In 2020, nearly 300,000 women had cervical cancer in the United States (U.S.). In 2023, about 13,963 women were newly diagnosed with cervical cancer, and 4,310 died of cervical cancer (1).
The vast majority of cervical cancer (>95%) is caused by the human papillomavirus (HPV), the most common sexually transmitted infection in the U.S. (2). One in 10 people in the U.S. develops persistent HPV infections, putting them at risk for cervical cancer. HPV vaccination can prevent 90% of cervical cancers from occurring. With an average total expenditure per patient of $56,250 during the first year after diagnosis—reaching $97,000 annually at the end-of-life—cervical cancer treatment costs are considerable for society (3, 4). Thus, early detection is crucial for reducing cervical cancer deaths and alleviating the economic burden associated with treatment (5).
Women who are not appropriately screened are at the highest risk of developing cervical cancer (6–8). Cervical cancer can be detected with three types of screenings: (1) an HPV test; (2) a Papanicolaou test (also called a Pap smear or cervical cytology); and (3) an HPV/Pap co-test that combines HPV and Pap tests. Effective strategies ensuring all women are screened at appropriate intervals are essential for reducing U.S. cervical cancer incidence and mortality. To that end, the U.S. Preventive Services Task Force (USPSTF) has developed specific guidelines for cervical cancer screening based on women's risk and age: every 3 years with a Pap test for women ages 21–29; every 3 years with a Pap test for women ages 30–65; every 5 years for those with high-risk HPV testing, or every 5 years with both tests. The USPSTF recommends against cervical cancer screening for women aged <21, those with a history of hysterectomy, those without a history of cervical cancer or high-grade precancerous lesion, those aged >65, those who have had adequate screening, and women with low risk for cervical cancer. See Table 1 for the most recent 2018 guidelines (9, 10).

Table 1. USPSTF guidelines for cervical cancer screening.
Although these guidelines can improve cervical cancer detection and prevention, screening services can be costly, with costs varying widely due to screening under-use and inadequate use (3, 11–15). A previous study has identified an excess cost of $166,100 over 5 years for each ineligible woman (e.g., <21 and >65 years or those with a hysterectomy) who underwent screening (15). Equally important is identifying unscreened women among the eligible population to ensure equitable access to treatments and reduce excess costs. Therefore, research that identifies factors associated with use, inadequate use, and non-use of cervical cancer screenings is urgent for public health and economic imperatives, as well as achieving equitable access to screenings for hard-to-reach and underserved women.
Screening rates are below the Healthy People 2030 targets of 79.2% (73.9% in 2021) based on the most recent guidelines (objective C-09) and 11.5% (from 5.3% in 2020) for receipt of appropriate evidence-based clinical preventive services (objective AHS-08) (10). Multiple sociodemographic, healthcare access, and health status factors such as race/ethnicity, education and income levels, marital status, sexual orientation, and rurality (16–18) contribute to use, inadequate use, and non-use of cervical cancer screening rates in certain populations. While previous studies have primarily examined factors predicting cervical cancer screening in silos, fewer studies have employed a robust theoretical framework to investigate the confluence of various sociodemographic, healthcare access, and health factors in explaining adherence to USPSTF cervical cancer screening guidelines among women in the U.S. Our study fills this gap by examining predisposing, enabling, and needs factors contributing the most to disparities in cervical cancer screening rates, and more specifically, the use, non-use, and inadequate use of cervical cancer screenings.
1.1 Theoretical framework, research objectives and hypothesesTo better understand non-adherence to the USPSTF cervical cancer guidelines, we applied Andersen's Behavioral Model of Health Services Use (Andersen's Model) (19, 20) by examining predisposing, enabling, and needs factors associated with use, non-use, or inadequate use of cervical cancer screening services guidelines among age-eligible women in the U.S. More specifically, predisposing factors refer to socio-demographic characteristics that “predispose” women to adhere cervical cancer screening guidelines. Enabling factors are those that “enable” or, to the contrary, impede women's adherence to these guidelines. Needs factors are subjective or objective health needs that incite women to get screened. We hypothesized a significant association between these factors and non-use or inadequate use of cervical cancer screening services among women eligible for cervical cancer screening.
2 Materials and methods 2.1 Data sourceThis study is based on a secondary data analysis of the National Health Interview Survey (NHIS, 2019) of noninstitutionalized and civilian participants living in 50 states and the District of Columbia. NHIS is an annual survey that compiles cross-sectional data on physical and mental health status, functioning, health insurance coverage, health services utilization, and sociodemographic characteristics of the U.S. population. For this study, the Adult file was used. This file contains data obtained from an adult aged 18 or older randomly selected from a household. A suitable proxy is chosen if the selected participant is incapable of responding due to physical or mental limitations (21).
2.2 Study population and inclusion criteriaAccording to the 2019 NHIS, 31,997 adults aged ≥18 (17,261 women) were interviewed. Among them, 10,117 women aged 21–65 reported having had a cervical cancer screening test, and 1,489 reported not having had the test. Among them, 8,580 age-eligible women without a hysterectomy had a cervical cancer screening, whereas 1,248 age-eligible women without a hysterectomy did not. After consideration of inclusion/exclusion criteria and missing data, the final analytical sample included 7,331 women aged 21–65, including (1) a first group (the Users) composed of 4,744 age-eligible women who were properly screened (recommended screening method) within the timeframe recommended by USPSTF; (2) a second group (the Non-Users) composed of 1,248 age-eligible women without a hysterectomy who could have been screened but were not; and (3) a third group (the Inadequate-Users) composed of 1,339 age-eligible women who were non-adherent either because they were improperly screened or untimely screened (i.e., outside of the time window recommend by USPSTF). See Figure 1 for a flow chart of the study sample.
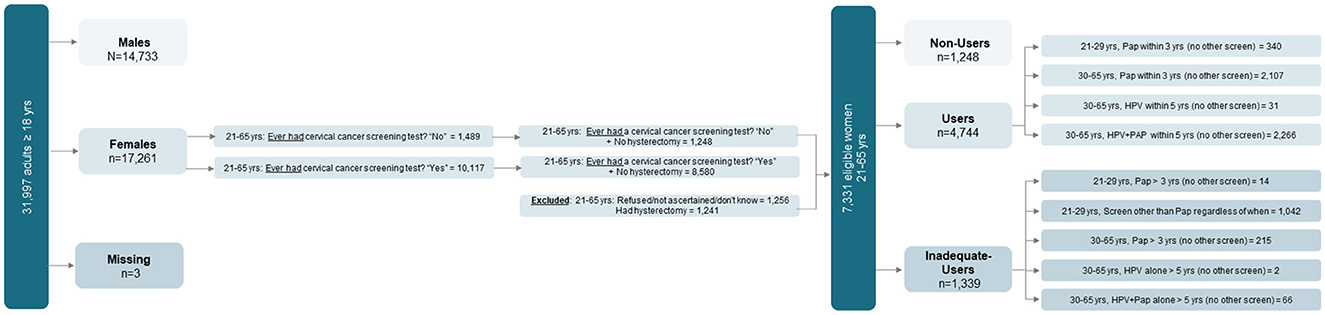
Figure 1. Flow chart of eligible women classified as Users, Non-Users, and Inadequate-Users based on adherence to the United States Preventive Services Task Force (USPSTF) guidelines for cervical cancer screening. Source: 2019 National Health Interview Survey (NHIS)—National Center of Health Statistics, Centers for Diseases Control and Prevention.
2.3 Measures 2.3.1 OutcomeThe dependent variable is a three-category variable that captures: (1) all age-eligible women who were adherent Users (i.e., between the ages of 21–29 who had a Pap test within 3 years with no other screening; or between the ages of 30–65 who had a Pap test within 3 years with no other screening; or between the ages of 30–65 who had HPV within 5 years with no other screening; or 30–65 years who had Pap + HPV co-screening within 5 years with no other screening); (2) all age-eligible women who were Non-Users; and (3) all eligible women who were Inadequate-Users.
2.3.2 Explanatory variablesPredisposing (age 21–29 vs. 30–65, sex, sexual orientation, race/ethnicity, education, marital status, region), enabling (employment status, federal poverty level [FPL], public assistance, health insurance coverage, medical financial hardship, usual source of care, number of children in household), and needs (wellness visits within past year, number of health care visits in the past year, self-reported health status, anxiety, past cancer diagnosis, breast examination by health professionals, mammography, HPV vaccine, disability, body mass index [BMI], smoking status).
2.4 Descriptive and regression analysesTo assess adherence to the USPSTF cervical cancer screening guidelines among eligible participants, percentages and 95% confidence intervals (CI) for categorical variables and means and standard errors (SE) for continuous variables were computed.
After running a multicollinearity test to detect among explanatory variables, mammography, usual sources of care, and breast examination by health professionals were excluded based on the variance inflation factor (VIF). The test was recomputed among the remaining variables and generated a mean VIF of 1.46. Multinomial logistic regression—with odds ratios (OR) and 95% CIs—was used to estimate the odds of being a Non-user or an Inadequate-User vs. a User accounting for the women's predisposing, enabling, and needs factors. The group of eligible women who followed USPSTF guidelines (the adherent Users) was used as the base outcome. All analyses employed sampling weights, stratification, and primary sampling unit variables to account for NHIS complex design by using the svy function in STATA/SE 17 (22).
3 ResultsSix in 10 eligible women (59.8%) were adherent Users (met the USPSTF screening guidelines), two in 10 were Non-Users (19.5%), and two in 10 were Inadequate-Users (20.7%). The mean age of adherent Users was 44.8 years; Non-Users and Inadequate-Users were younger (average age of 40.4 and 31.3 years, respectively). Other predisposing, enabling, and needs characteristics of adherent Users, Non-Users, and Inadequate-Users are presented in Table 2.

Table 2. Descriptive analysis: cervical cancer screening among eligible women aged 21–65. Predisposing, enabling, and needs factors among Users, Non-Users, and Inadequate-Users based on the USPSTF cervical cancer guidelines: NHIS, 2019.
3.1 Explaining non-use and inadequate use of cervical cancer screening services among age-eligible womenMultinomial logistic regression was conducted to examine among eligible women (i.e., 21–65 years without a hysterectomy) the odds of being a Non-User vs. User and the odds of being an Inadequate-User vs. User, accounting for women's predisposing, enabling, and needs factors. Only the statistically significant associations pre-determined at α = 0.05 are reported below. See Table 3 for all significant and non-significant associations.
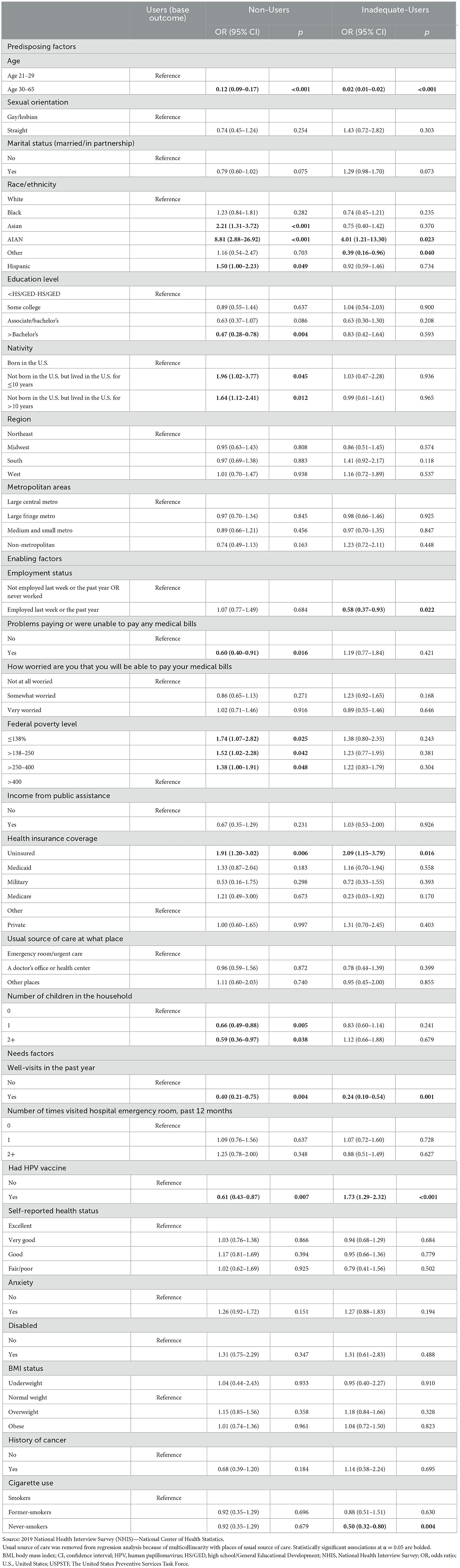
Table 3. Predisposing, enabling, and needs factors associated with cervical cancer screening among Users, Non-Users, and Inadequate-Users: results from multinomial logistic regression.
3.1.1 The Non-Users vs. Users 3.1.1.1 Predisposing factorsCompared with women aged 21–29, those aged 30–65 had lesser odds of being Non-Users than Users (OR: 0.12, CI: 0.09–0.17). Being of Hispanic, Asian, and AIAN race/ethnicity vs. White increased the odds of being a Non-User (OR: 1.50, CI: 1.00–2.23; OR: 2.21, CI: 1.31–3.72; OR: 8.81, CI: 2.88–26.92, respectively). Having more than a bachelor's degree vs. <high school/General Educational Development decreased the odds of being a Non-User (OR: 0.47, CI: 0.28–0.78). Foreign-born women with <10 years in the U.S. had twice the odds of being Non-Users than those born in the U.S. (OR: 1.96, CI: 1.02–3.77), and these odds were also higher for those with ≥10 years in the U.S. (OR: 1.64, CI: 1.12–2.41).
3.1.1.2 Enabling factorsWomen who reported having problems paying medical bills had lesser odds of being Non-Users as compared with those without such hardships (OR: 0.60, CI: 0.40–0.91). Compared with women at 400% FPL, those at ≤138%, >138%−250%, and >250%−400% FPL had increased odds of being Non-Users (OR: 1.74, CI: 1.07–2.82); (OR: 1.52, CI: 1.02– 2.28); (OR: 1.38, CI: 1.00–1.91). Uninsured women vs. those with private insurance had greater odds of being Non-Users (OR: 1.91, CI: 1.20–3.02). Compared with women without children in the household, those with 1 or 2+ children had lesser odds of being Non-Users [(OR: 0.66, CI: 0.49–0.88) and (OR: 0.59, CI: 0.36–0.97), respectively].
3.1.1.3 Needs factorsPast-year well visits were associated with decreased odds of being Non-Users (OR: 0.40, CI: 0.21–0.75). Past HPV vaccination was also associated with lesser odds of being Non-Users (OR: 0.61, CI: 0.43–0.87).
3.1.2 The Inadequate-Users vs. Users 3.1.2.1 Predisposing factorsWomen aged 30–65 had lesser odds of being Inadequate-Users of cervical cancer services than their younger counterparts (OR: 0.02, CI: 0.01–0.02). Whereas AIAN women had increased odds of being Inadequate-Users (OR: 4.01, CI: 1.21–12.30), those of “other” races had decreased odds of being Inadequate-Users than White women (OR: 0.39, CI: 0.16–0.96).
3.1.2.2 Enabling factorsEmployed women had greater odds of being Inadequate-Users than unemployed ones (OR: 0.58, CI: 0.37–0.93). Uninsured women had twice the odds of being Inadequate-Users than their counterparts with private insurance coverage (OR: 2.09, CI: 1.15–3.79).
3.1.2.3 Needs factorsPast-year well visits were associated with decreased odds of being Inadequate-Users (OR: 0.24, CI: 0.10–0.54). Past HPV vaccination was associated with increased odds of being Inadequate-Users (OR: 1.73, CI: 1.29–2.32). Never-smokers had more decreased odds of being Inadequate-Users than smokers (OR: 0.50, CI: 0.32–0.80).
4 DiscussionCervical cancer kills thousands of women every year. Screening is a double-edged sword; over-screening limits screening services availability for eligible women (especially underserved women) (23). Under-screening puts eligible women at higher risk for undiagnosed cervical cancer and related mortality. The goal of this study was to determine factors associated with population-level non-use and inadequate use of cervical cancer screenings among eligible women using a robust theoretical framework.
Our findings showed age as a significant predisposing factor of non-use and inadequate use among eligible women. Women aged 30–65 years were less likely to be Non-Users or Inadequate-Users than their younger counterparts. Research on age has largely focused on whether screening outside the eligible age window (<21 or >65) should be expanded (24–26). A previous study based on 2013 and 2015 NHIS data showed the proportion of women aged 41–70 not recently screened increased with age, especially among women approaching the “stopping” age (27). Our finding that 21–29 year old women were more likely to adhere to the USPSTF guidelines suggests a trend reversal, as a 2000–2010 report from the Centers for Disease Control and Prevention (CDC) showed the proportion of women who never had a Pap test increased from 6.6% to 9.0% among those aged 22–30 who should have been screened every 3 years (18). However, experts have underlined the importance for continued screening among younger populations—especially the 21–25 year-old group—at higher risk for underscreening due to many barriers (e.g., fragmented healthcare, uninsurance or under-insurance, low employment, and diminutions of pelvic examinations for cervical cancer screening despite recommendations) (28).
Our study also found racial/ethnic differences in non-use and inadequate use among minority groups. AIAN women were more likely to be Non-Users and Inadequate-Users, whereas Asian and Hispanic women were more likely to be Non-Users relative to White women. Previous studies have revealed women receiving screening tests outside of guidelines were more likely to identify as Black and Hispanic (16, 23). However, our study showed Black women were not more or less likely to be Non-Users or Inadequate-Users than their White counterparts. The stark White-Black differences in death by cervical cancer may be partly explained by the fact that care continuity after screening is often inadequate for Black women vs. White women though the former group is screened for cervical cancer at rates similar to the latter groups. Differences in treatment may also be important contributing factors. Black women are 41% more likely to have cervical cancer than White women and are 75% more likely to die from it (10, 29). Among all racial/ethnic groups, black women have the lowest 5-year relative survival rate of cervical adenocarcinoma despite being the group with lowest incidence rates (30). This indicates the urgent need to examine factors other than uptake of screening programs (e.g., differential access to treatments, mechanistic influence of enabling and needs factors among this population) that may be at the core of these observed inequities in cervical cancer outcomes.
Our finding that foreign-born women with <10 years of residence in the U.S. were also more likely to be Non-Users is consistent with a previous research study based on pooled NHIS data (2005, 2008, 2013, 2015) finding that foreign-born women ≥18 years were more than twice as likely to have never received a Pap test compared with U.S.-born women. Previous studies have also shown higher level of education predisposing women to more cervical screening as our study has found (16).
In terms of enabling factors, our study showed that women experiencing financial hardships (problems paying medical bills) were less likely to be Non-Users than adherent Users. This finding is inconsistent with a recent convenience sampling study of 970 women aged 21–65 which did not find a significant association between financial hardship (material, psychosocial, and behavioral aspects) and screening rates (31). Although we did not find inadequate use of screening to be significantly associated with financial hardship, adherence to cervical cancer screening may still be associated with problems paying bills. For preventive health services, such as cervical cancer screening, the Patient Protection and Affordable Care Act (ACA Section-2713) requires all ACA Marketplace and non-grandfathered private health plans to abolish patients cost-sharing (coinsurance or copayments) irrespective of whether the patient meets his/her deductible. However, previous research has underscored the fact that “full coverage” often encompasses only the initial test that screens for cancer, but the cancer screening process may require further testing to establish malignancy. Women may still be faced with financial barriers to completing the diagnostic process for cervical cancer following an abnormal initial test result (14). In that vein, our study still showed that indigent or uninsured women have greater likelihood of being Non-Users than Users and employed women are less likely to be Inadequate-Users than Users, independent of financial hardship. This corroborates previous studies on the associations between underscreening and over-screening and income, employment, and insurance coverage (17, 23, 31).
Our study revealed women with one or more children in the household vs. none are less likely to be Non-Users. According to the American Cancer Society, women who have had children (≥3) are at an increased risk of cervical cancer compared with those who have not, probably due to the increased exposure to HPV infection with sexual activity (32).
Usual source of care was a significant enabler of non-use or inadequate use. The literature shows inconsistencies regarding healthcare providers' adherence to USPSTF guidelines as over-screening is common practice among them (33–35).
The finding that well-visits in the past year (needs factor) are associated with lesser likelihood of non-use or inadequate use is consistent with the literature (36). Indeed, well-woman visits including annual check-up for gynecological and reproductive health focus on preventative care. Cervical screenings, Pap smears, and breast exams are three of the most common tests performed during well-woman visits.
Finally, our study unveiled an intriguing finding regarding HPV vaccination. While HPV vaccination was associated with lesser odds of non-use, it was associated with greater odds of inadequate use among eligible women. Studies have shown HPV vaccination is associated with decreased risk of cervical cancer (37) and have found women who receive HPV vaccination are more likely to participate in cervical cancer screening (depending on age of vaccination) (38, 39). It is plausible that women who have received the HPV vaccine may have a heightened sense of being protected against the virus (less at risk) and may be less prone to regimentally follow the recommended timeline for vaccinations. However, a study has shown HPV vaccinated Danish women perceived cervical cancer risk to be greater than unvaccinated women did, but the study found no associations between perceived cervical cancer risk and intention to participate in screening (40). The National Cancer Institute and the CDC encourage past HPV vaccinated populations to continue screening for cervical cancer because HPV vaccines do not protect against all HPV types that can cause cancer (41, 42). Vaccinated women are advised to follow the same cervical cancer screening guidelines as unvaccinated women. Future studies on screening recommendations in terms of harms and benefits for vaccinated women are warranted. Furthermore, studies on risk perceptions of cervical cancer screening among HPV vaccinated women stratified by factors associated with inadequate use (e.g., age group, race/ethnicity, uninsurance) are needed.
4.1 Study strengths and limitationsFindings from this study should be appraised in light of the following limitations: first, this study used cross-sectional data, and thus, causality cannot be established. Other limitations relate to recall bias, especially as the time window recommended by USPSTF becomes larger and as women age. We were limited with self-reported data, whereas medical records could have provided data less tainted by recall bias. Furthermore, the language around Non-Users and Inadequate-Users shifts the “blame” onto women, whereas healthcare providers might be the culprit for over or underscreening. The data used in our study did not allow us to clearly disentangle whether non-use or inadequate use was intentionally born by women or imposed by their providers. Despite these limitations, by using a conceptual framework and population data to explain factors associated with non-use and inadequate use separately, our study provides a more nuanced and complex explanation of cervical screening utilization among a representative sample of women. Future studies should explore mechanistic pathways (mediation and moderation) that lead to U.S. women's non-use and inadequate utilization of cervical cancer screening services.
5 ConclusionPredisposing, enabling, and needs factors are differently associated with non-use and inadequate use of cervical cancer screening among eligible women aged 21–65 years. Understanding factors associated with the use, non-use, and inadequate use of cervical cancer screening is crucial to avoid or curb unnecessary tests, increased costs to both society and individuals, and the ill allocation of limited resources.
Data availability statementPublicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhis/2019nhis.htm. No datasets were generated for this study. Any analysis, interpretation, and/or conclusion based on the NHIS 2019 data is solely that of the authors. Opinions, conclusions, and recommendations expressed herein do not necessarily represent those of the National Center for Health Statistics or CDC, which are responsible for the data.
Ethics statementEthical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributionsM-RN: Methodology, Writing—original draft. PM: Writing—review & editing. EH: Writing—review & editing. NP-J: Writing—review & editing. HF: Conceptualization, Writing—review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by University of Arkansas for Medical Sciences Translational Research Institute funding awarded through the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) [UL1 TR003107]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References3. Blanco M, Chen L, Melamed A, Tergas AI, Khoury-Collado F, Hou JY, et al. Cost of care for the initial management of cervical cancer in women with commercial insurance. Am J Obstet Gynecol. (2021) 224:286.e1–11. doi: 10.1016/j.ajog.2020.08.039
PubMed Abstract | Crossref Full Text | Google Scholar
5. Melnikow J, Henderson JT, Burda BU, Senger CA, Durbin S, Weyrich MS. Screening for cervical cancer with high-risk human papillomavirus testing updated evidence report and systematic review for the US preventive services task force. JAMA. (2018) 320:687–705. doi: 10.1001/jama.2018.10400
PubMed Abstract | Crossref Full Text | Google Scholar
7. Vesco K, Whitlock E, Eder M, Lin J, Burda B, Senger C, et al. Screening for Cervical Cancer: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Rockville, MD (2011). Contract No.: 11-05156-EF-1.
8. Vesco K, Whitlock E, Eder M, Burda B, Senger C, Lutz K. Risk factors and other epidemiologic considerations for cervical cancer screening: a narrative review for the US Preventive Services Task Force. Ann Intern Med. (2011) 155:698–705. doi: 10.7326/0003-4819-155-10-201111150-00377
PubMed Abstract | Crossref Full Text | Google Scholar
11. Arora S, Tuffaha H. Cost-effectiveness analysis of human papillomavirus vaccines for the prevention of cervical cancer in India. Asia Pac J Clin Oncol. (2023). doi: 10.1111/ajco.13962. [Epub ahead of print].
PubMed Abstract | Crossref Full Text | Google Scholar
12. Esselen KM, Feldman S. Cost-effectiveness of cervical cancer prevention. Clin Obstet Gynecol. (2013) 56:55–64. doi: 10.1097/GRF.0b013e3182823797
Crossref Full Text | Google Scholar
13. Gamboa OA, Murillo RH, Gonzalez M. Cost-effectiveness analysis of primary and secondary prevention strategies for cervical cancer in Colombia. Value Health. (2013) 16:A669–736. doi: 10.1016/j.jval.2013.08.1932
Crossref Full Text | Google Scholar
14. Ruff A, Harper DM, Dalton V, Fendrick AM. Coverage for the entire cervical cancer screening process without cost-sharing: lessons from colorectal cancer screening. Women Health Issues. (2023) 33:113–6. doi: 10.1016/j.whi.2022.11.009
PubMed Abstract | Crossref Full Text | Google Scholar
15. Teoh D, Hultman G, Dekam M, Vogel RI, Downs LS, Geller MA, et al. Excess cost of cervical cancer screening beyond recommended screening ages or after hysterectomy in a single institution. J Low Genit Tract Di. (2018) 22:184–8. doi: 10.1097/LGT.0000000000000400
PubMed Abstract | Crossref Full Text | Google Scholar
16. Kepka D, Breen N, King JB, Meissner HI, Roland KB, Benard VB, et al. Demographic factors associated with overuse of pap testing. Am J Prev Med. (2014) 47:629–33. doi: 10.1016/j.amepre.2014.07.034
PubMed Abstract | Crossref Full Text | Google Scholar
17. Almeida CM, Rodriguez MA, Skootsky S, Pregler J, Steers N, Wenger NS. Cervical cancer screening overuse and underuse: patient and physician factors. Am J Manag Care. (2013) 19:482–9.
PubMed Abstract | Google Scholar
18. Saraiya M, King J, Thompson T, Watson M, Ajani U, Li J, et al. Cervical cancer screening among women aged 18-30 years-United States, 2000-2010 (reprinted from MMWR, vol 51, pg 1038-1042, 2013). JAMA. (2013) 309:868–70. doi: 10.1001/jama.2013.107
PubMed Abstract | Crossref Full Text | Google Scholar
20. Narcisse MR, Felix H, Long CR, Hudson T, Payakachat N, Bursac Z. et al. Frequency and predictors of health services use by Native Hawaiians and Pacific Islanders: evidence from the US National Health Interview Survey. BMC Health Serv Res. (2018) 18:575. doi: 10.1186/s12913-018-3368-3
Crossref Full Text | Google Scholar
22. StataCorp. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC; 2021.
23. Franklin M, Webel A, Kaelber D, Evans J, Kelley C. Prevalence of cervical cancer overscreening review of a wellness registry. Comput Inform Nurs. (2020) 38:459–65. doi: 10.1097/CIN.0000000000000610
PubMed Abstract | Crossref Full Text | Google Scholar
24. Silver MI, Anderson ML, Beaber EF, Haas JS, Kobrin S, Pocobelli G, et al. De-implementation of cervical cancer screening before age 21. Prev Med. (2021) 153:106815. doi: 10.1016/j.ypmed.2021.106815
PubMed Abstract | Crossref Full Text | Google Scholar
26. Vash-Margita A, Kobernik EK, Flagler EN, Quint EH, Dalton VK. National trends in cervical cancer screening in adolescents. J Pediatr Adol Gynec. (2021) 34:717–24. doi: 10.1016/j.jpag.2021.02.097
Crossref Full Text | Google Scholar
27. White MC, Shoemaker ML, Benard VB. Cervical cancer screening and incidence by age: unmet needs near and after the stopping age for screening. Am J Prev Med. (2017) 53:392–5. doi: 10.1016/j.amepre.2017.02.024
PubMed Abstract | Crossref Full Text | Google Scholar
28. Moscicki AB, Perkins RB, Saville M, Brotherton JML. Should cervical cancer screening be performed before the age of 25 years? J Low Genit Tract Di. (2018) 22:348–51. doi: 10.1097/LGT.0000000000000434
PubMed Abstract | Crossref Full Text | Google Scholar
29. Christy K, Kandasamy S, Majid U, Farrah K, Vanstone M. Understanding black women's perspectives and experiences of cervical cancer screening: a systematic review and qualitative meta- synthesis. J Health Care Poor Underserved. (2021) 32:1675–97. doi: 10.1353/hpu.2021.0159
PubMed Abstract | Crossref Full Text | Google Scholar
30. Cohen CM, Wentzensen N, Castle PE, Schiffman M, Zuna R, Arend RC, et al. Racial and ethnic disparities in cervical cancer incidence, survival, and mortality by histologic subtype. J Clin Oncol. (2023) 41:1059. doi: 10.1200/JCO.22.01424
PubMed Abstract | Crossref Full Text | Google Scholar
31. Kasting ML, Haggstrom DA, Lee JL, Dickinson SL, Shields CG, Rawl SM. Financial hardship is associated with lower uptake of colorectal, breast, and cervical cancer screenings. Cancer Cause Control. (2021) 32:1173–83. doi: 10.1007/s10552-021-01465-7
PubMed Abstract | Crossref Full Text | Google Scholar
33. Denson V, Keele R. Cervical cancer screening practices of volunteer providers in faith-based clinics. Jnp-J Nurse Pract. (2016) 12:27–34. doi: 10.1016/j.nurpra.2015.09.005
Crossref Full Text | Google Scholar
34. Moss JL, Roy S, Shen C, Cooper JD, Lennon RP, Lengerich EJ, et al. Geographic variation in overscreening for colorectal, cervical, and breast cancer among older adults. JAMA Netw Open. (2020) 3:e2011645. doi: 10.1001/jamanetworkopen.2020.11645
PubMed Abstract | Crossref Full Text | Google Scholar
35. Schoenborn NL, Massare J, Park R, Pollack CE, Choi Y, Boyd CM. Clinician perspectives on overscreening for cancer in older adults with limited life expectancy. J Am Geriatr Soc. (2020) 68:1462–8. doi: 10.1111/jgs.16415
PubMed Abstract | Crossref Full Text | Google Scholar
38. Paynter CA, Van Treeck BJ, Verdenius I, Lau AW, Dhawan T, Lash KA, et al. Adherence to cervical cancer screening varies by human papillomavirus vaccination status in a high-risk population. Prev Med Rep. (2015) 2:711–6. doi: 10.1016/j.pmedr.2015.07.011
PubMed Abstract | Crossref Full Text | Google Scholar
39. Boone SD, Pinkston CM, Baumgartner KB, Baumgartner RN, Harper SM, Bonham AJ, et al. Associations between prior HPV4 vaccine doses and cervical cancer screening participation. Cancer Epidemiol. (2016) 42:108–14. doi: 10.1016/j.canep.2016.04.003
PubMed Abstract | Crossref Full Text | Google Scholar
40. Hestbech MS, Gyrd-Hansen D, Kragstrup J, Siersma V, Brodersen J. How does HPV vaccination status relate to risk perceptions and intention to participate in cervical screening? a survey study. BMC Public Health. (2016) 16:708. doi: 10.1186/s12889-016-3397-y
留言 (0)