNA. Coronaviruses (including SARS-CoV-2) use non-canonical translation mechanisms to enhance their coding capacity and adjust the expression levels of individual viral proteins. For example, translation of pp1ab depends on the presence of the programmed ribosomal frame shift (PRF) at position −1 upstream of orf1a termination codon, thus extending the pp1a open reading frame pp1a from orf1b. As a result, up to 2-times overproduction of the protein encoded by orf1a relative to orf1b was detected (Malone et al., 2022). Thus, viral RNA enables rapid translation of selected reading frames, while newly synthesized NSPs regulate replication and transcription of the SARS-CoV-2 genome. All translated proteins and synthesized genomic copies of the coronavirus are transported to the endoplasmic reticulum (ER) and the Golgi apparatus (GA), and new virions are self-assembled, allowing them to escape the cell through exocytosis (V’kovski et al., 2021).
3 General relationships between SARS-CoV-2 and mitochondria from cellular perspectiveThe current research showed a wide variety of mitochondrial activities that are severely affected by SARS-CoV-2 infection. Particularly, those findings have firmly established the key role of mitochondria in COVID-19 pathogenesis due to the characterization of (1) subtle interactions between these organelles, the immune system, and mitochondrial damage-related molecular patterns, (2) alterations in mitochondrial functionality and motility, (3) multiple interactions between SARS-CoV-2 proteins and the host mitochondrial proteome, and (4) participation of mitochondria in cellular signaling severely affected by SARS-CoV-2 (Shoraka et al., 2023b). Mitochondrial-mediated antiviral immunity represents the first line of patient defense and mitochondria-related gene expression, mitochondrial functions, and related metabolic pathways are altered in patients with COVID-19 (Duan et al., 2022). Furthermore, accelerated by senescence multiple mitochondrial dysfunctions, including ROS increase, oxidative damage, increased mitogenome mutation rate, OXPHOS deregulation, as well as decrease in ATP production, coenzyme Q (CoQ) level, and failure in antioxidative protection mechanisms, contribute to factors that affect the severity of COVID-19 and lead to increased mortality. The other well-known factors cover age, immunosuppression, hypertension, diabetes, as well as population factors, related to its high density, and anatomical and transmissibility factors (Moreno Fernández-Ayala et al., 2020; Shenoy, 2020; Ganji and Reddy, 2021).
ER membranes were initially shown to be the main centers for the formation of membrane vesicles containing SARS-CoV-2 particles. However, by analogy with other double membrane vesicle (DMV)-inducing viruses, it became evident that mitochondria and various lipid droplets also participate in the biogenesis of SARS-CoV-2 (Roingeard et al., 2022). Some heat shock proteins associated with ER (for example, HSPA5) in ER stress caused by SARS-CoV-2 infection, can move to the cell surface as well as to mitochondrial and nuclear membranes. HSPA5 protein participates in signaling pathways that alleviate SARS-CoV-2 entry and also plays a critical role in the SARS-CoV-2 invasion among cancer patients (Li et al., 2023b). Furthermore, mitochondrial dysfunctions in human diseases, for example, mitochondrial myopathies and neuropathies, ethylmalonic aciduria, Friedreich ataxia, hereditary spastic paraplegia 7, Wilson disease, ageing, cancer, and lung fibrosis, can also affect symptoms and mortality of COVID-19, which also indicates the important role of mitochondria in the development of COVID-19 (Sharma and Sarode, 2022).
From the beginning of the COVID-19 pandemic, mitochondria were suggested to play a critical role in the endocytic pathway that allows SARS-CoV-2 replication and survival in cells, because many viruses have evolved to hijack immunometabolic and mitochondrial functions, resulting in bioenergetic cell deficiencies, dysfunctional mitophagy, Ca2+ and ROS imbalance, and other consequences, including preference for aerobic glycolysis to favor SARS-CoV-2 replication (Chapter 5; Nunn et al., 2020; Wang et al., 2020). Mitochondrial response of cell cultures related with the activation of the innate immune and alterations in the protein quality control differs between various coronaviruses (Kohli et al., 2022; Nunn et al., 2022). It was also proposed that mitochondria-endosome interactions are critical for lysosome degradation functions and that mitochondrion may play an important role in SARS-CoV-2 replication (Chapter 8; Swain et al., 2021).
Reactivation of some viruses (e.g., Epstein-Barr [EB] virus) is also associated with severely hijacked mitochondrial functions, such as “long COVID.” “Long COVID” represents post-acute sequelae of COVID-19 (PASC) that can affect at least half of COVID survivors in half of the year after recovery. It is characterized by continuous pulmonary and neurologic symptoms, mental health and mobility impairments, difficulty concentrating, generalized anxiety disorders, fatigue, and muscle weakness, and some multisystemic disorders (Groff et al., 2021). EB virus becomes dormant in a large percentage of the population and was shown to induce the expression of the ACE2 receptor and the entry of SARS-CoV-2 into epithelial cells (Verma et al., 2021). Therefore, it was speculated that EB virus may co-invade COVID-19 patients coincidentally or be truly reactivated due to the impaired immunity of the patient. The association of EB virus infection with mitochondrial aberrations and post-infective fatigue (similar to PASC symptoms), reported by Vernon et al. (2006), could play a key role in the susceptibility and recovery of COVID. This would affect mitochondria in a similar way to SARS-CoV-2 infection; therefore, care should be taken when discussing cellular effects in coronavirus because they can interfere with the effects of EB infection. Among patients mildly suffering from COVID-19, reactivation of the EB virus could further enhance a longer-term secondary syndrome if mitochondrial aberrations are still present (Nunn et al., 2021), indicating the importance of the current physiological status of mitochondria in this process.
Interactions of viral proteins with mitochondria accelerate the overreaction of immunity resulting in permanently increased inflammatory processes, which is one of the ‘long COVID’ features (Yokota et al., 2021; Chen et al., 2023a). One of the more fascinating factors that may affect such a relationship are prion diseases. Stefano et al. (2023a) reported a quite surprising link between the pathophysiological sequelae of “long COVID” and the pathogenesis of prion diseases. Authors proposed a mechanism in which SARS-CoV-2 targets mitochondria and promotes their dysfunction, speculating that ROS burst under SARS-CoV-2 infection can lead to misfolding of prion proteins that can be further accelerated, thus increasing neurodegenerative symptoms in selected tissues, and leading to “long COVID.” In this model “long COVID” with multiple behavioral changes resulting from neuron alterations and neurodegeneration, may arise from prion mobilization and/or SARS-CoV-2 infection. Neurodegeneration that comes from prion misfolding may therefore mask the “true” effects of SARS-CoV-2. According to Stefano et al. (2023a), the pathophysiological effects of “long COVID” may involve the induction of spontaneous production of infectious prion species, especially in individuals susceptible to its origin. The authors concluded that such prion mobilization may explain some “long COVID” symptoms. Recently, aberrant mitochondrial quality control (including dysfunctional mitophagy) has been suggested to contribute to the pathogenesis of numerous diseases, including prion and scrapie infections, that are associated with the an increased pool of intramitochondrial ROS and decreased mitochondrial membrane potential (Kim et al., 2022). However, the unexpected link between prion action, SARS-CoV-2 infection, “long COVID,” and mitochondrial biogenesis needs to be further explored.
In COVID-19, very devastative inflammatory responses accompanied by an increase in the level of pro-inflammatory serum cytokines and chemokines, as well as an increase in neutrophil and macrophage count and the decrease in the endogenous antiviral response from CD8+ T cells, NK cells, and gammaDelta T cells (Jaiswal et al., 2020; Potter et al., 2020). Hyperinflammation is induced by the NLR3 pathway by N-protein of SARS-CoV-2, which promotes interactions between NLRP3 and ASC proteins and further results in the IL-1β and IL-6 activation (Pan et al., 2021). Activation of the NLRP3 inflammasome, which contributes to the severity of COVID-19 was studied in in vitro infected macrophages and monocytes, in mouse models, and in lung cells with severe COVID-19 (Dutta et al., 2022). Recently, it was suggested that SARS-CoV-2 employs non-canonical activation of NLRP3 mediated by caspase 4/11 (Rodrigues and Zamboni, 2023).
A controlled ROS pool in mitochondria is needed to maintain the continuity of multiple processes and signaling cascades. However, an elevated level of ROS leads to chronic oxidative stress, which can cause cell damage (Giorgi et al., 2018). ROS of mitochondrial origin are important modulators of the inflammatory response in numerous cells, including epithelial cells, and the crosstalk between epithelial and endothelial cells specifically modulates alveolar-capillary injury under COVID-19 (Wang et al., 2020). As chronic state may be initiated by SARS-CoV-2 infection of the epithelium, Chang et al. (2021) proposed that SARS-CoV-2 activates ROS-mediated feedback loops that cause permanent alterations in epithelial redox status and functions, promoting cardiovascular disease and lung injury after COVID-19 recovery. Inflammatory responses lead to the rare event known as the cytokine release syndrome or “cytokine storm,” resulting not only in abnormal coagulation, excessive oxidation, organ damage, immune deficiencies, intravascular coagulation, and organ failure, but also in numerous mitochondrial dysfunctions. Interestingly, pro-inflammatory cytokines induce the activity of indoleamine 2,3-dioxygenase (IDO), which produces kynurenine activating the aryl hydrocarbon receptor (AhR) that modulates mitochondrial metabolism (Anderson et al., 2020). However, its contributions to the COVID-19 pathogenesis still need more studies. In general, the initial phase of pro-inflammatory cytokines inhibits the endogenous antiviral response (Anderson et al., 2020). Cell inflammasome is severely activated by SARS-CoV-2 and mitochondrial dysfunctions accompany chronic inflammation, the “cytokine storm,” inhibited IFN-I release, and the affected immunological responses (Moreno Fernández-Ayala et al., 2020). One of the gatekeepers that regulates mitochondrial-inflammasome interdependencies is the mitochondrial protein NLRX1, a member of the NOD-like receptor family and a potent pattern recognition receptor; it also affects the inflammatory response in COVID-19 (Pickering and Booty, 2021).
The enhanced mitochondrial senescence is accompanied by reprogramming of the immune system, increased oxidative stress, increased mutation ratio in the mitogenome, and increased deregulation of OXPHOS, as well as with a substantial decrease in the pool of naturally occurring antioxidant compounds, as well as CoQ10 and ATP levels. The ultrastructure of aged mitochondria and mitochondria under SARS-CoV-2 infection is also severely affected, e.g., in lung cells and retina; these deficiencies are accelerated by COVID-19, by the appearance of swollen mitochondria, in some cases containing numerous lipid droplets in matrix (Chow et al., 2021; Nardacci et al., 2021; Araujo-Silva et al., 2023). In general, mitochondrial senescence contributes to the malignant prognosis of COVID-19 and to the continuous activation of the inflammasome.
4 Aberrations across systems and tissues in COVID-19, and their relevance to mitochondria“Life cycle” of SARS-CoV-2 can lead to a higher viral load in certain tissues and cell lines. COVID-19 was believed to be primarily associated with serious aberrations in the respiratory system; however, over time, multiple evidence of the devastating role of SARS-CoV-2 in other systems, including the central nervous system was collected (Stefano et al., 2021a; Stefano et al., 2021b; Denaro et al., 2022).
Tissues with slow blood circulation and neurons that require oxygen for OXPHOS activity and ATP synthesis seem to be ideal targets for coronavirus infection, allowing for the high level of replication of SARS-CoV-2, for example, in some brain tissues that exhibit slightly hypoxic conditions. The energy metabolism of neurons may become compromised under SARS-CoV-2 infection, where cells with high oxygen demand become dysfunctional. It was proposed that targeting of neuronal mitochondria by SARS-CoV-2 is selective, induces a “brain fog,” and results in behavioral changes (Stefano et al., 2021b).
The general mechanism of neuropsychiatric manifestations in COVID-19 is very complex; mitochondrial aberrations, leading to systemic downregulation of metabolic activity and cellular bioenergetics within central nervous system cells, were recently investigated (Stefano et al., 2021b; Stefano et al., 2022). ACE2 receptors are also present in neuronal and glial and epithelial cell plasma membranes. The α-cobratoxin domain of S-protein allows interaction with α7 nicotinic acetylcholine receptors (Quiles et al., 2020; Valdés-Aguayo et al., 2021a), however this domain prevents mitochondrial-driven apoptosis if the SARS-CoV-2 replication cycle has not yet finished (Kalashnyk et al., 2021). In dopaminergic brain neurons, ACE2 activity releases angiotensin heptapeptide (Ang-1-7) and alamandine at high levels. Both products bind to the Mas-related gene receptor (Mrg) at the outer mitochondrial membrane (OMM) that is responsible for NO synthesis. Both the abundances of ACE2 and MrgE decrease with age. As ACE2 is bound by S-protein of SARS-CoV-2, ACE2/MrgE/NO signaling pathway that releases NO interferes with coronavirus infection (Valenzuela et al., 2021).
The SARS-CoV-2 sZeta and sGamma variants differentially altered the expression profiles of inflammatory protein genes in rat hippocampus cells exposed to sera containing these variants; moreover, a significant decrease in the expression of mitochondrial biogenesis genes was observed in hippocampi treated with serum samples derived from patients infected with sZeta and sGamma and from individuals with post-COVID syndrome, while a reduction in mitochondrial dynamics was only observed in the latter case (Montenegro et al., 2023).
The effects of coronavirus infection on the nervous system could be seen not only in neurons, but also in the endothelium and microglial cells, and microglia link SARS-CoV-2 infection especially notable with mitochondrial biogenesis, “long COVID” events and chronic inflammation (Stefano et al., 2021a). On the other hand, aberrations of cerebral endothelium respiration are related to mitochondrial damage induced by S1- and Trimer proteins (Kim et al., 2021). When microglial cells were treated with S-protein or inactivated SARS-CoV-2 virions, specific alterations in mitochondrial biogenesis occurred through increased ROS levels, leading to a decrease in mitogenome copies and increased phospholipid saturation (Pliss et al., 2022). Those harmful responses within the central nervous system can be further exacerbated among patients suffering from additional diseases, for example, neurodegenerative ones (Denaro et al., 2022). Since multiple neurological disorders are associated with COVID-19 pathogenesis, damaged mitochondria (with severely affected functionality) and migrasomes (migration-dependent membrane-bound vesicles containing cell content) are present in brain cells infected with SARS-CoV-2; the oxidative stress in brain cells can affect innate immunity leading to impaired cognitive ability (Denaro et al., 2022; Lin et al., 2022; Zhang et al., 2022; Büttiker et al., 2023). The interactome analyses employing viral and neural mitochondrial proteins support these relationships (Maurya et al., 2022). Autopsies from deceased patients indicated a high viral load in the central nervous system, particularly in the brain and Swain et al. (2021) proposed a mechanism of mitochondrial and mTOR dysfunction in infected neurons, where a high level of ROS/Fe accelerates inflammasome hyperactivation and leads to the increased apoptosis. Peluso et al. (2022) proposed that the abundance of SARS-CoV-2 N- and S1-proteins in plasma neuron-derived and astrocyte-derived extracellular vesicles could serve as long-prognostic biomarkers. In particular, SARS-CoV-2 penetrates the nervous system, not only the brain, but also invades enteric neurons and the spinal cord, resulting in cell damage due to inflammation, oxidative stress, mitochondrion dysfunction, and poorly controlled proteostasis (Kaundal et al., 2021).
SARS-CoV-2 infection is related to brain-blood barrier dysfunctions caused by “cytokine storm” and hyperactive immunity, and it also initiates thrombosis in the bloodstream (by activating specific prothrombotic factors). Furthermore, the activity of the brain-blood barrier can be altered among patients with neurodegenerative disorders suffering from SARS-CoV-2 infection, while the physiological state of mitochondria is also negatively altered (Kaundal et al., 2021; Denaro et al., 2022). Recent reports also link the ageing of the blood-brain barrier with increased susceptibility to SARS-CoV-2 infection, which also employ mitochondrial functions (Adesse et al., 2022; Denaro et al., 2022).
Mitochondrial dysfunctions due to SARS-CoV-2 infection alter the functioning of other tissues and organs. Bartolomeo et al. (2022) indicated a preferentially increased susceptibility to SARS-CoV-2 infection in liver, intestine, and breast-derived cell lines, which was connected not only with aberrations in the numerous OXPHOS protein level (including ATP synthase, COX and NDUFS2 subunits), but also with the higher expression level of the TMPRSS2 protein. As mitochondria dominate in hepatocyte metabolism, COVID-induced liver injury (either due to cytopathic effects or due to “the inflammatory burst”) was suggested to be a consequence of mitochondrial dysfunctions in hepatocytes (Morio et al., 2021; Akbari and Taghizadeh-Hesary, 2023). SARS-CoV-2 seriously affects hepatocyte mitochondria, using them for replication and leading to decreased OXPHOS activity (Akbari and Taghizadeh-Hesary, 2023). Multi-organ damage during COVID-19 also affects the kidneys due to acute kidney injury. Mechanisms of kidney injury in COVID-19 comprise systemic immune and inflammatory responses induced by viral infection, systemic hypoxia, reduced renal perfusion, endothelial damage and direct epithelial infections, and mitochondria are key organelles that contribute to the deregulation of the anti-inflammatory response in kidney cells under SARS-CoV-2 infection, where increased glycolysis rate and the reduced oxidation of fatty acids occurred (Kellum et al., 2020).
Placenta belongs to other organs seriously affected by SARS-CoV-2. Mandò et al. (2021) reported multiple aberrations in placenta cells driven by SARS-CoV-2 presence which covered various alterations in the mitochondrial biogenesis including decrease in the mitogenome copy number and the antioxidant protein level. The downregulation of the OXPHOS protein genes (e.g., NDUFA9, SDHA, COX4I1) and the motility genes (e.g., DNM1L, FIS1) was also notable. These effects were attributed to the worsening intrauterine environment, leading to the deepening of placental oxidative stress and the decrease in mitochondrial performance. Additionally, Liu et al. (2022) noted a general increase in abnormal mitochondria and an elevated mitogenome content in placentas of mothers infected with SARS-CoV-2, which was speculated to be associated with transient early fine motor abnormalities among newborns. Gabanella et al. (2022) also studied the effects of SARS-CoV-2 in trophoblasts and found the presence of the coronavirus genome within host mitochondria, which was associated with global reprogramming of mitochondrial net and mitochondrial activities. Furthermore, Sun et al. (2022) speculated that SARS-CoV-2 may possibly also affect female fertility by hijacking mitochondrial activity.
5 SARS-CoV-2 sensing through mitochondria- from MAVS to respiratory aberrations, OXPHOS deficiencies and perturbations in iron homeostasisThe presence of SARS-CoV-2 RNA is detected by the RIG-I receptor (retinoic acid-inducible gene I) receptor, which activates MAVS (mitochondrial antiviral signaling protein), a part of the mitochondrial signalosome complex located in OMM (Figure 3). MAVS affects the level of antiviral IFN that regulates viral replication by interaction with a viral inhibitor (viperine; Kloc et al., 2020). Recently, numerous SARS-CoV-2 proteins have been shown to facilitate MAVS degradation, e.g., ORF10. Under overexpression, it participates in ORF10-induced autophagy that includes the degradation of the mitochondrial signalosome. ORF10 can also induce mitophagy through interaction with the Nip3-like protein X (NIX) mitophagy receptor, which can also subject MAVS to degradation. Through these activities, ORF10 facilitates SARS-CoV-2 replication (Li et al., 2022).
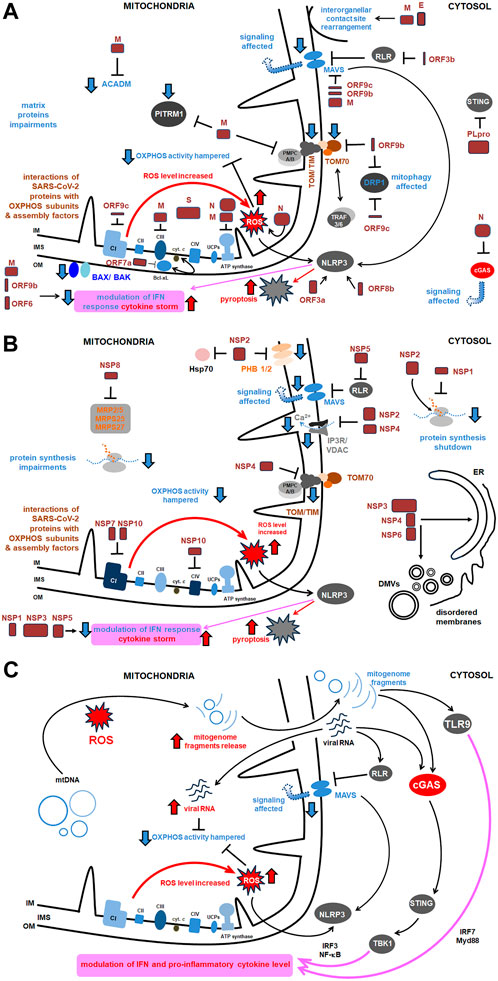
Figure 3. The comprehensive scheme of interactions of SARS-CoV-2 with host mitochondrial proteins and diverse mitochondrial activities. Key proteins as well as the most relevant pathways affected under SARS-CoV-2 infection were shown. More details in the text. (A) Main interactions between structural proteins of SARS-CoV-2 and host mitochondrial proteins and activities; (B) Main interactions between virus NSPs and mitochondrial proteins and activities (the remaining ones are presented in Table 1); (C) Main signaling routes for SARS-CoV-2 RNA and the released mitogenome fragments in the host cell. ACADM, acyl CoA dehydrogenase medium chain; Bax/Bak, Bcl 2-like protein 4/BCL2 antagonist/killer 1; Bcl xL, B cell lymphoma extra large; CI, CIV, respiratory chain complexes; cGAS, cyclic GMP–AMP synthase; COX, cytochrome c oxidase; cyt., cytochrome; DMVs, double membrane vesicles; DRP, dynamin related protein; E, SARS-CoV-2 envelope protein; Hsp, heat shock protein; IFN, interferon; IM, inner membrane; IMS, intermembrane space; IP3R, inositol triphosphate receptor; IRF, interferon release factor; MAVS, mitochondrial antiviral signaling; MRP, multidrug response protein; MTP, mitochondrial peptidase; Myd, myeloid differentiation primary response; N, nucleocapsid protein; NF-κB, nuclear factor kappa-light- chain-enhancer of activated B cells; NLRP, nucleotide-binding oligomerization domain; leucine rich repeat and pyrin domain containing; NSP, non-structural protein; OM, outer membrane; ORF, open reading frame; OXPHOS, oxidative phosphorylation; PITRM1, pitrilysin metallopeptidase; PHB, prohibitin; PLpro, polyprotein; RLR, RIG-I-like receptor; ROS, reactive oxygen species; S, ‘spike’ protein; STING, stimulator of interferon genes; TBK, TANK binding kinase; TIM, translocase of the inner membrane; TLR, Toll-like receptor; TOM, translocase of the outer membrane; TRAF, tumor necrosis factor receptor–associated factor; UCP, uncoupling protein; VDAC, voltage dependent anion channel.
SARS-CoV-2 infection affects the expression of several cellular genes. Perturbations in mitochondrial activity, inflammation, and autophagy pathways were specifically observed for SARS-CoV-2 infected cells; they were associated with downregulation of mechanistic target of rapamycin (mTOR) components, as well as mitochondrial ribosomal and mitochondrial complex I (CI) subunits, and proteins for lysosome acidification (Singh et al., 2020b). Using single cell RNA-seq data from human bronchial epithelial cells, colon and ileum organoids, Gutiérrez and Elena (2022) showed distinctness of expression profiles for a variety of mitochondrial genes under SARS-CoV-2 infection. SARS-CoV-2 affects mitochondrial respiratory metabolism, switching from OXPHOS activity to glycolysis, and, in general, it indirectly alters the immune system response (Kloc et al., 2020). SARS-CoV-2 leads to downregulation of numerous nuclear OXPHOS genes, especially those coding CI subunits (Figure 3). SARS-CoV-2 can affect the expression level of mitochondrial protein genes as early as 2 h after infection (Miller et al., 2021; Archer et al., 2022). As most of the mitochondrial proteome is encoded by nuclear genes, they are particularly contributing to the pathogenesis of COVID-19.
Carotid bodies allow for oxygen detection and proper chemoreflex, and decreased OXPHOS and MAVS activities, together with ROS burst, severely alter their functions, leading to hypoxemia. However, aberrations in mitochondrial biogenesis affect damage in the respiratory system, where not only oxygen sensing, but also pulmonary artery constriction is impaired and vascular damage progresses (Ackermann et al., 2020; Archer et al., 2020; Gattinoni et al., 2020). These responses are accompanied by a decrease in the IF level and an increase in the level of oxidized biomolecules and a severe inflammation (Burtscher et al., 2020). In the human nasal epithelium, Yeung-Luk et al. (2023) investigated tissue remodeling, which includes relatively early aberrations in mitochondrial activity, decreases in oxygen consumption and extracellular pH, and increases in actin polymerization that led to general remodeling of the cytoskeleton.
Mitochondrial dysfunctions were also studied in peripheral blood monocytes from COVID-19 patients. ATP synthesis, respiratory rate in state 3, basal respiration rate, and maximal respiratory capacity in these cells were all decreased (Ajaz et al., 2020; Gibellini et al., 2020). Additionally, SARS-CoV-2 led to the accumulation of advanced glycation end products. Interestingly, the proton leak across the mitochondrial membrane was also less effective. Gibellini et al. (2020) also reported the subsequent increase in tumor necrosis factor (TNF) and IFN synthesis, and the increase in mitochondrial size under COVID-19 progression, which appeared slightly less round and larger. According to Gibellini et al. (2020) results, mitochondria of monocytes from COVID-19 patients were heterogenous in size, swelled, and with electron-lucent matrix. They had increased both area, as well as external perimeter and Feret’s diameter by 4.3, 1.2 and 1.2 times, respectively. The roundness of these mitochondria significantly decreased, however, the aspect ratio increased almost by 1.1 times. SARS-CoV-2 infection of the lung epithelial cell line increased pyruvate kinase muscle isoform 2 (PKM2); however, the PKM2-specific stabilizer restored glycolytic aberrations (Allen et al., 2022). HIF-1α (hypoxia inducible factor 1α) together with the ORF3a protein, plays an important role during SARS-CoV-2 infection and the development of the pro-inflammatory response (Figure 3) (Tian et al., 2021) and induce the expression of glycolytic genes. Finally, the energetic deficit and the glycolytic switch increase the pro-inflammatory response, decrease the level of IFN and the rate of lymphocyte T proliferation, and severe COVID-19 symptoms; the higher glucose level favors SARS-CoV-2 infection with vast glycolysis (Codo et al., 2020). The level of interleukin-6 (IL-6) increased in blood mononuclear cells among both SARS-CoV-2 infected and deceased patients (Ajaz et al., 2020). Among severely affected COVID-19 patients Romão et al. (2022) noted that the elevated SARS-CoV-2 load induced an increase in mitochondrial ROS level associated with the altered pattern of CD14+ monocytes, with decreased mitochondrial membrane polarization.
In general, the studies discussed above support the relevance of monocytes in the pathogenesis of COVID-19. Generally, in leukocytes, under SARS-CoV-2 infection, inflammation and oxidative damage are associated with affected mitochondrial functions and increased probability of apoptosis; these replies explain the rapid changes, that overactivated the immune system (De la Cruz-Enríquez et al., 2021). Regarding other blood elements, human platelets (often enlarged) in COVID-19 patients contain hyperpolarized mitochondria and significantly reduced intracellular Ca2+ pool, however expression profiles of OXPHOS genes are not affected, despite permanent inflammation (Yasseen et al., 2022).
The level of OXPHOS proteins in neural cells is also markedly reduced in some cases of COVID-19. Peluso et al. (2022) noted that the lower abundance of CI subunit 6, CIII subunit 10, VDAC1 and the neuroprotective humanin was associated with PASC with neuropsychiatric manifestations. Emerging evidence is collected to support the idea that disruption of NAD+ metabolism and subsequent mitochondrial dysfunctions accompanying the integration of the SARS-CoV-2 genome may, in fact, contribute to the pathogenesis of post-acute sequelae of SARS-CoV-2 (Block and Kuo, 2022).
Mitochondria also belong to key organelles involved in cellular iron (Fe) turnover. This element is present within these organelles among the well-known hem residues, Fe-S clusters, and Fe-binding proteins, including ferritin. In severe COVID-19, an increased iron level was detected in hepatocytes among patients with liver injury during SARS-COV-2 infection; therefore, it was suggested that hepatic iron overload could enhance liver injury associated with COVID-19 (Del Nonno et al., 2021). The increased level of free iron within mitochondria is a consequence of pro-inflammatory processes, and its overaccumulation promotes further oxidative stress, leading to lipid peroxidation and decreased glucose tolerance. Mitochondria are sensitive to oxidative stress under elevated iron level, which is often a consequence of high ferritin level (hyperferritinemia) and is associated with severe outcomes of COVID-19 (Saleh et al., 2020; Mahroum et al., 2022). Deepened oxidative stress leads to ferroptosis, mitochondrial dysfunctions, and finally organ damage. Interestingly, some microbiota and platelet dysfunctions (with depolarized mitochondria and other organellar aberrations) could be enhanced by mitochondrial dysfunctions that also progress them, leading to hypercoagulopathy, reported during COVID-19 progression. Surendran et al. (2022) observed spatiotemporal deregulation of the mitochondrial isoform of heme oxygenase 1 (HMOX1), proving the relevance of Fe metabolism in the pathogenesis of COVID-19. Lipid peroxidation, resulting from the iron release from Fe-storing proteins, is induced by tissue acidosis and intramitochondrial ROS burst in COVID-19 and leads to further mitochondrial damage. Jovandaric et al. (2022), based on recent reports, speculated that such mitochondrial aberrations could result in increased mortality of newborns and pregnant women under SARS-CoV-2 infection. Furthermore, in COVID-19, in addition to the increase in intramitochondrial iron level, the distribution of Mn is also altered, which affects Mn-superoxide dismutase (Mn-SOD) activity (Denorme et al., 2020; Edeas et al., 2020; Saleh et al., 2020).
6 Complexity of SARS-CoV-2 protein interactions and activityFlynn et al. (2021) have analyzed the SARS-CoV-2 interactome, which consists of multiple cellular proteins. The most abundant families comprised RNA-binding proteins, heterogenous ribonucleoprotein particles (hnRNPs), the translation apparatus, metabolic enzymes, cytoskeleton components, and intracellular vesicles protein components. However, most SARS-CoV-2 proteins also interact with host mitochondrial proteins (Figure 3). The interaction of SARS-CoV-2 with mitochondria allows the initial opening of the mitochondrial permeability transition pore, mitochondrial membrane depolarization, and the ROS release (Shang et al., 2022). Some of the mentioned below proteins affect mitochondria, including antiviral signaling, mitochondrial motility, ion homeostasis, as well as expression profiles of genes for various mitochondrial proteins; all that illustrates complexity of their action.
One of the best-known interactions between coronavirus and host cell is driven by S-protein. It is responsible for viral transmission and pathogenesis and modulates the host immune response. S-protein forms a complex composed of two proteins of (S1/S2)3 stoichiometry, with the surface subunit S1 and the S2 protein interacting directly with the membrane. S-protein precursor is hydrolyzed by a furin-like protease, resulting in the formation of mature S1 and S2 subunits. The S1 subunit contains an N-terminal domain (NTD) and a receptor binding domain (RBD). In contrast, the S2 subunit has a fusion peptide (FP), heptad repeat 1 (HR1), heptad repeat 2 (HR2), transmembrane domain (TM), as well as a cytoplasmic tail at the C end (CT) (Figure 1) (Ghimire et al., 2022). S-protein binds the opportunistic angiotensin-converting enzyme-2 receptor (ACE-2) and the transmembrane serine protease (TMPRSS2) to the cell plasma membrane. This interaction is followed by internalization of S1 RBD and the formation of active trimer of S-protein. Endosomal vesicles are generated by membrane fusion, which allows viral entry by endocytosis (Kim et al., 2021). However, an alternative pathway for virus penetration was proposed. For example, ACE2/“spike”-independent infection of T lymphocytes is probably mediated by the lymphocyte function-associated antigen (LFA-1) expressed in these cells (Shen et al., 2022).
According to Zekri-Nechar et al. (2022), S-protein subunits affected mitochondrial membrane potential, decreased respiratory rate, and increased mitochondrial fission rate in human pulmonary microvascular endothelial cells (HPMEC). Cao et al. (2023) showed that S-protein induces long-term transcriptional suppression of mitochondrial metabolic genes (including ATP synthase and CI subunit genes) and upregulates genes for factors related to the stress pathway and genes for glucose metabolism. Consequently, cardiac impairments (including cardiac fibrosis) develop in obese mice with PASC; moreover, lipid and obesity cause selective accumulation of SARS-CoV-2 in heart, aorta, and adipose tissues. In general, ROS and intramitochondrial Ca2+ levels increased in human cardiomyocytes under SARS-CoV-2 infection (Huynh et al., 2023). Furthermore, Lesiów et al. (2023) showed that peptides derived from S-protein together with Cu2+ synergistically induce an increase in intramitochondrial ROS. This finding highlights the relevance of interactions between SARS-CoV-2 proteins with metals, which contribute to lung damage in COVID-19.
M-protein, which is the most abundant of the transmembrane proteins, is associated with the morphology of SARS-CoV-2 and key steps of its replication. Yang et al. (2022) suggested that the structural M-protein of SARS-CoV-2 exhibits pro-apoptotic properties in the lung epithelium and causes lung edema. M-protein stabilizes the B cell lymphoma 2 ovarian killer (BOK) by inhibiting its degradation and promotes the translocation of BOK mitochondria. Furthermore, M-protein negatively affects mitochondrial MAVS aggregation and signaling (Fu et al., 2021) and downregulates the IFNβ and IFN-stimulated gene expression (Sui et al., 2021). It also interacts with the ATPase Na+/K+ transporting subunit β1 (ATP1B1), the vacuolar ATPase H+ transporting V1 subunit A (ATP6V1A), acyl-CoA dehydrogenase (ACADM), ALADIN protein, mitochondrial peptidase processing subunits (PMPCA, PMPCB), pitrilysin metallopeptidase (PITRM1) and the coenzyme Q8B (COQ8B) (Singh et al., 2020b).
E-protein, a conserved viroporin, is involved in the release of viral particles from cells and modulation of the activity of membrane ion channels. Together with M-protein, it regulates intracellular trafficking of S-protein as well as its intracellular processing (Boson et al., 2021), and affects Ca2+ homeostasis, and participates in the rearrangement of interorganellar contact sites, leading to mitochondrial remodeling (Poggio et al., 2023).
N-protein affects viral replication, transcription, and assembly together with SARS-CoV-2 NSPs and suppress host cell apoptosis by interaction with MCL-1 anti-apoptotic protein; this may explain SARS-CoV-2 effective replication in asymptomatic patients without respiratory dysfunction (Hirabara et al., 2022; Pan et al., 2023). N-protein also disrupts the assembly of cGAS with its co-factor G3BP1, which reduces IFN-I signaling (Cai et al., 2023). This reply opposes the accumulation of mitogenome fragments that activate the cGAS pathway (Chapter 7). N-protein also increases the mitochondrial ROS level, affects ATP synthesis, increases CI and CIII activity and upregulates the expression level of mitochondrial genes for their subunits; it stabilizes mitochondrial transcriptional complexes and thus belongs to SARS-CoV-2 proteins seriously affecting host mitochondria (Yu et al., 2023).
Other SARS-CoV-2 proteins, including ORF3a, ORF9c, and ORF10 bind to various mitochondrial proteins, including components of the mitochondrial PTP complex, cyclophilin D, SPG-7 paraplegin, ANT transporter, ATP synthase, and uncharacterized CCDC58 protein (Figure 3) (Ramachandran et al., 2022). ORF3a, a monomer or dimer protein on the plasma membrane, which is synthesized in the ER, recruits some host proteins that induce mitochondrial damage and ROS production. This facilitates HIF-1α production and obviously accelerates further ROS production, release of mitogenome fragments by NIM811-sensitive mitochondrial-permeability-pore (Chapter 7) and NLRP3 inflammasome. ORF3a also interferes with proteins that regulate mitochondrial fission (Tian et al., 2021; Guarnieri et al., 2023; Jiao et al., 2023). ORF3b protein interacts with the RIG-I-like receptor (RLR), which also affects MAVS signaling. ORF3c targets mitochondria and evades host immunity by restricting IFN-β production, however, not by changing JAK-STAT signaling (Stewart et al., 2023). ORF7a interacts with Bcl-XL, thus affecting programmed cell death (PCD). The proteins ORF3a and ORF8b interact with NLP3, which regulates the pro-inflammatory cascade and pyroptosis (Figure 3).
ORF9b protein, together with ORF6, inhibits, by the direct action, IFN signaling within the infected cell. ORF9b inhibits the activities of the poly (rC) binding protein (PCBP2) and the E3 ligase of the HECT domain (AID4E3) necessary for the activation of the MAVS signalosome, which affects mitochondrial anti-viral signaling (Malavolta et al., 2020). ORF9b protein also induces the formation of membrane vesicles containing mitogenome fragments; moreover, it causes extensive mitochondrial remodeling (Chapter 9) and, together with NSP4, forms OMM macropores, which influences mitochondrial motility and ultrastructure (Faizan et al., 2022). ORF9c protein became another object of the studies. It directly inhibits the MAVS signalosome and the Drp1 protein (which promotes mitochondrial elongation over fission) and ORF9c binds to the NDUFAF1 and NDUFB9 proteins, altering the functioning of OXPHOS. Consequently, pro-inflammatory factors are released, neutrophiles and monocytes are hyperactivated, antiviral signaling, and host kinase activities are altered, OXPHOS gene expression is severely downregulated and mitochondrial senescence is enhanced (Figure 3; Malavolta et al., 2020).
Regarding the activity of various NSPs, NSP1, NSP2, NSP3, and NSP5 are particularly important for viral biogenesis. NSP1, which belongs to the initial synthesized SARS-CoV-2 proteins in the host cell, participates in translational shutdown (inhibits host protein synthesis) and host mRNA decay only after ribosome engagement with the transcript (Alanagreh et al., 2020; Shehata and Parker, 2023). NSP1 through inhibition of translation and induction of mRNA degradation targets the translated transcript pool. The N terminal part of NSP1 stabilizes the binding of the C-terminus and nonspecifically blocks the mRNA channel in the 40S ribosomal subunit, suppressing host gene expression. Interestingly, the fragment of the natively unstructured C-terminus of NSP1 adopts a secondary structure upon binding of ribosomes and is capable of Cu2+ binding. Targeting stem-loop 1 of the 5′UTR of SARS-CoV-2 can accelerate NSP1 activity by switching cells to coronavirus protein translation (Mendez et al., 2021; Zhao et al., 2021; Morales et al., 2022; Vora et al., 2022). NSP1, in general, suppresses IFN signaling (Thoms et al., 2020; Fisher et al., 2022).
NSP2 have been shown to hamper intracellular cell signaling by interaction with two prohibitin isoforms (PHB1 and PHB2); it was initially speculated that NSP2 may be involved in altering the host cell environment (Cornillez-Ty et al., 2009). According to Graham et al. (2005), in SARS-CoV-1 this protein is not necessary for infection. However, in SARS-CoV-2, NSP2 enables the “life cycle” of the virus and impairs IFN production by binding to the GIGYF2 protein, which is a translation repressor (through interaction with the cap binding complex) of Ifnb1 transcripts (Xu et al., 2022). Furthermore, Naeli et al. (2023) showed that NSP2 can also increase miRNA-mediated silencing of cellular transcripts. However, the exact role of NSP2 in translation is still unclear. On the contrary, the Korneeva et al. (2023) report states that translation increased even in the HEK293T cell line that overexpressed NSP2 both under hypoxia and normal growth conditions. NSP2 also affects VDAC2 biogenesis and increases the abundance of stomatin-like 2 (STOML2), which subsequently decreases apoptosis. Together with NSP4, NSP2 affects mitochondrial Ca2+ homeostasis (see below). Davies et al. (2020) revealed sets of commonly and uniquely interacting partners of NSP2 proteins from SARS-CoV-2. The SARS-CoV-2 interactome comprised proteins indispensable for mitochondrial biogenesis, including PHBs, VDAC2 isoform and STOML2 protein. Furthermore, the Hsp70 chaperone system participated in interactions with NSP2/NSP4 proteins, as well as with components of the inositol triphosphate receptor (IP3R) ubiquitination system (see below) and proteins indispensable for vacuolar ATPase biogenesis.
In addition to affecting the host’s innate immune response, the NSP3 protein (known also as PLpro, papain-like protease) plays a role in polyprotein cleavage and is a component of SARS-CoV-2 replication transcription complexes (Khan et al., 2021; Ju et al., 2023). Furthermore, PLpro also cleaves host ubiquitin and ISG15 ubiquitin-like protein modifications affecting innate immunity (Calleja et al., 2022). NSP3 and N-protein interactions are essential for the processing of SARS-CoV-2 genomic RNA as well as SARS-CoV-2 fitness and virulence. At least 14 amino acid residues of NSP3 were suggested to be indispensable for virus RNA synthesis (Khan et al., 2021; Li et al., 2023a). Shi et al. (2021) showed that NSP3 exhibits dual subcellular localization (in ER and mitochondria).
NSP4 transmembrane glycoprotein forms DMVs associated with replication complexes (Gadlage et al., 2010). Furthermore, NSP4 (together with NSP2) through the recruitment of the ERLIN1/2 and RNF170 proteins (present in the ER membranes and close to the contact sites) severely affect mitochondrial Ca2+ homeostasis at the ER-mitochondrial junctions (Figure 3). These proteins generally block Ca2+ efflux through the IP3R receptor from the ER to mitochondria by interaction of the ERLIN1/2 with the E3 ligase and targeting of the IP3R receptor to polyubiquitination, after its interaction with RNF170, which has fatal consequences for Ca2+ transport (Davies et al., 2020). In cardiomyocytes infected by SARS-CoV-2, mitochondrial Ca2+ homeostasis was severely altered by its cycling alterations (Ramachandran et al., 2022). According to these findings, the level of mitochondrial Ca2+ uniporter in neurons was decreased in “long COVID” (Peluso et al., 2022). Furthermore, NSP4 also affects TIM functioning (leading to import impairments) and regulates the level of the negative regulator of cell proliferation. Both NSP4 and ORF9c proteins belong to key coronavirus proteins that affect numerous cellular processes. In particular, the expression of MERS-CoV nsp3 and nsp4 genes, as well as SARS-CoV-2, was necessary to form double membrane vesicles (DMVs; Oudshoorn et al., 2017). According to Angelini et al. (2013), overexpression of NSP3, NSP4, and NSP6 in tissue cultures also resulted in DMV and disordered membrane appearance (Chapter 6).
NSP5 (also known as Mpro, main protease for processing SARS-CoV-2 polyproteins) together with N-protein of SARS-CoV-2 block formation of “stress granules” and affect RIG-I signaling by disrupting RIG-I-MAVS complex (Zheng et al., 2022; Ju et al., 2023). In addition, it activates NF-κB signaling pathway through specific SUMOylation of the MAVS signalosome, which increases its stability and further triggers NF-κB pathway (Li et al., 2021). NSP5 targets RIG-I and MAVS in two distinct ways: it cleaves off the 10 most N-terminal residues of RIG-I and deprives it of the ability to activate MAVS and promotes proteosome-mediated degradation of MAVS (Liu et al., 2021). NSP5 also cleaves the melanoma-associated antigen D2 (MAGED2), ubiquitously expre
留言 (0)