The human T-lymphotropic virus type 1 (HTLV-1) causes a persistent infection in an estimated 5–10 million people globally, leading to various clinical manifestations. These range from severe conditions like adult T-cell leukemia/lymphoma (ATLL) to HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) and other inflammatory diseases (1). Regarding HTLV-2, it is estimated that 800,000 people globally are infected with this virus, which is prevalent in people who inject drugs and American indigenous populations, particularly in Amazon region of Brazil (2, 3). Although HTLV-2 has been associated with rare cases of myelopathy, its pathological role is still unclear (4, 5). Despite these clinical and epidemiological differences, both viruses are transmitted by unprotected sexual intercourse, injecting drug use or transfusion/transplantation of contaminated blood/tissue or organ, and from mother to child, mainly through breastfeeding (1, 5).
There are more than 10.77 million people living in prison worldwide, and the world prison population may well be in excess of 11.5 million (6). Brazil has the third largest prison population in the world (835,643 in December 2022) (7). Incarcerated individuals frequently endure precarious conditions. These include overcrowded cells, inadequate ventilation and lighting, poor infrastructure, and limited access to basic sanitation. Additionally, issues like malnutrition, illicit drug use, and restricted access to health services are prevalent (8, 9), and create an environment conducive to the spread of infectious diseases (10), including bloodborne and sexually transmitted infections (STIs) (11, 12).
This study, recognizing the significant vulnerability of incarcerated individuals and the limited research on HTLV-1/2 in Brazilian prisons (13–17), aims to investigate the prevalence of HTLV-1/2 infection in Goiás State’s major penitentiary complex, Central-West Brazil, and compare these findings with prevalence data and related studies from other regions in Brazil.
Methods Study populationA cross-sectional study was conducted in prisoners of the major penitentiary complex of Goiás State, located in the metropolitan region of Goiânia (capital of the State of Goiás), Central-West Brazil. The study was conducted among prisoners in four units of the Prison Complex of Aparecida de Goiânia in Goiás State, Brazil. These units included the Coronel Odenir Guimarães Penitentiary, Consuelo Nasser Women’s Penitentiary, Provisional Prison House, and Triage Center.
Sample size was calculated based on an estimated HTLV-1/2 infection prevalence of 1.3% among Brazilian prisoners (13, 14, 16), a 95% confidence level (α < 0.05), 80% statistical power (β = 20%), a 1% precision, and a design effect of 1.5%. This calculation yielded a minimum sample size of 740 participants, therefore the study included 910 prisoners.
From May 2018 to January 2020, all prisoners were invited to participate in this study, and they were recruited with the help of prison security agents and with the assistance of inmate health monitors, who were responsible for managing health-related requests in the prisons. Prisoners aged 18 years or older were included in the study. Individuals who showed aggressive behavior and those who appeared to be under the influence of psychotropic drugs were excluded from the study.
Ethical aspects and sample collectionThis study was approved by the Research Ethics Committee of the Clinical Hospital, Federal University of Goiás (reference number 80757617.9.1001.5078). All participating subjects provided their voluntary agreement by signing the free and informed consent form.
Data and blood samples were collected at a private location in each unit of the Prison Complex of Aparecida de Goiânia. All participants were previously informed about the aims of the study, and those who agreed to participate signed the informed consent form. A structured and comprehensive questionnaire was employed to gather data on participants’ sociodemographic characteristics, risk behaviors for HTLV-1/2 infection, and medical history. Then, a sample of venous blood (10 mL) was collected from each participant for laboratory tests.
Laboratory testsSerum samples from all participants were tested for anti-HTLV-1/2 (Murex HTLV-I + II, DiaSorin, Dartford, UK) by enzyme-linked immunosorbent assay (ELISA). Seropositive samples were confirmed using a line immunoassay (INNO-LIA HTLV I/II, Fujirebio, Europe N.V., Belgium). Briefly, this assay uses strips containing four non-typed-specific antigens (two gag: p19 I/II, p24 I/II; and two env: gp46 I/II and gp21 I/II) for anti-HTLV confirmation. Additionally, three typed-specific antigens for HTLV-1 (gag p19-I and env gp46-I) and for HTLV-2 (env gp46-II) are used for differentiation of HTLV-1 and HTLV-2 antibodies. Samples that tested anti-HTLV positive by LIA (reactive with at least two of the following confirmation bands: p19 I/II or p24 I/II or gp46 I/II and gp21 I/II) were subsequently considered positive for anti-HTLV-1 (if the intensities of p19-I and gp46-I bands were higher than that of the gp46-II band) or anti-HTLV-2 (if the gp46-II band was more intense than the p19-I and gp46-I bands). All assays were conducted according to the respective manufacturer’s instructions.
Anti-HTLV-1/2 positive samples were tested for human immunodeficiency virus 1 (HIV-1) co-infection using a four-generation ELISA for the simultaneous detection of HIV-1 p24 antigen and anti-HIV-1/2 antibodies (HIV Ag/Ab ELISA 4a Generation test, Wiener Lab, Rosario, Argentina); hepatitis B virus (HBV) co-infection using an ELISA for hepatitis B surface antigen (HBsAg, Biokit S.A., Bioelisa, Spain); and for hepatitis C virus (HCV) exposure using an ELISA for antibodies to the HCV (anti-HCV, Bioelisa-Bioclin®, Quibasa, Brazil).
Data analysisData were analyzed using the IBM Statistical Package for the Social Sciences (SPSS) (IBM SPSS Statistics for Windows, Version 20.0). Descriptive analyses were performed using frequency distributions, mean values, and standard deviations. Prevalence of anti-HTLV-1/2 was estimated using a confidence interval of 95% (95% CI).
ResultsTable 1 details the sociodemographic characteristics of the study participants. The majority were male (83.1%), self-identified as heterosexual (93.4%), aged between 25 and 39 years (56.1%; mean age of 31.98 ± 9.69 years), and of brown ethnicity (56.2%). Nearly half were single (49.3%), with 41.4% reporting 9 years or less of formal education.
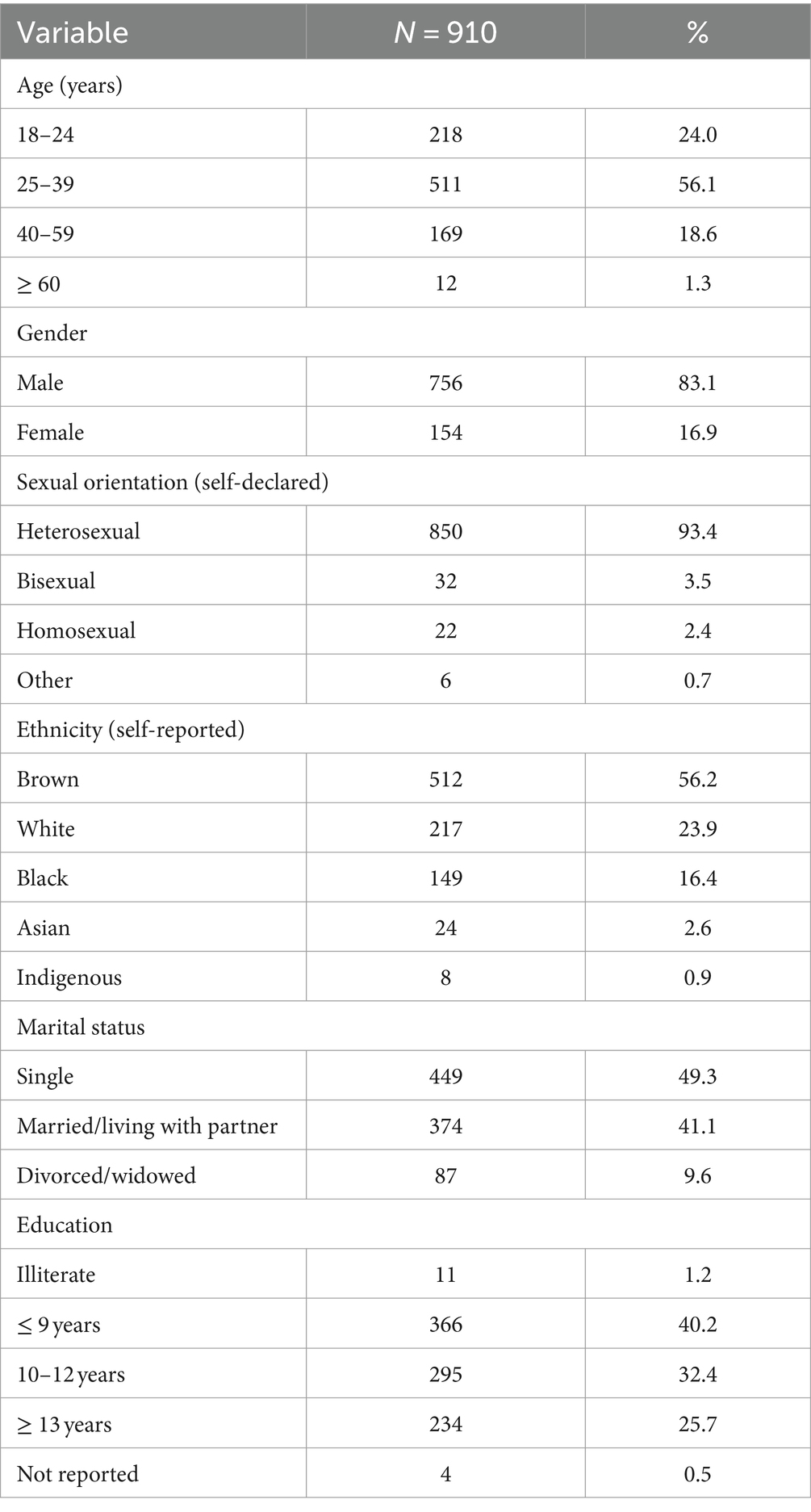
Table 1. Demographic characteristics of the prison study population.
As shown in Table 2, a minority of prisoners reported receiving blood transfusions (11.8%). While a significant portion used illicit drugs (85.2%), only a small fraction (4.2%) engaged in injecting drug use. High-risk sexual behaviors were common: 75.3% reported more than 10 sexual partners in lifetime; the prevalence of vaginal, oral, and anal intercourse was 98.1, 90.4, and 78.3%, respectively; 83.9% engaged in homosexual intercourse; 77.1% had sex with drug-using partners; and 67.1% with sex workers. Condom use was infrequent or occasional for 85.4% of the participants. Additionally, 37.1% reported group sex, and 32.3% reported sex for money. A history of STIs was noted in 25.7% of participants.

Table 2. Behavioral characteristics reported by 910 prisoners of the major penitentiary complex of Goiás State, Central-West Brazil.
Among the 910 prisoners enrolled, 3 (0.33%; 95% CI: 0.07–0.96) were repeatedly anti-HTLV-1/2 positive by ELISA. After confirmatory testing (LIA), two were positive for HTLV-1 (0.22%) and one was positive for HTLV-2 (0.11%). The distribution of anti-HTLV-1/2 by gender revealed a higher seroprevalence among female prisoners (1.3%; 2/154) than among male prisoners (0.13%; 1/756). None of the seropositive individuals reported symptoms associated with HTLV-1/2 infection at the time of interview.
Regarding the seropositive individuals, detailed in Table 3, the two HTLV-1 positive cases included a 55-year-old male (PLG-440) and a 21-year-old female (PLG-611), both reporting unprotected sexual intercourse with multiple partners and a history of STIs. The HTLV-2 positive case, a 69-year-old female (PLG-240), had unprotected intercourse only with her sole partner and a notable history of being breastfed for over 6 months by her mother and three other women. None of the seropositive individuals reported blood transfusions or injecting drug use. In addition, one HTLV-1 positive case (PLG-611) was co-infected with HIV-1, and there were no HTLV-HBV or HTLV-HCV coinfected participants.
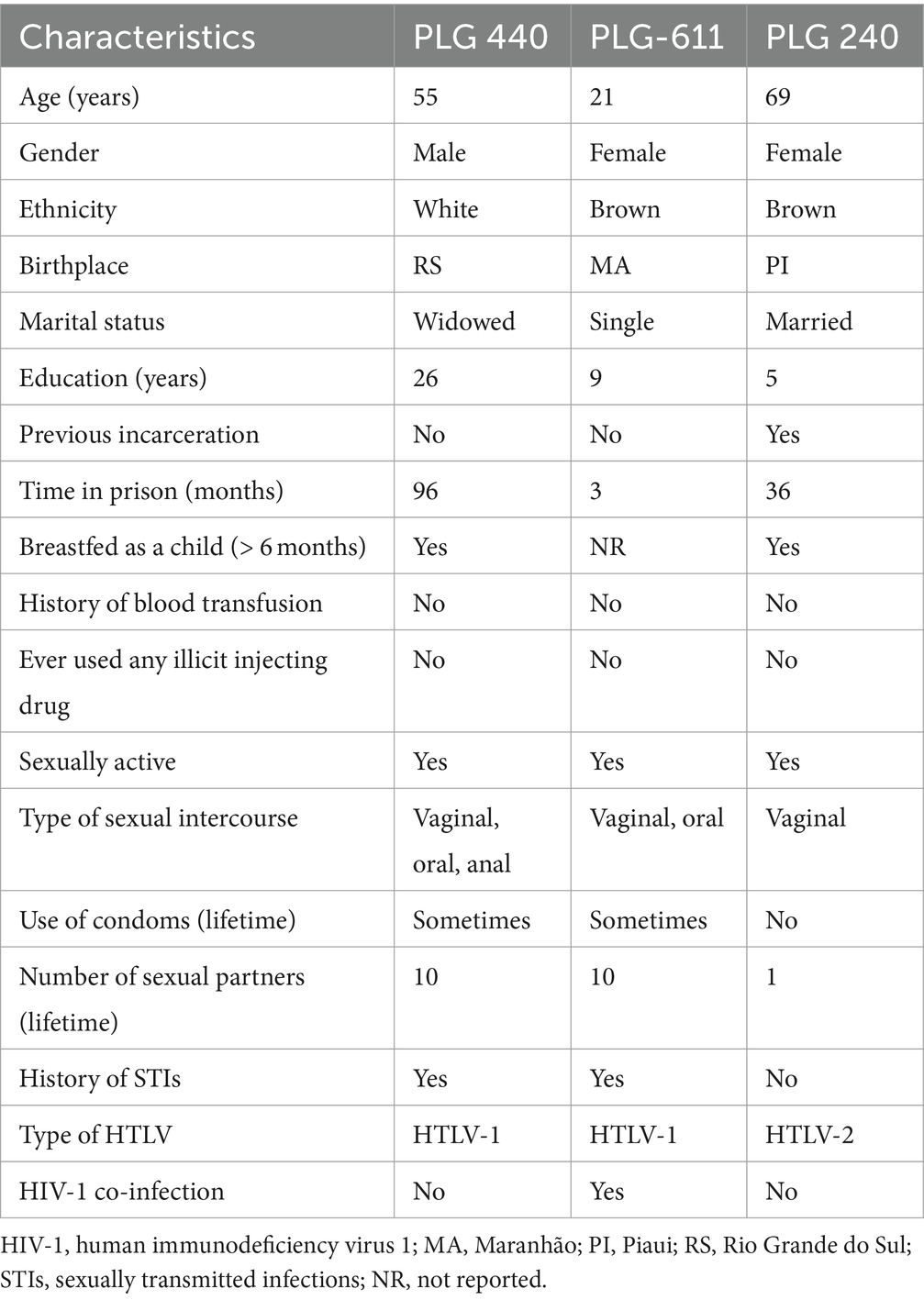
Table 3. Characteristics of HTLV-1/2-positive prisoners of the major penitentiary complex of Goiás State, Central-West Brazil.
DiscussionThis study, the second to focus on HTLV-1/2 infection in Central-West Brazil’s prison population, addresses a critical issue in a strategic region. This region’s borders with other Brazilian states, Bolivia, and Paraguay make it a pivotal area for drug trafficking and criminal activities (18). In addition, our data were compared with the limited data available from other Brazilian regions in order to guide prevention actions and successful control of HTLV-1/2 and other infections in prison settings.
The sociodemographic characteristics of the studied population were similar to those reported by Melo Bandeira et al. (17) in prisoners in Mato Grosso do Sul State, Central-West Brazil, such as mostly adults aged 25–39 years, males, self-reported as brown ethnicity, single, and with less than 9 years of formal education.
Few participants reported a history of blood transfusions, with most transfusions occurring after November 1993, the onset of mandatory anti-HTLV-1/2 screening in Brazilian blood banks (19). Despite widespread illicit drug use, injecting drug use was infrequent, both in this study and among other incarcerated individuals (15, 17, 20). The majority engaged in high-risk sexual behaviors, including having multiple sexual partners and engaging in various types of sexual intercourse (vaginal, oral, and anal). Relationships with drug-using partners or sex workers were also common, alongside low condom usage. A quarter of the participants also reported a history of STIs, aligning with findings from other Brazilian prison studies (15–17, 20, 21), reinforcing the alarming vulnerability of incarcerated people to STIs and the importance of screening, treatment, and prevention strategies targeting this specific population.
The observed seroprevalence of HTLV-1/2 in this study (0.33%; 95% CI: 0.07–0.96) was significantly higher than the rate among local blood donors (0.09%) (22). Nevertheless, relative to other Brazilian prison populations (Table 4), this prevalence was similar to that estimated in prisoners in Mato Grosso do Sul State (0.40%; CI 95%: 0.15–0.87) (17) and falls within the confidence interval reported for incarcerated adolescents in Salvador, Bahia (1.01%; CI 95%: 0.21–2.92) (16). In prior studies, however, Broutet et al. (13) observed a higher seroprevalence (1.41%; CI 95%: 0.52–3.03) in Fortaleza, Ceará. Also, Catalan-Soares et al. (14) determined a seroprevalence of 1.59% (95% CI: 0.04–8.53) among male prisoners in Manhuaçu, Minas Gerais, in a study that included a smaller number of participants.
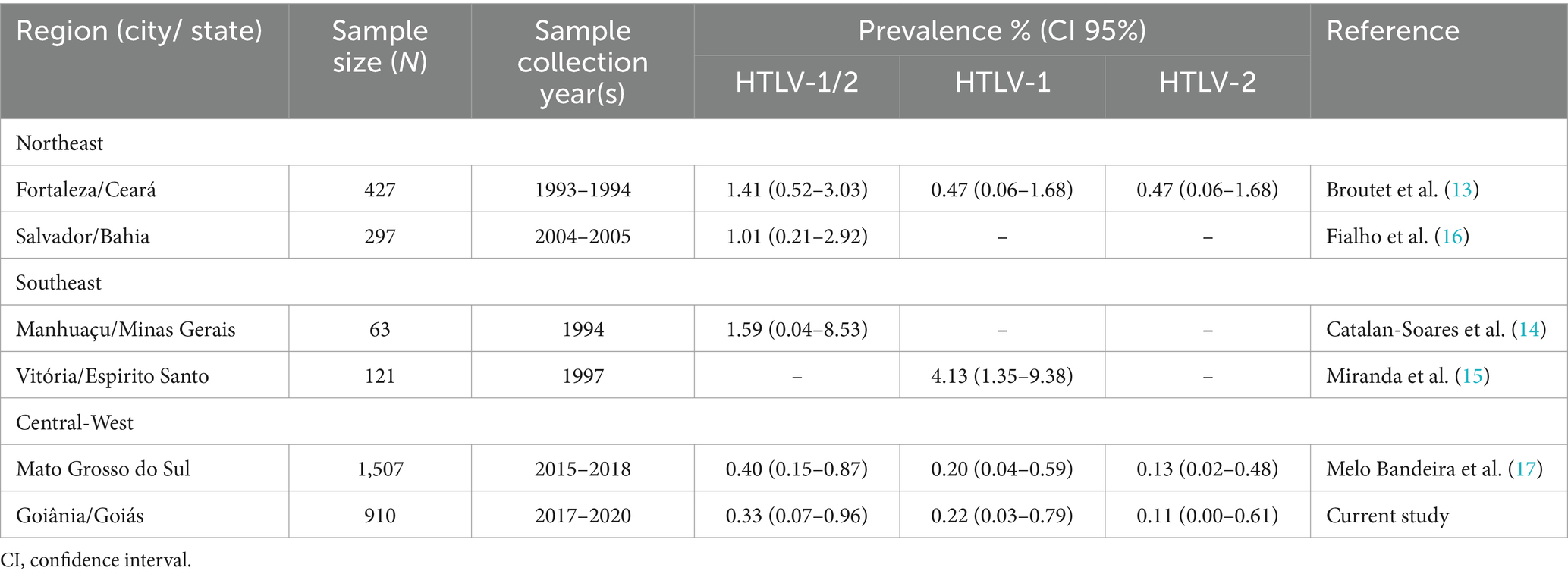
Table 4. Prevalence rates of HTLV-1/2, HTLV-1 and HTLV-2 infections among prisoners in Brazil.
Internationally, the HTLV-1/2 seroprevalence in this study was lower than those reported among specific groups of prisoners, such as male inmates in Maryland, USA (0.93%; CI 95%: 0.55–1.47) (23), HIV-infected inmates in Mozambique (1.55%; CI 95%: 0.67–3.04) (24), immigrants in prison in Italy (2.52%; CI 95%: 1.09–4.91) (25), and prisoners in Tijuana, Mexico (7.07%; CI 95%: 4.79–10.00) (26), but a study in a Danish prison reported no cases of HTLV-1/2 infection (27), indicating varied prevalence rates among prisoners globally.
The distribution of HTLV-1/2 infection by gender showed a higher seroprevalence among female prisoners, which was consistent with that reported in Mozambique (24), possibly reflecting the more efficient transmission of virus from male to female during sexual intercourse (28). On the other hand, this finding differs from previous reports in Brazil which found a higher prevalence of HTLV-1/2 in male prisoners, possibly reflecting their high-risk behaviors (16, 17).
In the major penitentiary complex of Goiás State, we detected anti-HTLV-1 (0.22%) and anti-HTLV-2 (0.11%). This finding aligns with previous research in Fortaleza, Ceará, where anti-HTLV-1 (0.47%) and HTLV-2 (0.47%) were found among prisoners (13). More recently, Melo Bandeira et al. (17) also found the presence of HTLV-1 (0.20%) and HTLV-2 (0.13%) in people living in prisons in Mato Grosso do Sul. Relative to other studies on HTLV-1/2 infection in Goiás, HTLV-1 and 2 dual infections were observed only in patients with pulmonary tuberculosis (29), a population among whom one quarter reported previous incarceration.
Both HTLV-1 seropositive prisoners (PLG-440 and PLG-611) reported unprotected sex with multiple partners and history of STIs. These risky sexual behaviors may contribute to the observed seropositivity for this infection among the studied population since unprotected sexual intercourse and a higher number of partners increase the risk of HTLV-1 transmission (1, 28, 30). In addition, one of these individuals (PLG-611) was co-infected with HIV-1. As HTLV-1 and HIV-1 share common transmission routes, co-infection can occur in endemic areas, as reported in other prison populations (17, 24, 25).
Additionally, two seropositive individuals, one with anti-HTLV-1 (PLG-440) and the other with HTLV-2 (PLG-240), reported being breastfed during childhood. Notably, PLG-240 was breastfed for over 6 months by her mother and three other women in Piaui State, a detail that aligns with findings from a previous study on blood donors and their families in Piaui, where long-term breastfeeding during childhood was linked to HTLV-1/2 infection (31). In fact, HTLV-1/2 is transmitted primarily through infected bodily fluids including breast milk (1, 5). As reported elsewhere (32–34), it is important to note cross-breastfeeding as a potential risk to transmit HTLV-1/2 infection in addition to mother-to-child transmission. Therefore, implementing antenatal screening for HTLV-1/2 and interventions to limit or avoid breastfeeding by women living with HTLV-1/2 are essential to prevent the spread of this infection in endemic regions.
This study has some limitations. First, all interviews were performed face-to-face; therefore, some findings are subject to response biases, especially those regarding sexual behaviors/practices, illicit drug use, etc. Otherwise, some strategies were used to minimize potential biases, including previously trained interviewers and a private place for interviews in each unit of the Prison Complex of Aparecida de Goiânia. Also, the exclusion of individuals who appeared to be under the influence of psychotropic drugs from the study may bias the results potentially underestimating the prevalence; however, only a few prisoners were excluded considering this criterion. Due to health restrictions imposed by the COVID-19 pandemic, it was not possible to collect another blood sample from the three anti-HTLV-1/2 positive individuals for HTLV proviral DNA detection, and ultimately the seropositive individuals were transferred to other cities. Despite these limitations, this study provides valuable epidemiological information on HTLV-1/2 in an incarcerated population in Central-West Brazil.
ConclusionThis study revealed a relatively low seroprevalence of HTLV-1/2 infection in the major penitentiary complex of Goiás State, Central-West Brazil. Importantly, it also indicated the presence of HTLV-1 and HTLV-2 in this setting. The prevalence of high-risk behaviors, particularly sexual behaviors, and the interactions of incarcerated individuals with the external community, underscore the critical need for targeted programs in prisons. These programs should focus on the diagnosis, control, and prevention of HTLV-1/2 and other STIs.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Research Ethics Committee of the Clinical Hospital, Federal University of Goiás. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributionsMO: Conceptualization, Writing – review & editing, Methodology. MaM: Conceptualization, Methodology, Writing – review & editing. NF: Methodology, Writing – review & editing, Formal analysis. ÁS: Methodology, Writing – review & editing. JM: Methodology, Writing – review & editing. TM: Methodology, Writing – review & editing. MáM: Writing – review & editing, Conceptualization, Writing – original draft. RM: Conceptualization, Writing – original draft, Writing – review & editing, Supervision.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the National Council on Scientific and Technological Development/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-public call Universal 01/2016 process 408492/2016-3), and Foundation for Research Support in Goias/Fundação de Amparo à Pesquisa do Estado de Goiás (FAPEG-public call 03/2015).
AcknowledgmentsThe authors thank the prison workers and the members of the Virology Laboratory of the Institute of Tropical Pathology and Public Health for their assistance in carrying out the study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Murphy, EL, Cassar, O, and Gessain, A. Estimating the number of HTLV-2 infected persons in the world. Retrovirology. (2015) 12:O5. doi: 10.1186/1742-4690-12-S1-O5
Crossref Full Text | Google Scholar
3. Ishak, R, de Oliveira Guimarães Ishak, M, Abreu, IN, Machado, LFA, Lima, SS, Queiroz, MAF, et al. Long-term prevalence follow-up (1967-2022) of HTLV-2 among vulnerable indigenous populations in the Amazon region of Brazil. Front Microbiol. (2023) 14:1217134. doi: 10.3389/fmicb.2023.1217134
PubMed Abstract | Crossref Full Text | Google Scholar
4. Blanco, S, Barile, ME, Frutos, MC, Vicente, ACP, and Gallego, SV. Neurodegenerative disease in association with sexual transmission of human T-cell lymphotropic virus type 2 subtype b in Argentina. Trans R Soc Trop Med Hyg. (2022) 116:622–7. doi: 10.1093/trstmh/trab173
PubMed Abstract | Crossref Full Text | Google Scholar
5. Rosadas, C, Menezes, MLB, Galvão-Castro, B, Assone, T, Miranda, AE, Aragón, MG, et al. Blocking HTLV-1/2 silent transmission in Brazil: current public health policies and proposal for additional strategies. PLoS Negl Trop Dis. (2021) 15:e0009717. doi: 10.1371/journal.pntd.0009717
PubMed Abstract | Crossref Full Text | Google Scholar
8. Bartos, MSH. Access to healthcare in Brazilian prisons: why is it important to look at the bureaucracy and policy implementation? Int J Public Health. (2023) 68:1605266. doi: 10.3389/ijph.2023.1605266
PubMed Abstract | Crossref Full Text | Google Scholar
9. Paiva, JPS, Leal, TC, Silva, LFD, Santos, LG, Santana, GBA, Machado, MF, et al. Health in prison: coronavirus disease 2019's challenges in the Brazilian criminal justice system. Rev Assoc Med Bras. (2023) 69:186–90. doi: 10.1590/1806-9282.20210889
PubMed Abstract | Crossref Full Text | Google Scholar
10. Walter, KS, Dos Santos, PCP, Gonçalves TOda Silva, BO, da Silva, SA, de Cássia, LA, et al. The role of prisons in disseminating tuberculosis in Brazil: a genomic epidemiology study. Lancet Reg Health Am. (2022) 9:100186. doi: 10.1016/j.lana.2022.100186
PubMed Abstract | Crossref Full Text | Google Scholar
11. Benedetti, MSG, Nogami, ASA, Costa, BBD, Fonsêca, HIFD, Costa, IDS, Almeida, IS, et al. Sexually transmitted infections in women deprived of liberty in Roraima, Brazil. Rev Saude Publica. (2020) 54:105. doi: 10.11606/s1518-8787.2020054002207
PubMed Abstract | Crossref Full Text | Google Scholar
12. Tanaka, TSO, Cesar, GA, Rezende, GR, Puga, MAM, Weis-Torres, SMDS, Bandeira, LM, et al. Molecular epidemiology of HIV-1 among prisoners in Central Brazil and evidence of transmission clusters. Viruses. (2022) 14:1660. doi: 10.3390/v14081660
PubMed Abstract | Crossref Full Text | Google Scholar
13. Broutet, N, de Queiroz, SA, Basilio, FP, Sá, HL, Simon, F, and Dabis, F. Prevalence of HIV-1, HIV-2 and HTLV antibody, in Fortaleza, Ceara, Brazil, 1993-1994. Int J STD AIDS. (1996) 7:365–9. doi: 10.1258/0956462961918103
PubMed Abstract | Crossref Full Text | Google Scholar
14. Catalan-Soares, BC, Almeida, RT, and Carneiro-Proietti, AB. Prevalence of HIV-1/2, HTLV-I/II, hepatitis B virus (HBV), hepatitis C virus (HCV), Treponema pallidum and Trypanosoma cruzi among prison inmates at Manhuaçu, Minas Gerais state, Brazil. Rev Soc Bras Med Trop. (2000) 33:27–30. doi: 10.1590/S0037-86822000000100004
PubMed Abstract | Crossref Full Text | Google Scholar
15. Miranda, AE, Vargas, PM, St Louis, ME, and Viana, MC. Sexually transmitted diseases among female prisoners in Brazil: prevalence and risk factors. Sex Transm Dis. (2000) 27:491–5. doi: 10.1097/00007435-200010000-00001
Crossref Full Text | Google Scholar
16. Fialho, M, Messias, M, Page-Shafer, K, Farre, L, Schmalb, M, Pedral-Sampaio, D, et al. Prevalence and risk of blood-borne and sexually transmitted viral infections in incarcerated youth in Salvador, Brazil: opportunity and obligation for intervention. AIDS Behav. (2008) 12:S17–24. doi: 10.1007/s10461-008-9409-x
PubMed Abstract | Crossref Full Text | Google Scholar
17. Melo Bandeira, L, Moreira Puga, MA, Croda, J, Pompílio, MA, Amianti, C, Rocha de Rezende, G, et al. Human T-lymphotropic virus-1/2 infection in Central Brazil prisons: a multicenter study. Front Microbiol. (2022) 12:740245. doi: 10.3389/fmicb.2021.740245
PubMed Abstract | Crossref Full Text | Google Scholar
19. Brasil. Ministério da Saúde. Portaria n° 1.376, de 19 de novembro de 1993. Brasília-DF: Diário Oficial da União (1993).
20. Santos, BF, de Santana, NO, and Franca, AV. Prevalence, genotypes and factors associated with HCV infection among prisoners in northeastern Brazil. World J Gastroenterol. (2011) 17:3027–34. doi: 10.3748/wjg.v17.i25.3027
PubMed Abstract | Crossref Full Text | Google Scholar
21. Sousa, KAA, Araújo, TME, Teles, SA, Rangel, EML, and Nery, IS. Factors associated with HIV prevalence in a prison population. Rev Esc Enferm USP. (2017) 51:e03274. doi: 10.1590/S1980-220X2016040903274
PubMed Abstract | Crossref Full Text | Google Scholar
22. Pessoni, LL, Aquino, ÉC, and Alcântara, KC. Prevalence and trends in transfusion-transmissible infections among blood donors in Brazil from 2010 to 2016. Hematol Transfus Cell Ther. (2019) 41:310–5. doi: 10.1016/j.htct.2019.03.009
PubMed Abstract | Crossref Full Text | Google Scholar
23. Vlahov, D, Lee, H, Taylor, E, Canavaggio, M, Canner, C, Burczak, J, et al. Antibody to human T-lymphotropic virus type I/II (HTLV-I/II) among male inmates entering Maryland prisons. J Acquir Immune Defic Syndr. (1988) 3:531–5. doi: 10.1097/00126334-199003050-00010
Crossref Full Text | Google Scholar
24. Augusto, Â, Augusto, O, Taquimo, A, Nhachigule, C, Siyawadya, N, Tembe, N, et al. First description of HTLV-1/2 seroprevalence in HIV-infected inmates in Mozambique. J Med Virol. (2017) 89:1498–502. doi: 10.1002/jmv.24801
PubMed Abstract | Crossref Full Text | Google Scholar
25. Ansaldi, F, Comar, M, D'Agaro, P, Grainfenberghi, S, Caimi, L, Gargiulo, F, et al. Seroprevalence of HTLV-I and HTLV-II infection among immigrants in northern Italy. Eur J Epidemiol. (2003) 18:583–8. doi: 10.1023/a:1024655228893
PubMed Abstract | Crossref Full Text | Google Scholar
26. Guerena-Burgueno, F, Benenson, AS, Sepulveda-Amor, J, Ascher, MS, Vugia, DJ, and Gallo, D. Prevalence of human T cell lymphotropic virus types 1 and 2 (HTLV-1/2) in selected Tijuana subpopulations. Am J Trop Med Hyg. (1992) 47:127–32. doi: 10.4269/ajtmh.1992.47.127
PubMed Abstract | Crossref Full Text | Google Scholar
27. Christensen, PB, Krarup, HB, Niesters, HG, Norder, H, and Georgsen, J. Prevalence and incidence of bloodborne viral infections among Danish prisoners. Eur J Epidemiol. (2000) 16:1043-9. 1. doi: 10.1023/a:1010833917242
PubMed Abstract | Crossref Full Text | Google Scholar
29. Kozlowski, AG, Carneiro, MA, Matos, MA, Teles, SA, Araújo, JA, Otsuki, K, et al. Prevalence and genetic characterization of HTLV-1 and 2 dual infections in patients with pulmonary tuberculosis in central-West Brazil. Mem Inst Oswaldo Cruz. (2014) 109:118–21. doi: 10.1590/0074-0276130230
Crossref Full Text | Google Scholar
30. Rosadas, C, Brites, C, Arakaki-Sanchez, D, Casseb, J, and Ishak, R. Brazilian protocol for sexually transmitted infections 2020: human T-cell lymphotropic virus (HTLV) infection. Rev Soc Bras Med Trop. (2021) 54:e2020605. doi: 10.1590/0037-8682-605-2020
PubMed Abstract | Crossref Full Text | Google Scholar
31. Ribeiro, IP, Kozlowski, AG, Dias de Matos, MA, da Costa, E, Silva, ÁM, Dos Santos Carneiro, MA, et al. HTLV-1 and -2 in a first-time blood donor population in northeastern Brazil: prevalence, molecular characterization, and evidence of intrafamilial transmission. J Med Virol. (2018) 90:1651–7. doi: 10.1002/jmv.25231
PubMed Abstract | Crossref Full Text | Google Scholar
32. Rosadas, C, and Taylor, GP. Current interventions to prevent HTLV-1 mother-to-child transmission and their effectiveness: a systematic review and meta-analysis. Microorganisms. (2022) 10:2227. doi: 10.3390/microorganisms10112227
PubMed Abstract | Crossref Full Text | Google Scholar
33. Abreu, IN, Lima, CNC, Sacuena, ERP, Lopes, FT, da Silva Torres, MK, Santos, BCD, et al. HTLV-1/2 in indigenous peoples of the Brazilian Amazon: Seroprevalence, molecular characterization and sociobehavioral factors related to risk of infection. Viruses. (2022) 15:22. doi: 10.3390/v15010022
PubMed Abstract | Crossref Full Text | Google Scholar
34. Boccolini, CS, Reis, NB, Farias, DR, Berti, TL, Lacerda, EMA, Castro, IRR, et al. Cross-breastfeeding and milk donation in Brazil. Cad Saúde Pública. (2023) 39:e00082322. doi: 10.1590/0102-311XEN082322
留言 (0)