Pancreatic hepatoid carcinoma (HC) represents a distinct carcinoma variant exhibiting hepatocellular carcinoma (HCC)-like features, often associated with elevated serum alpha-fetoprotein (AFP), and remains uncommon in clinical practice (1). Prone to vascular invasion and liver or lymph node metastasis, HC is considered a highly aggressive malignant tumor with a poor prognosis (2). Despite being first described in 1987 (3), only around 41 cases have been documented in the literature to date (4). However, comprehensive large-scale clinical investigations on pancreatic HC are still notably lacking, leading to a lack of consensus regarding its diagnosis and treatment protocols.
In this study, we present a case of pancreatic HC accompanied by multiple lymph node metastases, followed by early liver metastases post-pancreaticoduodenectomy. The patient underwent adjuvant therapy with Teysuno (also known as S-1) alongside immunotherapy utilizing Durvalumab. This case underscores the urgent need for further research and clinical exploration to establish standardized diagnostic and therapeutic approaches for pancreatic HC.
Case description: diagnosis and treatmentA 45-year-old Chinese woman was admitted to our center in 2022 for abdominal pain accompanied by jaundice. The patient has a history of chronic Viral hepatitis B without receiving regular antiviral therapy. The results of laboratory tests after admission were as follows: the quantification of hepatitis B virus DNA: 4.25×102 IU/mL, alpha fetoprotein (AFP): 5229.00 ng/mL, protein induced by vitamin K absence or Antagonist-II (PIVKA-II): 176.32 mAu/mL, carbohydrate antigen 19-9 (CA19-9): 12.95 U/mL, carcinoembryonic antigen (CEA): 1.00 ng/mL. Abdominal enhanced computed tomography (CT) and magnetic resonance imaging (MRI) revealed an irregular mass measuring approximately 47×35mm in the head of the pancreas, with uneven abnormal enhancement characteristics. Additionally, multiple lymph nodes in the hilar and retroperitoneal regions were found to be enlarged, along with dilation of the distal pancreatic duct, upper common bile duct, and intrahepatic bile ducts (Figure 1). Consequently, percutaneous transhepatic cholangial drainage (PTCD) was performed on the patient to alleviate jaundice. Furthermore, endoscopic ultrasound (EUS) and fine needle aspiration biopsy (FNA) were carried out. The subsequent pathological examination suggested the presence of a pancreatic malignant tumor, possibly a poorly differentiated AFP-producing carcinoma, high-grade neuroendocrine tumor, or pancreatic acinic cell carcinoma.
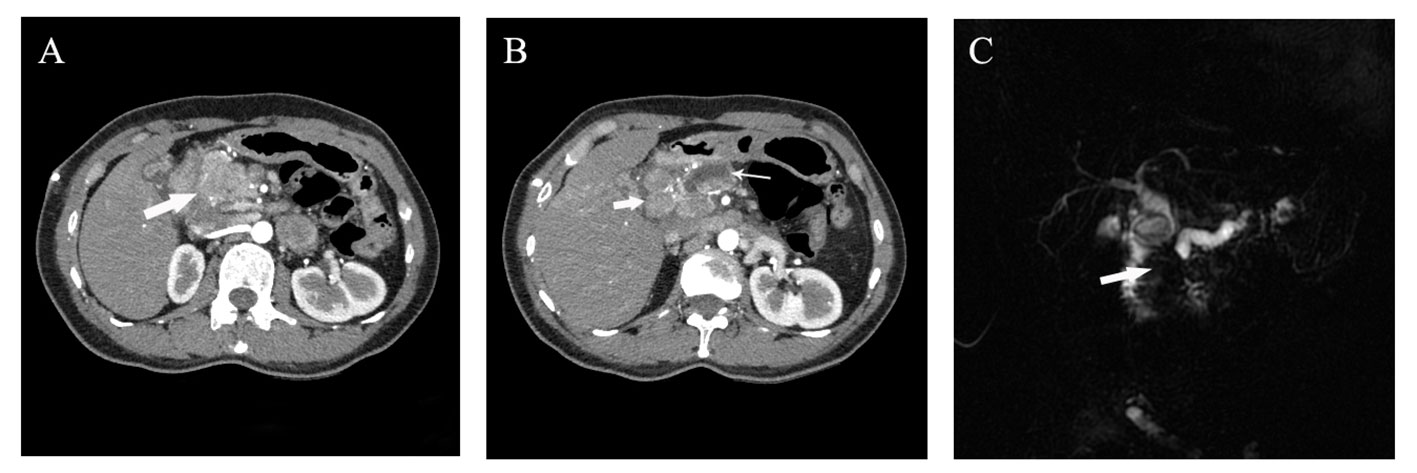
Figure 1 Preoperative imaging examination of the patient. (A, B) Abdominal arterial phase CT imaging suggested the presence of a 4 cm irregular mass with inhomogeneous enhancement in the pancreatic head (denoted by thick arrow in A), along with enlarged lymph nodes around the head of the pancreas (approximately 2cm in diameter, thick arrow in B) and an dilated main pancreatic duct with maximum width of 8mm (denoted by thin arrow in B); (C) Magnetic resonance imaging indicated truncation of both the common bile duct and the main pancreatic duct resulting from a mass in the pancreatic head.
Eventually, the patient underwent pancreaticoduodenectomy and abdominal lymph node dissection after the jaundice had subsided. The final pathological examination confirmed the presence of poorly differentiated pancreatic hepatoid adenocarcinoma invading the mucosal layer of the duodenal wall. Additionally, there were multiple peripancreatic lymphatic node metastases (12/12). The immunohistochemical results were as follows (Figure 2): AFP (+), Glypican-3 (+), Hep Par-1(-), Arginase-1 (-), CK7 (+), CK8/18 (+), CK19 (+), CK20 (-), E-Cadherin (+), EMA(+), Vimentin (-), β-catenin (+), P53 (+), RB1 (+), Ki-67 (Li approximately 40%). Thereby, the patient was diagnosed with advanced pancreatic HC, staged at T4N2M0, following the 8th AJCC/UICC staging system for pancreatic ductal adenocarcinoma.
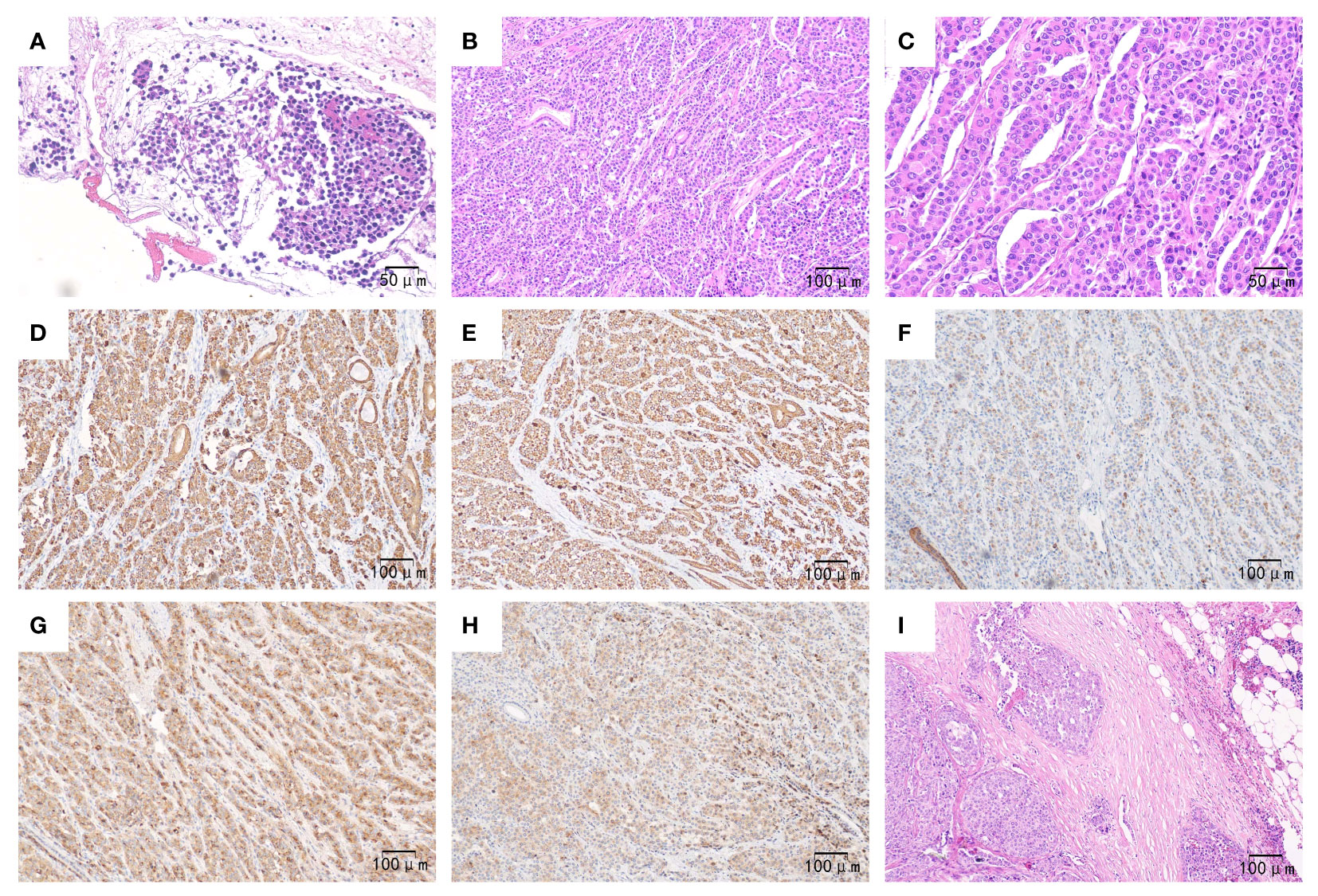
Figure 2 Pathological outcomes of the patient. Preoperatively, (A) (×200): Hematoxylin-eosin staining revealed heteromorphic tumor cells in the pancreas tissue obtained from fine needle aspiration. Postoperatively, (B) (×100): Hematoxylin-eosin staining showed that the poorly differentiated tumor exhibited hepatoid differentiation, with neoplastic cells growing in a sheet-like or trabecular pattern along with sinusoids. (C) (×200): Hematoxylin-eosin staining demonstrated polygonal or cubic eosinophilic cells with abundant cytoplasm and enlarged, prominent nuclei. (D–H) (×100): Immunohistochemistry staining revealed tumor cells expressing CK7, CK8/18, CK19 (with weak positivity), AFP, and Glypican-3, respectively; (I) (×100). Hematoxylin-eosin staining displayed heteromorphic tumor cells proliferating within the lymph node.
The patient recovered well from the surgery, and her serum AFP levels returned to normal. And then, the patients started combination chemotherapy with gemcitabine and albumin-bound paclitaxel a month after surgery. However, after undergoing standard chemotherapy for about 2 months, the patient exhibited elevated serum AFP levels (591.80 ng/mL) and the presence of scattered round nodules in the liver on the MRI which were considered as metastatic lesions.
Sequentially, ultrasound-guided microwave ablation was performed to treat the newly discovered liver masses. Considering the patient’s weak physical condition and intolerance to prior chemotherapy regimen, Teysuno monotherapy was recommended to replace previous adjuvant chemotherapy (gemcitabine plus albumin-bound paclitaxel). To better eradicate any potential residual metastatic lesions, durvalumab was added as an exploratory treatment. Throughout the treatment process, the patient complained of no serious discomfort, although the laboratory test suggested mild hypothyroidism. Consequently, levothyroxine supplementation and regular thyroid function testing were provided. Positron emission tomography/computed tomography (PET/CT) the patient received five months after chemotherapy plus immunotherapy indicated that there was no significant increase in liver and retroperitoneal lymph nodes’ metabolic activity, suggesting that the tumor activity was basically suppressed. Additionally, the patient’s serum AFP level has decreased to 90.66ng/mL. It is worth mentioning that the patient has successfully survived for one year after the surgery.
DiscussionSince its first report by Ishikura et al. in 1970 (5), scholars have subsequently discovered that hepatoid carcinoma can develop in various organs besides the stomach, including the esophagus, lung, gallbladder, pancreas, colorectum, ovary, and others (1). However, reports of pancreatic hepatoid carcinoma remain relatively rare even in present times. Consequently, there is still a lack of relevant studies on the unique pathogenesis and progression mechanisms of pancreatic HC. Additionally, there is no international consensus on the standardized diagnosis and treatment of this disease. Therefore, further exploration is needed to enhance our clinical understanding of pancreatic HC.
Excluding cases diagnosed with pancreatic ectopic hepatocellular carcinoma (HCC) and AFP-producing pancreatic carcinoma, a total of 42 cases of pancreatic HC were reported in the literature. These cases have been pooled and summarized in Tables 1, 2. The majority of these patients were male (69.05%) and older than 50 years (57.14%).Their ages ranged from 21 to 83 years, with a median age of 53 years. The most common clinical manifestation among these patients was abdominal and/or back pain (30.95%), followed by weight loss (19.05%) and jaundice (16.67%). Approximately one-third of these patients were asymptomatic. 19 out of the 31 patients (61.29%) who underwent preoperative serum AFP examinations were found to have elevated levels. On the other hand, only 24.13% of the patients (7/29) with available preoperative data on serum CEA showed elevated levels. Among these cases, the majority (54.76%) of the tumors were located in the body or tail of the pancreas, and 40.48% were found to have other histological components alongside, including neuroendocrine tumors (10/42), ductal adenocarcinoma (3/42), serous cystic adenoma (2/42), and acinar cell carcinoma (1/42). Additionally, 42.86% (18/42) of the patients had metastatic lesions, with liver metastases observed in 13 cases.
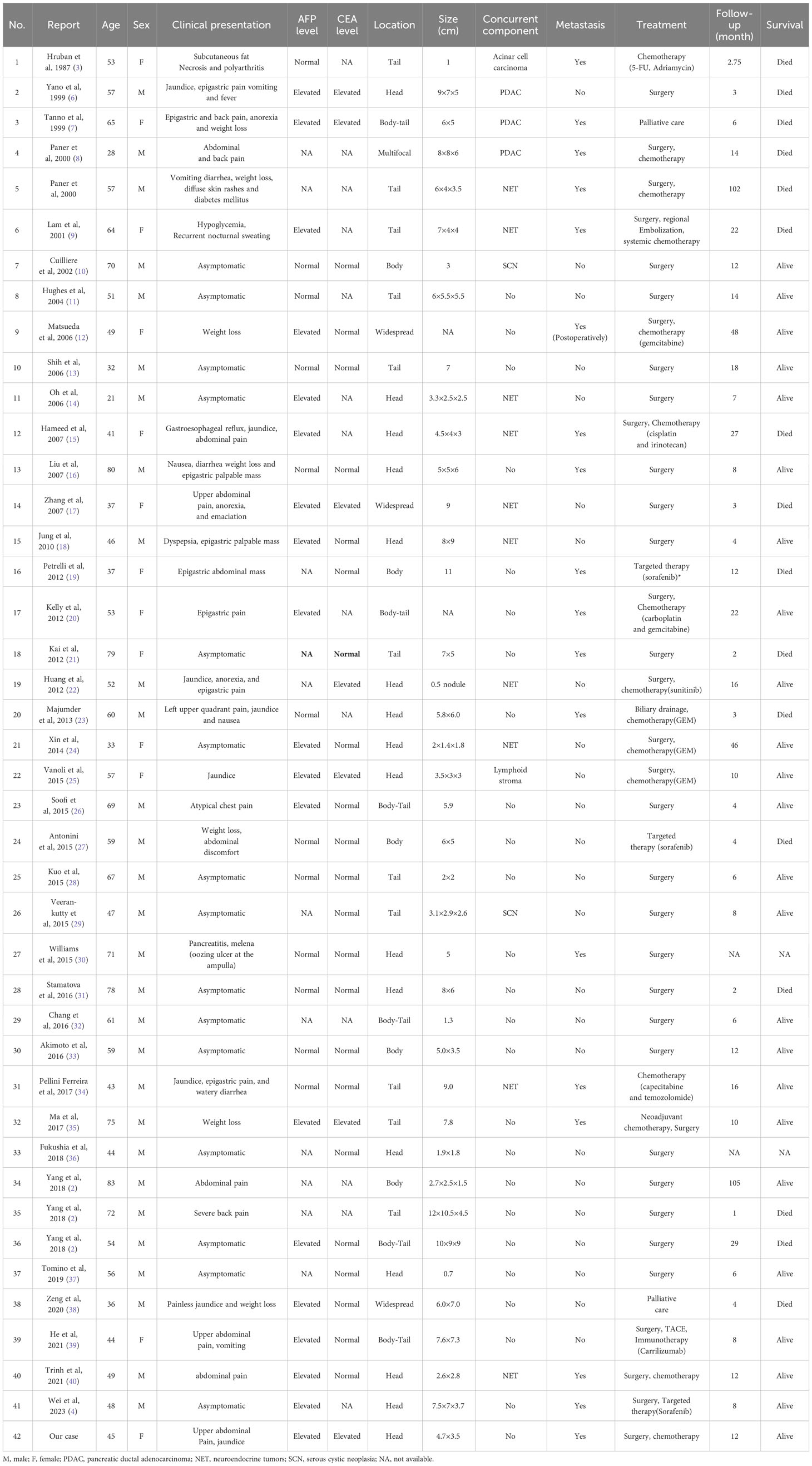
Table 1 Summary of clinical characteristics of 42 cases of pancreatic hepatoid carcinomas reported in the literature.
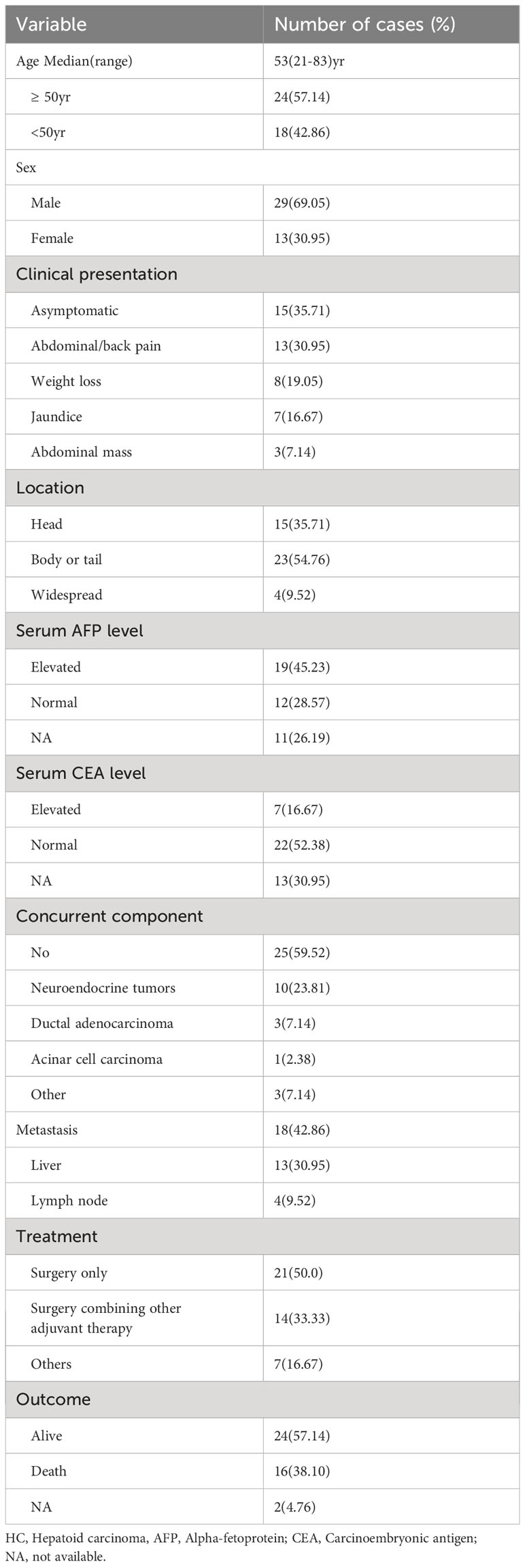
Table 2 Clinical and biochemical characteristics of the 42 cases of pancreatic HC.
In a retrospective study of 271 patients with HC in various organs, it was found that 84.8% of patients had elevated serum AFP levels (41). Therefore, initially HC was thought to be an AFP-producing tumor (42). However, it is important to note that not all cases of HC express AFP, and likewise, AFP-producing tumors are not always linked to hepatoid differentiation. Generally, AFP-producing tumors are defined as those with elevated serum AFP or positive expression of AFP in immunohistochemistry examination, whereas HC is a tumor that exhibits hepatocellular carcinoma differentiation features (43). Although serum AFP levels are typically elevated in most cases of pancreatic HC, it has been reported that elevated serum AFP can also be observed in other types of pancreatic tumor, such as acinar cell carcinoma (16), ductal adenocarcinoma (44), neuroendocrine tumor (45), and pancreatoblastomas (46). As demonstrated in our case, serum AFP can serve to evaluate the effectiveness of treatment and identify tumor recurrence during follow-up (47).
Lacking characteristic imaging features, the diagnosis of pancreatic HC heavily relies on histopathology. Similar to HCC, the typical hepatoid differentiation area of pancreatic HC comprises polygonal or cubic eosinophilic cells growing in a sheetlike or trabecular pattern with sinusoids with abundant cytoplasm and enlarged, prominent nuclei. The presence of bile production, which serves as substantial evidence of hepatocyte lineage differentiation, is a more definitive diagnostic feature (1). Commonly, immunohistochemistry is employed in the differential diagnosis of pancreatic HC. A series of hepatocyte markers are expressed in pancreatic HC, including AFP, Hepatocyte antigen (Hep Par1), Glypican-3, and Arginase-1, with positivity rates of 67%, 96%, 78%, and 75% respectively (37). Actually, pancreatic HC needs to be distinguished from other types of pancreatic cancer such as ductal adenocarcinoma, acinar cell carcinoma, neuroendocrine carcinoma, etc. Additionally, it must also be differentiated from pancreatic metastases of HCC or HCC originating from ectopic liver tissues, which may be more common (with an incidence of 2.7% to 5.6%) than primary pancreatic HC (48). In our case, the diagnosis of pancreatic HC could not be confirmed based on the preoperative EUS-FNA pathology, as it is challenging to differentiate it from acinar cell carcinoma or neuroendocrine carcinoma. Since no obvious intrahepatic neoplastic lesions were detected on the preoperative imaging(both enhanced CT and MRI), the diagnosis of pancreatic HC was formally established, based on the combination of microscopic morphological features and immunohistochemical results after thorough postoperative pathological examination.
83.33% (35/42) of patients reported in the available literature received surgical treatment, demonstrating the superiority of surgery over non-surgical treatment. Generally, radical surgical resection is considered the preferred treatment option (23). Although there are few relevant studies currently, adjuvant chemotherapy may be a viable treatment to improve the prognosis for those instances that are inoperable. The role of targeted therapy and immunotherapy in this disease is still uncertain. Similar pathomorphologic features suggest that the biological behavior of pancreatic HC may share more similarities with HCC. Several reports have highlighted the success of sorafenib in treating pancreatic HC, indicating that sorafenib may offer short-term therapeutic advantages for patients with unresectable metastatic pancreatic HC (19). He et al. reported that a patient with pancreatic HC received transarterial chemoembolization (TACE) combined with postoperative immunotherapy (karelizumab), which brought about positive outcomes (relief of abdominal symptoms, a gradual decrease in serum AFP, and no recurrence observed at the 8-month follow-up) (39). Durvalumab, as one of the PD-L1 inhibitors, is mainly applied to the treatment of non-small cell lung cancer and bile tract cancer. Recently, studies on the role of Durvalumab in hepatocellular carcinoma and pancreatic cancer have been ongoing (49, 50). Basso et al. reported a case of hepatoid adenocarcinoma of the lung responding to PD-L1 inhibitor (durvalumab) therapy despite no PD-L1 expression (51). After a multidisciplinary discussion, the patient was recommended to receive durvalumab immunotherapy on the basis of S1 single-agent chemotherapy following microwave ablation for liver metastases, even though pertinent testing indicated PD-L1 negative. The patient is still in close follow-up with no significant progress. Indeed, there is a lack of validated markers to accurately predict tumor responsiveness to immunotherapy. The exact role of PD-L1 inhibitor in the HC is also unclear. Regardless, it is an exploration in the treatment of HC, and more research is required to evaluate the role of immunotherapy in HC.
ConclusionThe case we reported indicated that the combination of microwave ablation with adjuvant therapy of S1 and durvalumab postoperatively might improve the prognosis and quality of life, providing a potential option for HC.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by the institutional review board of the Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsXH: Writing – review & editing, Writing – original draft, Methodology, Investigation. XG: Writing – review & editing, Investigation, Data curation. YY: Writing – review & editing, Supervision, Resources, Funding acquisition.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grant from the Wuhan applied and basic research project of Wuhan Science and Technology Bureau (2022020801010442).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Li M, Mei YX, Wen JH, Jiao YR, Pan QR, Kong XX, et al. Hepatoid adenocarcinoma-clinicopathological features and molecular characteristics. Cancer Lett. (2023) 559:216104. doi: 10.1016/j.canlet.2023.216104
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Yang C, Sun L, Lai JZ, Zhou L, Liu Z, Xi Y, et al. Primary hepatoid carcinoma of the pancreas: A clinicopathological study of 3 cases with review of additional 31 cases in the literature. Int J Surg Pathol. (2019) 27:28–42. doi: 10.1177/1066896918783468
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Hruban RH, Molina JM, Reddy MN, Boitnott JK. A neoplasm with pancreatic and hepatocellular differentiation presenting with subcutaneous fat necrosis. Am J Clin Pathol. (1987) 88:639–45. doi: 10.1093/ajcp/88.5.639
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Wei D, Xu B, Zhu H, Wang J, Jin Z, Chen W, et al. Extended pancreatoduodenectomy with portal vein and inferior vena cava resection and reconstruction combined with sorafenib for hepatoid carcinoma of the pancreas: A strategy of superextended surgery combined with targeted therapy. Hepatobiliary Surg Nutr. (2023) 12:637–40. doi: 10.21037/hbsn-23-74
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Ishikura H, Fukasawa Y, Ogasawara K, Natori T, Tsukada Y, Aizawa M. An afp-producing gastric carcinoma with features of hepatic differentiation. A Case Rep Cancer. (1985) 56:840–8. doi: 10.1002/1097-0142(19850815)56:4<840::aid-cncr2820560423>3.0.co;2-e
CrossRef Full Text | Google Scholar
6. Yano T, Ishikura H, Wada T, Kishimoto T, Kondo S, Katoh H, et al. Hepatoid adenocarcinoma of the pancreas. Histopathology. (1999) 35:90–2. doi: 10.1046/j.1365-2559.1999.0728f.x
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Tanno S, Obara T, Fujii T, Izawa T, Mizukami Y, Saitoh Y, et al. Alpha-fetoprotein-producing adenocarcinoma of the pancreas presenting focal hepatoid differentiation. Int J Pancreatol. (1999) 26:43–7. doi: 10.1385/ijgc:26:1:43
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Lam K, Lo C, Wat M, Fan ST. Malignant insulinoma with hepatoid differentiation: A unique case with alpha-fetoprotein production. Endocr Pathol. (2001) 12:351–4. doi: 10.1385/ep:12:3:351
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Cuilliere P, Lazure T, Bui M, Fabre M, Buffet C, Gayral F, et al. Solid adenoma with exclusive hepatocellular differentiation: A new variant among pancreatic benign neoplasms? Virchows Arch. (2002) 441:519–22. doi: 10.1007/s00428-002-0683-0
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Matsueda K, Yamamoto H, Yoshida Y, Notohara K. Hepatoid carcinoma of the pancreas producing protein induced by vitamin K absence or antagonist ii (Pivka-ii) and alpha-fetoprotein (Afp). J Gastroenterol. (2006) 41:1011–9. doi: 10.1007/s00535-006-1889-8
PubMed Abstract | CrossRef Full Text | Google Scholar
13. Shih NN, Tsung JS, Yang AH, Tsou MH, Cheng TY. A unique pancreatic tumor with exclusive hepatocytic differentiation. Ann Clin Lab Sci. (2006) 36:216–21.
PubMed Abstract | Google Scholar
14. Oh HJ, Cheung DY, Kim TH, Kim SS, Kim MS, Kim JI, et al. [a case of hepatoid carcinoma of the pancreas]. Korean J Gastroenterol. (2006) 47:389–93.
PubMed Abstract | Google Scholar
15. Hameed O, Xu H, Saddeghi S, Maluf H. Hepatoid carcinoma of the pancreas: A case report and literature review of a heterogeneous group of tumors. Am J Surg Pathol. (2007) 31:146–52. doi: 10.1097/01.pas.0000213370.79300.e1
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Lin YC, Lee PH, Yao YT, Hsiao JK, Sheu JC, Chen CH. Alpha-fetoprotein-producing pancreatic acinar cell carcinoma. J Formos Med Assoc. (2007) 106:669–72. doi: 10.1016/S0929-6646(08)60025-X
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Zhang Y, Zhou P YS. Hepatoid carcinoma of the pancreas: A case report. Chin J Clin Oncol. (2007) 4:445–7. doi: 10.1007/s11805-007-0445-5
CrossRef Full Text | Google Scholar
18. Jung JY, Kim YJ, Kim HM, Kim HJ, Park SW, Song SY, et al. Hepatoid carcinoma of the pancreas combined with neuroendocrine carcinoma. Gut Liver. (2010) 4:98–102. doi: 10.5009/gnl.2010.4.1.98
PubMed Abstract | CrossRef Full Text | Google Scholar
19. Petrelli F, Ghilardi M, Colombo S, Stringhi E, Barbara C, Cabiddu M, et al. A rare case of metastatic pancreatic hepatoid carcinoma treated with sorafenib. J Gastrointest Cancer. (2012) 43:97–102. doi: 10.1007/s12029-011-9264-2
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Kelly PJ, Spence R, Dasari BV, Burt AD, Taylor M, Loughrey MB. Primary hepatocellular carcinoma of the pancreas: A case report and review of the heterogeneous group of pancreatic hepatoid carcinomas. Histopathology. (2012) 60:1012–5. doi: 10.1111/j.1365-2559.2011.04129.x
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Kai K, Nakamura J, Ide T, Masuda M, Kitahara K, Miyoshi A, et al. Hepatoid carcinoma of the pancreas penetrating into the gastric cavity: A case report and literature review. Pathol Int. (2012) 62:485–90. doi: 10.1111/j.1440-1827.2012.02814.x
PubMed Abstract | CrossRef Full Text | Google Scholar
22. Huang SC, Chang HC, Yeh TS, Ng KF, Chen TC. Hepatoid microcarcinoma of the pancreas: A case report and review of the literature. Chang Gung Med J. (2012) 35:285–91. doi: 10.4103/2319-4170.106142
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Xin BB, Li JA, Han X, Zhao J, Ji Y, Lou WH, et al. Successful treatment of a case with pancreatic neuroendocrine carcinoma with focal hepatoid differentiation: A case report and literature review. Int J Clin Exp Med. (2014) 7:3588–94.
PubMed Abstract | Google Scholar
25. Vanoli A, Argenti F, Vinci A, La Rosa S, Viglio A, Riboni R, et al. Hepatoid carcinoma of the pancreas with lymphoid stroma: first description of the clinical, morphological, immunohistochemical, and molecular characteristics of an unusual pancreatic carcinoma. Virchows Arch. (2015) 467:237–45. doi: 10.1007/s00428-015-1788-6
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Soofi Y, Kanehira K, Abbas A, Aranez J, Bain A, Ylagan L. Pancreatic hepatoid carcinoma: A rare form of pancreatic neoplasm. Diagn Cytopathol. (2015) 43:251–6. doi: 10.1002/dc.23195
PubMed Abstract | CrossRef Full Text | Google Scholar
27. Antonini F, Angelelli L, Rubini C, Macarri G. Endoscopic ultrasound diagnosis of a primary hepatoid carcinoma of the pancreas. Endoscopy. (2015) 47 Suppl 1 UCTN:E367–8. doi: 10.1055/s-0034-1392430
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Veerankutty FH, Yeldho V, Tu SA, Venugopal B, Manoj KS, Vidhya C. Hepatoid carcinoma of the pancreas combined with serous cystadenoma: A case report and review of the literature. Hepatobiliary Surg Nutr. (2015) 4:354–62. doi: 10.3978/j.issn.2304-3881.2015.05.02
PubMed Abstract | CrossRef Full Text | Google Scholar
30. Williams NL, Palmer JD, Bar-Ad V, Anné PR, Sama AR, Weinstein JC, et al. Hepatoid carcinoma of the pancreas: A case report and review of the literature. Case Rep Pancreat Cancer. (2015) 1:3–6. doi: 10.1089/crpc.2015.29001.nlw
PubMed Abstract | CrossRef Full Text | Google Scholar
32. Chang JM, Katariya NN, Lam-Himlin DM, Haakinson DJ, Ramanathan RK, Halfdanarson TR, et al. Hepatoid carcinoma of the pancreas: case report, next-generation tumor profiling, and literature review. Case Rep Gastroenterol. (2016) 10:605–12. doi: 10.1159/000448064
PubMed Abstract | CrossRef Full Text | Google Scholar
33. Akimoto Y, Kato H, Matsumoto K, Harada R, Oda S, Fushimi S, et al. Pancreatic hepatoid carcinoma mimicking a solid pseudopapillary neoplasm: A challenging case on endoscopic ultrasound-guided fine-needle aspiration. Intern Med. (2016) 55:2405–11. doi: 10.2169/internalmedicine.55.6741
PubMed Abstract | CrossRef Full Text | Google Scholar
34. Pellini Ferreira B, Vasquez J, Carilli A. Metastatic hepatoid carcinoma of the pancreas: first description of treatment with capecitabine and temozolomide. Am J Med Sci. (2017) 353:610–2. doi: 10.1016/j.amjms.2016.12.006
PubMed Abstract | CrossRef Full Text | Google Scholar
35. Ma T, Bai X, Li G, Wei S, Liang T. Neoadjuvant modified-folfirinox followed by surgical resection of both the primary and metastatic tumors of a pancreatic hepatoid carcinoma with synchronous liver metastasis: A case report. Med (Baltimore). (2017) 96:e8413. doi: 10.1097/md.0000000000008413
CrossRef Full Text | Google Scholar
37. Tomino T, Ninomiya M, Matono R, Narutomi F, Oshiro Y, Watanabe K, et al. Pure pancreatic hepatoid carcinoma: A surgical case report and literature review. Surg Case Rep. (2019) 5:186. doi: 10.1186/s40792-019-0723-5
PubMed Abstract | CrossRef Full Text | Google Scholar
38. Zeng SX, Tan SW, Fong CTH, Liang Q, Zhao BL, Liu K, et al. Hepatoid carcinoma of the pancreas: A case report and review of the literature. World J Clin cases. (2020) 8:1116–28. doi: 10.12998/wjcc.v8.i6.1116
PubMed Abstract | CrossRef Full Text | Google Scholar
39. He J, Zhao Q, Liu Q, Li F, He L, Liu M, et al. Surgical resection of pancreatic hepatoid carcinoma followed by combined transarterial chemoembolization and immunotherapy: A case report. Onco Targets Ther. (2021) 14:4575–8. doi: 10.2147/ott.S323811
PubMed Abstract | CrossRef Full Text | Google Scholar
40. Trinh HS, Luong TH, Lai TT, Nguyen TK. Mixed pancreatic hepatoid carcinoma: A surgical case report and literature review. Int J Surg Case Rep. (2021) 83:105951. doi: 10.1016/j.ijscr.2021.105951
PubMed Abstract | CrossRef Full Text | Google Scholar
41. Su JS, Chen YT, Wang RC, Wu CY, Lee SW, Lee TY. Clinicopathological characteristics in the differential diagnosis of hepatoid adenocarcinoma: A literature review. World J Gastroenterol. (2013) 19:321–7. doi: 10.3748/wjg.v19.i3.321
PubMed Abstract | CrossRef Full Text | Google Scholar
42. Kinjo T, Taniguchi H, Kushima R, Sekine S, Oda I, Saka M, et al. Histologic and immunohistochemical analyses of A-fetoprotein–producing cancer of the stomach. Am J Surg Pathol. (2012) 36:56–65. doi: 10.1097/PAS.0b013e31823aafec
PubMed Abstract | CrossRef Full Text | Google Scholar
44. Hirai I, Kimura W, Kamiga M, Shibasaki H, Kawaguchi K, Fuse A. Intra- and postoperative radiation therapy for an alpha-fetoprotein-producing pancreatic carcinoma. J Hepatobiliary Pancreat Surg. (2003) 10:239–44. doi: 10.1007/s00534-002-0846-z
PubMed Abstract | CrossRef Full Text | Google Scholar
45. Zhu X, Yong H, Zhang L, Huang Y, Zheng J, Liu C, et al. Pure alpha-fetoprotein-producing neuroendocrine carcinoma of the pancreas: A case report. BMC Gastroenterol. (2015) 15:16. doi: 10.1186/s12876-015-0246-x
PubMed Abstract | CrossRef Full Text | Google Scholar
46. Iseki M, Suzuki T, Koizumi Y, Hirose M, Laskin WB, Nakazawa S, et al. Alpha-fetoprotein-producing pancreatoblastoma. A Case Rep Cancer. (1986) 57:1833–5. doi: 10.1002/1097-0142(19860501)57:9<1833::aid-cncr2820570921>3.0.co;2-p
CrossRef Full Text | Google Scholar
47. Inoue M, Sano T, Kuchiba A, Taniguchi H, Fukagawa T, Katai H. Long-term results of gastrectomy for alpha-fetoprotein-producing gastric cancer. Br J Surg. (2010) 97:1056–61. doi: 10.1002/bjs.7081
PubMed Abstract | CrossRef Full Text | Google Scholar
50. Renouf DJ, Loree JM, Knox JJ, Topham JT, Kavan P, Jonker D, et al. The cctg pa.7 phase ii trial of gemcitabine and nab-paclitaxel with or without durvalumab and tremelimumab as initial therapy in metastatic pancreatic ductal adenocarcinoma. Nat Commun. (2022) 13:5020. doi: 10.1038/s41467-022-32591-8
PubMed Abstract | CrossRef Full Text | Google Scholar
51. Basse V, Schick U, Guéguen P, Le Maréchal C, Quintin-Roué I, Descourt R, et al. A mismatch repair-deficient hepatoid adenocarcinoma of the lung responding to anti-pd-L1 durvalumab therapy despite no pd-L1 expression. J Thorac Oncol. (2018) 13:e120–e2. doi: 10.1016/j.jtho.2018.03.004
留言 (0)