Colorectal cancer (CRC) is one of the most commonly diagnosed cancers in the world. It has one of the highest rates of diagnosis and mortality of all cancer types, and the surgery-based combination therapy remains the treatment of choice for CRC (1, 2). The occurrence of colon cancer in splenic flexure is rare compared to other locations, accounting for merely 2-5% (3). Splenic flexure colon cancer (SFCC) is a tumor in the distal 1/3 of the transverse colon and the proximal 1/3 of the descending colon, which can be supplied with blood by the mid-colic and left colonic vessels due to its special feature of bidirectional blood supply (4, 5). In addition, lymphatic reflux can also flow to the superior and inferior mesenteric vessels. Due to the high degree of vascular variability, surgeons face uncertainty in determining the direction of the innervated vessels and lymphatic return. In addition, there are relatively few studies on the distribution of lymph node metastases and the value of lymph node dissection, with a high incidence of intestinal obstruction and postoperative complications in SFCC, as well as a low long-term survival rate (6). In contrast, colon adenocarcinoma of distal 2/3 descending colon, sigmoid colon and rectosigmoid junction origin, where the main lymphatic reflux is to the inferior mesenteric artery, is usually treated with standard left hemicolectomy or partial sigmoidectomy.
Extended right colectomy (ERC) is a common treatment for SFCC. Ligation of the ileocolic artery, the right colic artery, and the middle colic artery are needed, and depending on the location of the tumor, sometimes the left colon needs to be freed and the left colic artery ligated. Then, lymph nodes are dissected around the vessel, and finally, ileocolic anastomosis is performed. Other methods of treating SFCC include segmental colectomy (SC) and left colectomy (LC). SC requires ligation of the left branch of the middle colic artery and the left colic artery and colon-colon anastomosis of the remaining transverse colon and descending colon. LC requires high ligation of the inferior mesenteric artery and the left branch of the middle colic artery with transverse colon-rectal anastomosis. Because there is no consensus on the treatment of SFCC, the scope of surgical resection and lymph node dissection for splenic flexure carcinoma is still very controversial. This systematic review and meta-analysis focused on the surgical approach and the short- and long-term postoperative outcomes of SFCC.
2 Methods2.1 Search strategyThis meta-analysis was performed according to the PRISMA 2020 (7) and AMSTAR (8) guidelines. Up to March 3, 2024, two independent researchers searched the PubMed, Embase, Scopus, and Cochrane Library databases using the keywords “splenic flexure colon cancer”, “extended right hemicolectomy”, “extended right colectomy”, “segmental colectomy”, “left hemicolectomy”, and “left colectomy” and the search strategy was changed accordingly according to the different database requirements. Relevant literature was also searched from the cited literature of related studies.
2.2 Inclusion and exclusion criteriaStudies were included if they conformed to the principle of PICO (S) [participants, interventions, comparisons, outcomes, (study design)].
Inclusion and exclusion criteria were as follows:
(1). Participants: Patients were diagnosed with colon cancer located at the splenic flexure or between the distal 3/1 of the transverse colon and the proximal 3/1 of the descending colon.
(2). Interventions: ERC, SC or LC performed by either laparoscopic or open approaches.
(3). Comparison: ERC versus SC or ERC versus LC or SC versus LC
(4). Outcome:
● Primary outcome: anastomotic leakage, ileus, wound infection, total postoperative complications, severe postoperative complications, and reoperation.
● Secondary outcomes: lymph node harvest, R0 resection, postoperative mortality, 5-year overall survival (OS), 5-year disease-free survival (DFS), operation time, blood loss, hospital stay, recovery to regular diet, and first flatus.
(5). Exclusion criteria were studies of other surgical techniques unrelated to the extent of SFCC resection and studies in which the extent of SFCC resection was not compared. In addition, review articles, letters, technical notes and books were excluded.
2.3 Data extraction and quality assessmentTo assess the relevance of the retrieved studies, two reviewers independently screened their titles and abstracts, and all disagreements were resolved by discussion with a third reviewer. The following information was extracted from each included study: first author, year of publication, type of publication, count of patients, and patient characteristics (age, gender, body mass index, operation time, blood loss, hospital stay, recovery to regular diet, first flatus, anastomotic leakage, ileus, wound infection, total postoperative complication, severe postoperative complication, request reoperation, lymph node harvest, R0 resection, postoperative mortality, 5-year OS and 5-year DFS). To enhance sensitivity, certain reports were eliminated only if both reviewers eliminated specific reports. Later, the whole text within each study was examined, and these studies were included in this study during the screening process. The Newcastle−Ottawa Scale was used to ascertain the risk of bias, and reports achieving five or more stars were eligible (9). Randomized controlled trials used a tool made by the Cochrane Collaboration to examine the risk of bias (10).
2.4 Statistical analysisSTATA 17.0 was used to perform the statistical analysis. We used weighted mean differences (WMDs) and 95% confidence intervals (CIs) for continuous variables, whereas odds ratios (ORs) and 95% CIs were used for dichotomous variables. The random-effects model was implemented in this study to explore the statistical heterogeneity (I2>50% or P < 0.10). A fixed-effects model was implemented for further statistical analysis. Funnel plots and Egger’s test were performed to examine publication bias.
2.5 Information on the included studiesThe four databases yielded a total of 239 relevant studies; 3 studies identified through references list of the relevant articles. 35 studies were eliminated due to duplication, whereas the remaining literature was assessed by full-text assessment, and 13 studies were included. Among these, 13 were retrospective studies (6, 11–22) (Figure 1). In total, 5,918 splenic flexure cancer (SFC) patients were included, with 527 SFC patients in the ERC group, 1571 SFC patients in the SC group, and 3,820 SFC patients in the LC group. Table 1 depicts the basic information about the patients and describes the Newcastle−Ottawa scale is shown in Table 2.
Figure 1 CONSORT flowchart.
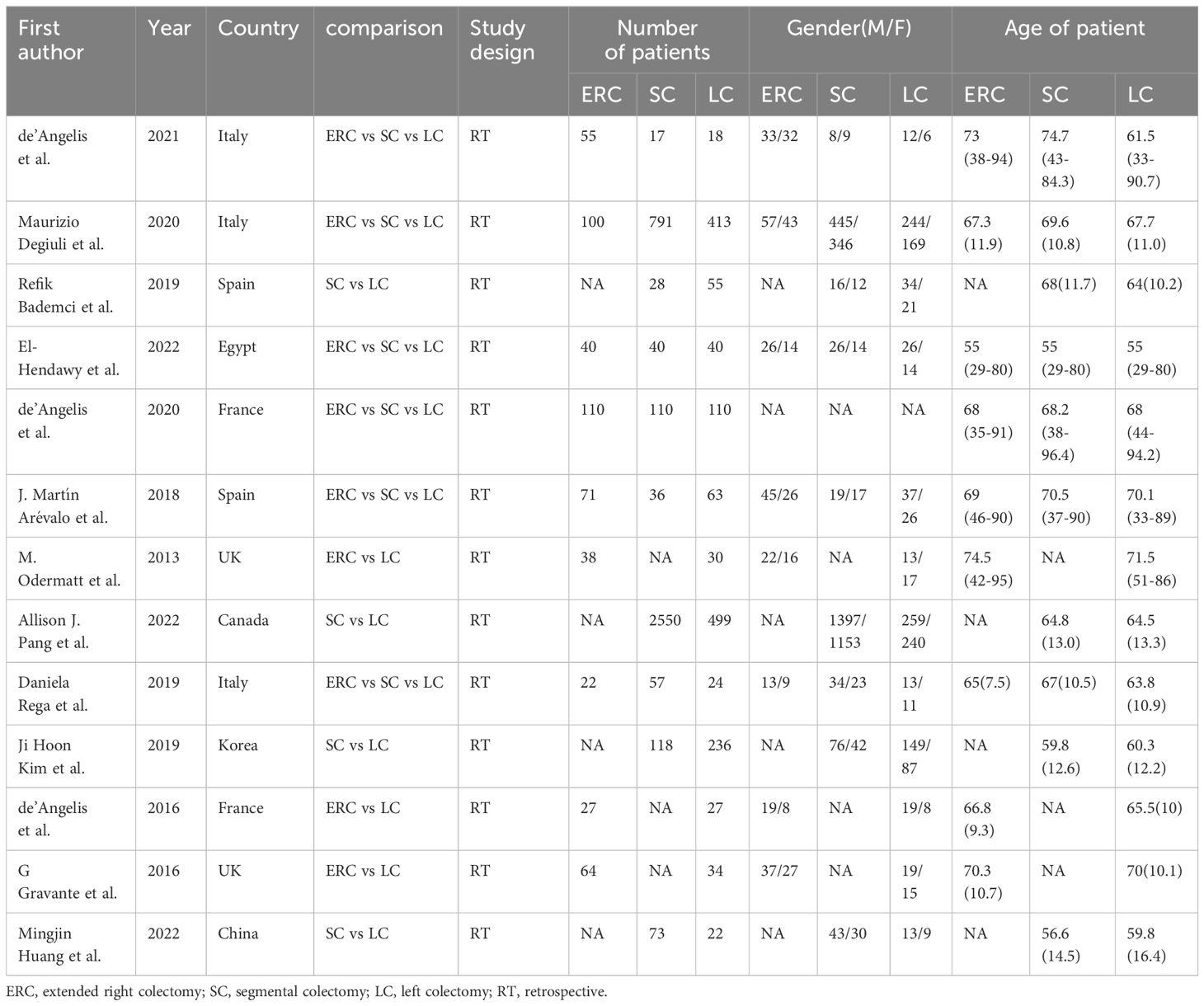
Table 1 The characteristic of included studies.
Table 2 Newcastle−Ottawa scale of included studies.
3 Results3.1 ERC versus SC3.1.1 ComplicationsAnastomotic leakage
Four studies with a total of 1266 patients compared anastomotic leakage between ERC and SC, with no significant differences between the two groups (OR: 0.91; 95% Cl: 0.47-1.78; Z=0.27, P=0.78), and no heterogeneity was observed; therefore, a random effects model was used (I2 = 0, P=0.64) (Figure 2.1A).
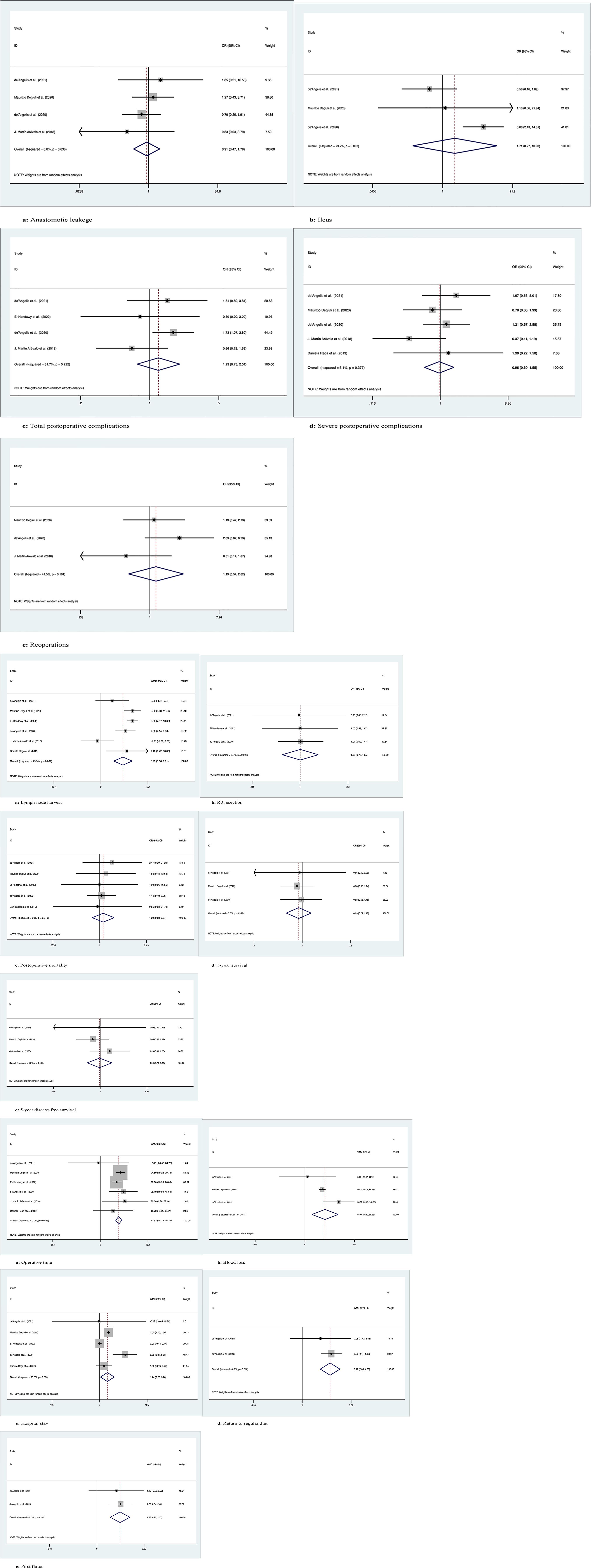
Figure 2 Meta-analytic graphs of extended right colectomy versus segmental colectomy. 1. (A–E) Complications; 2. (A–E) Pathology and long-term survival; 3. (A–E) Outcomes.
Ileus
The occurrence of postoperative ileus was recorded in a total of 1183 patients in 3 studies, with no significant difference between the two groups (OR: 1.71; 95% Cl: 0.27-10.98; Z=0.57, P=0.57), and high heterogeneity was observed using a random effects model (I2 = 79.7%, P=0.007) (Figure 2.1B).
Total postoperative complications
A total of 455 patients in four studies recorded the occurrence of total postoperative complications, with no significant difference between the two groups (OR: 1.23; 95% Cl: 0.75-2.01; Z=0.81, P=0.42), and low heterogeneity was observed, thus using a random effects model (I2 = 31.7%, P=0.22) (Figure 2.1C).
Severe postoperative complications
Five studies with a total of 1345 patients recorded the occurrence of serious postoperative complications (Clavien−Dindo grade ≥3), with no significant difference between the two groups (OR: 0.96; 95% Cl: 0.60-1.55; Z=0.16, P=0.88), due to the low heterogeneity observed, using a random effects model (I2 = 5.1%, P=0.38) (Figure 2.1D).
Reoperations
A total of 1218 patients in three studies were documented as requiring secondary surgery, with no significant difference between the two groups (OR: 1.19; 95% Cl: 0.54-2.62; Z=0.44, P=0.66), due to the low heterogeneity observed, using a random effects model (I2 = 41.5%, P=0.18) (Figure 2.1E).
3.1.2 Pathology and long-term survivalLymph node harvest
The number of lymph nodes harvested was recorded in a total of 1425 patients in six studies, with more lymph nodes harvested by ERC than by SC (OR: 6.29; 95% CI: 3.66-8.91; Z=4.69, P=0), and high heterogeneity was observed using a random effects model (I2 = 75.5%, P=0.001) (Figure 2.2A).
R0 resection
R0 resection was recorded in a total of 372 patients in the three studies, and there was no significant difference between the two groups (OR: 1.003; 95% CI: 0.75-1.35; Z=0.02, P=0.99). No heterogeneity was observed using a random effects model (I2 = 0, P=0.10) (Figure 2.2B).
Postoperative mortality
Postoperative mortality was recorded in a total of 1342 patients in five studies, with no significant difference between the two groups (OR: 1.29; 95% CI: 0.58-2.88; Z=0.63, P=0.53) and no heterogeneity observed using a random effects model (I2 = 0, P=0.97) (Figure 2.2C).
5-year overall survival
Three studies followed 1183 patients over time and recorded 5-year OS with no significant difference between the two groups (OR: 0.93; 95% CI: 0.74-1.18; Z=0.57, P=0.57), and no heterogeneity was observed using a random effects model (I2 = 0, P=0.95) (Figure 2.2D).
5-year disease-free survival
Three studies followed 1183 patients over time and recorded 5-year disease-free survival with no significant difference between the two groups (OR: 0.99; 95% Cl: 0.78-1.25; Z=0.12, P=0.91), and no heterogeneity was observed using a random effects model (I2 = 0, P=0.44) (Figure 2.2E).
3.1.3 OutcomesOperation time
Six studies recorded operative times in 1425 patients, with longer operative times for ERC than for SC (WMD: 22.53; 95% Cl: 18.75-26.31; Z=11.68, P=0), with no heterogeneity observed, using a random effects model (I2 = 0, P=0.57) (Figure 2.3A).
Blood loss
Intraoperative blood loss was recorded in 1183 patients in three studies, and more blood loss was observed with ERC than with SC (WMD: 58.44; 95% CI: 20.20-96.68; Z=2.99, P=0.003), with high heterogeneity observed using a random effects model (I2 = 61.2%, P=0.08) (Figure 2.3B).
Hospital stay
Five studies documented the length of postoperative hospital stay in 1342 patients, which was longer for ERC than for SC (WMD: 1.74; 95% CI: 0.20-3.28; Z=2.21, P=0.03), with high heterogeneity observed using a random effects model (I2 = 93.8%, P=0) (Figure 2.3C).
Return to regular diet
Two studies recorded the time to return to regular diet after surgery in 292 patients, with longer recovery times for ERH than for SC (WMD: 3.17; 95% CI: 2.05-4.30; Z=5.53, P=0), with no heterogeneity observed, using a random effects model (I2 = 0, P=0.52) (Figure 2.3D).
First flatus
Two studies recorded the time to postoperative first flatus in 292 patients, and the time to first flatus was longer for ERC than for SC (WMD: 1.66; 95% CI: 0.96-2.37; Z=4.61, P=0), with no heterogeneity observed, using a random effects model (I2 = 0, P=0.78) (Figure 2.3E).
3.2 LC versus SC3.2.1 ComplicationsAnastomotic leakage
Eight studies with a total of 5116 patients compared anastomotic leakage between LC and SC, with no significant differences between the two groups (OR: 0.97; 95% Cl: 0.68-1.38; Z=0.16, P=0.87) and no heterogeneity observed using a random effects model (I2 = 0, P=0.98) (Figure 3.1A).
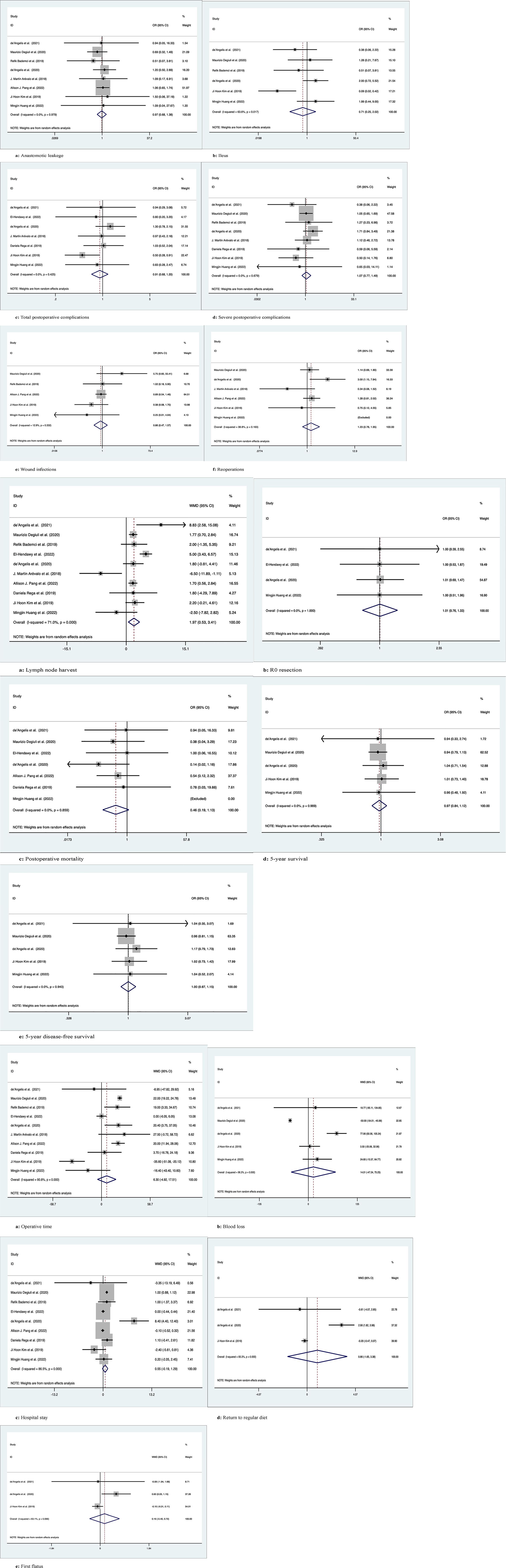
Figure 3 Meta-analytic graphs of left colectomy versus segmental colectomy. 1. (A–F) Complications; 2. (A–E) Pathology and long-term survival; 3. (A–E) Outcomes.
Ileus
A total of 1991 patients in six studies compared the occurrence of postoperative ileus, with no significant difference between the two groups (OR: 0.71; 95% CI: 0.25-2.02; Z=0.65, P=0.52), with high heterogeneity observed using a random effects model (I2 = 63.6%, P=0.02) (Figure 3.1B).
Total postoperative complications
Seven studies compared the occurrence of total postoperative complications in 941 patients, with no significant difference between the two groups (OR: 0.91; 95% CI: 0.68-1.20; Z=0.67, P=0.5), with low heterogeneity observed using a random effects model (I2 = 0, P= 0.43) (Figure 3.1C).
Severe postoperative complications
Eight studies documented severe postoperative complications (Clavien−Dindo grade ≥3) in 2148 patients, with no significant difference between the two groups (OR: 1.07; 95% Cl: 0.77-1.49; Z=0.4, P=0.69) and no heterogeneity observed using a random effects model (I2 = 0, P=0.68) (Figure 3.1D).
Wound infection
Five studies recorded postoperative wound infection in 4785 patients with no significant difference between the two groups (OR: 0.86; 95% CI: 0.47-1.57; Z=0.49, P=0.63) due to the low heterogeneity observed using a random effects model (I2 = 12.9%, P=0.33) (Figure 3.1E).
Reoperations
Six studies documented 5021 patients requiring reoperation after surgery, with no significant difference between the two groups (OR: 1.23; 95% CI: 0.78-1.95; Z=0.88, P=0.38), due to the low heterogeneity observed, using a random effects model (I2 = 38.8%, P=0.16) (Figure 3.1F).
3.2.2 Pathology and long-term survivalLymph node harvest
Ten studies counted the number of lymph nodes harvested in 5277 patients, with more lymph nodes harvested by LC than by SC (WMD: 1.97; 95% CI: 0.53-3.41; Z=2.68, P=0.007), due to the high heterogeneity observed, using a random effects model (I2 = 71%, P=0) (Figure 3.2A).
R0 resection
Four studies recorded R0 resection in 430 patients with no significant difference between the two groups (WMD: 1.01; 95% CI: 0.76-1.33; Z=0.04, P=0.97), and no heterogeneity was observed using a random effects model (I2 = 0, P=1) (Figure 3.2B).
Postoperative mortality
Seven studies recorded postoperative mortality in 4764 patients, with no significant difference between the two groups (WMD: 0.46; 95% CI: 0.19-1.13; Z=1.69, P=0.09) and no heterogeneity observed using a random effects model (I2 = 0, P=0.86) (Figure 3.2C).
5-year overall survival
Five studies followed and recorded 1908 patients for 5-year OS, with no significant difference between the two groups (OR: 0.97; 95% Cl: 0.84-1.12; Z=0.43, P=0.67) and no heterogeneity observed using a random effects model (I2 = 0, P=0.99) (Figure 3.2D).
5-year disease-free survival
Five studies followed and recorded 1908 patients for 5-year DFS, with no significant difference between the two groups (OR: 1.00; 95% Cl: 0.87-1.15; Z=0.03, P=0.98) and no heterogeneity observed using a random effects model (I2 = 0, P=0.94) (Figure 3.2E).
3.2.3 OutcomesOperation time
Ten studies counted operation time in 5277 patients, with no difference between the two groups (WMD: 6.30; 95% CI: -4.92-17.51; Z=1.1, P=0.27), and high heterogeneity was observed using a random effects model (I2 = 90.8%, P=0) (Figure 3.3A).
Blood loss
Five studies counted intraoperative blood loss in 1908 patients, with no significant difference between the two groups (WMD: 14.01; 95% CI: -47.24-75.25; Z=0.45, P=0.65), and high heterogeneity was observed using a random effects model (I2 = 96.3%, P=0) (Figure 3.3B).
Hospital stay
Nine studies recorded 5201 patients with no significant difference in postoperative hospital stay between the two groups (WMD: 0.55; 95% CI: -0.19-1.30; Z=1.45, P=0.15), with high heterogeneity observed using a random effects model (I2 = 86.5%, P=0) (Figure 3.3C).
Return to regular diet
Three studies recorded 609 patients who returned to a regular diet after surgery, with no significant difference between the two groups (WMD: 0.86; 95% CI: -1.65-3.38; Z=0.67, P=0.50), with high heterogeneity observed using a random effects model (I2 = 93.3%, P=0) (Figure 3.3D).
First flatus
Three studies recorded 609 patients with first postoperative flatus, with no significant difference between the two groups (WMD: 0.16; 95% CI: -0.40-0.72; z=0.57, p=0.57), with high heterogeneity observed using a random effects model (I2 = 63.1%, p=0.07) (Figure 3.3E).
3.3 ERC versus LC3.3.1 ComplicationsAnastomotic leakage
Seven studies recorded 1145 patients with postoperative anastomotic leakage, with no significant difference between the two groups (OR: 1.21; 95% Cl: 0.68-2.15; Z=0.66, P=0.51) and no heterogeneity observed using a random effects model (I2 = 0, P=0.67) (Figure 4.1A).
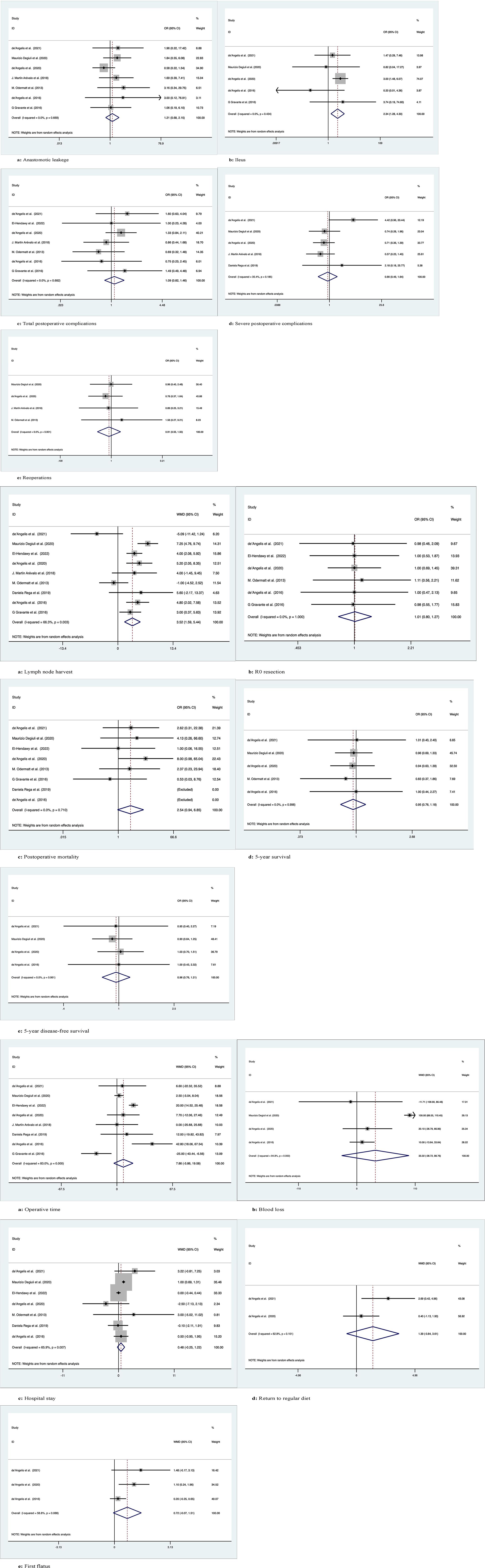
Figure 4 Meta-analytic graphs of extended right colectomy versus left colectomy. 1. (A–E) Complications; 2. (A–E) Pathology and long-term survival; 3. (A–E) Outcomes.
Ileus
Five studies recorded postoperative ileus in 958 patients, with more patients suffering from postoperative ileus with ERC than with LC (OR: 2.34; 95% CI: 1.28-4.30; Z=2.75, P=0.006), with no heterogeneity observed using a random effects model (I2 = 0, P=0.43) (Figure 4.1B).
Total postoperative complications
Seven studies recorded the occurrence of total postoperative complications in 712 patients, with no significant differences between the two groups (OR: 1.09; 95% CI: 0.82-1.46; Z=0.60, P=0.55) and no heterogeneity observed using a random effects model (I2 = 0, P=0.68) (Figure 4.1C).
Severe postoperative complications
Five studies documented the occurrence of severe postoperative complications (Clavien−Dindo grade ≥3) in 971 patients, with no significant difference between the two groups (OR: 0.90; 95% Cl: 0.49-1.64; Z=0.35, P=0.73), with low heterogeneity observed using a random effects model (I2 = 35.4%, P=0.19) (Figure 4.1D).
Reoperations
Four studies documented requiring reoperation after surgery in 935 patients, with no significant difference between the two groups (OR: 0.91; 95% CI: 0.55-1.50; Z=0.38, P=0.70) and no heterogeneity observed using a random effects model (I2 = 0, P=0.9) (Figure 4.1E).
3.3.2 Pathology and long-term survivalLymph node harvest
Nine studies recorded the number of lymph nodes harvested in 1271 patients, with more lymph nodes harvested for ERC than for LC (WMD: 3.52; 95% CI: 1.59-5.44; Z=3.58, P=0), with high heterogeneity observed using a random effects model (I2 = 66.3%, P=0.003) (Figure 4.2A).
R0 resection
Six studies recorded R0 resection in 593 patients with no significant difference between the two groups (WMD: 1.01; 95% CI: 0.80-1.27; Z=0.07, P=0.95), and no heterogeneity was observed using a random effects model (I2 = 0, P=1) (Figure 4.2B).
Postoperative mortality
Eight studies recorded postoperative mortality in 1152 patients, no significant difference between two groups of patients (OR: 2.54; 95% Cl: 0.94-6.85; Z=1.84, P=0.07), with no observed heterogeneity, using a random effects model (I2 = 0, P= 0.71) (Figure 4.2C).
5-year OS
Five studies followed and recorded 5-year OS in 928 patients over time, with no significant differences between the two groups (OR: 0.95; 95% Cl: 0.76-1.18; Z=0.47, P=0.64) and no heterogeneity observed using a random effects model (I2 = 0, P=1.0) (Figure 4.2D).
5-year DFS
Four studies followed and recorded 5-year DFS in 860 patients over time, with no significant differences between the two groups (OR: 0.96; 95% Cl: 0.76-1.21; Z=0.38, P=0.71) and no heterogeneity observed using a random-effects model (I2 = 0, P=0.96) (Figure 4.2E).
3.3.3 OutcomesOperation time
Eight studies recorded operation time in 1203 patients, with no significant difference between the two groups (WMD: 7.86; 95% CI: -3.86-19.58; Z=1.31, P=0.19), with high heterogeneity observed using a random effects model (I2 = 83%, P=0) (Figure 4.3A).
Blood loss
Four studies recorded intraoperative blood loss in 860 patients with no significant difference between the two groups (WMD: 35.02; 95% CI: -28.72-98.76; Z=1.08, P=0.28), with high heterogeneity observed using a random effects model (I2 = 94.8%, P=0) (Figure 4.3B).
Hospital stay
Seven studies documented the length of stay after surgery in 1054 patients, with no significant difference between the two groups (WMD: 0.48; 95% CI: -0.25-1.22; Z=1.30, P=0.19), with high heterogeneity observed using a random effects model (I2 = 65.9%, P=0.007) (Figure 4.3C).
Return to regular diet
Two studies recorded the time to return to regular diet after surgery in 293 patients, with no significant difference between the two groups (WMD: 1.39; 95% CI: -0.84-3.61; Z=1.22, P=0.22), with high heterogeneity observed using a random effects model (I2 = 62.8%, P=0.10) (Figure 4.3D).
First flatus
Three studies documented first postoperative flatus in 347 patients, no significant difference between two groups of patients (WMD: 0.72; 95% Cl: -0.07-1.51; Z=1.79, P=0.07), with high heterogeneity observed, using a random effects model (I2 = 58.8%, P=0.09) (Figure 4.3E).
The concise overview of the comparative outcomes after ERC, SC and LC are presented in Table 3.
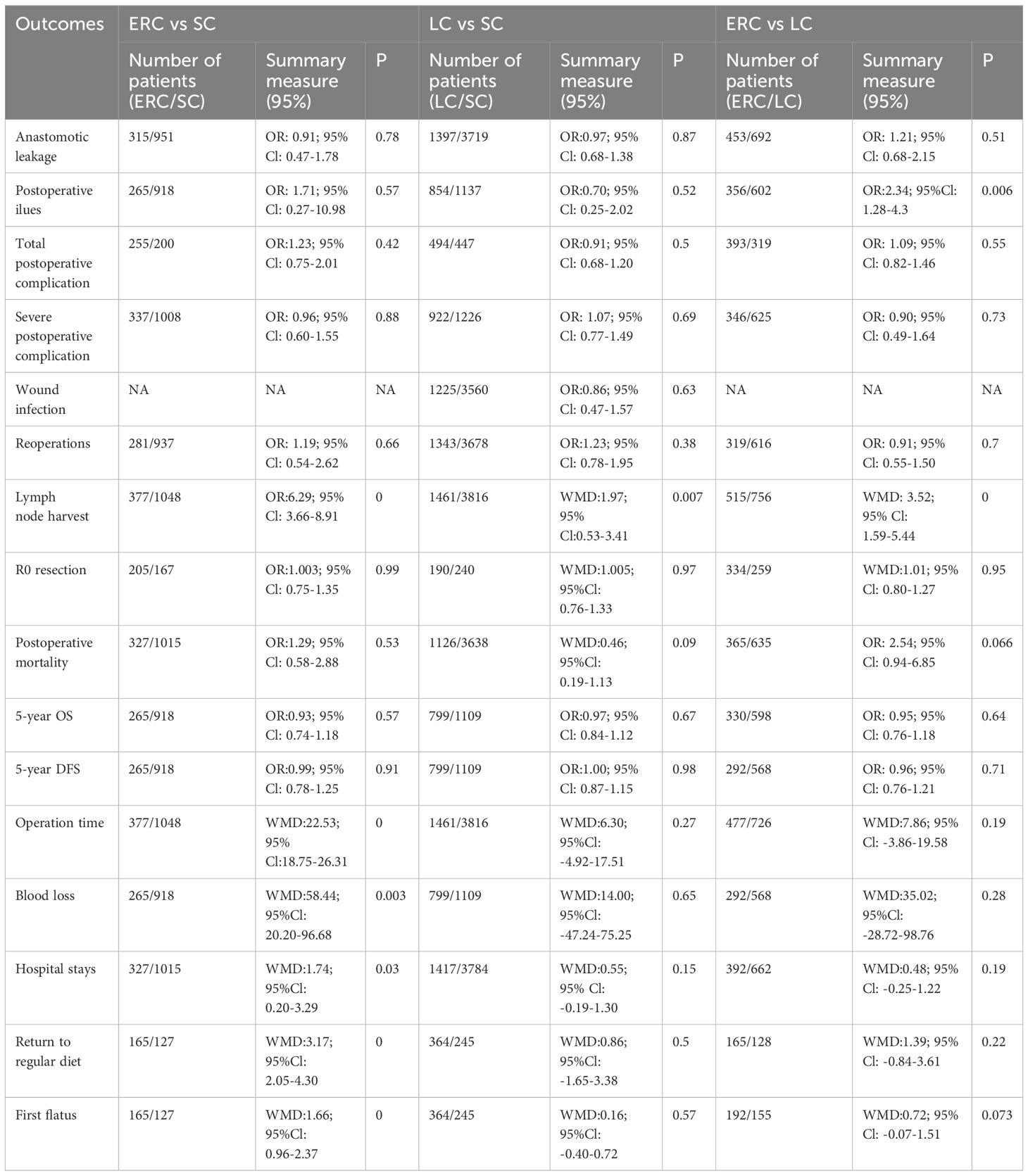
Table 3 Comparison of outcomes between ERC, SC and LC pooled results.
4 DiscussionWe performed a systematic review and meta-analysis after screening 13 articles with a total of 5918 patients to compare postoperative complications and long-term survival between ERC, SC, and LC. According to the results we observed, it is explainable that there is currently no consensus among the three surgical approaches to treat SFCC, as there are no significant differences in critical indicators such as total postoperative complications, severe postoperative complications, anastomotic leakage, 5-year OS and 5-year DFS rates. For the requirement of reoperation and R0 resection, a lack of significant differences among the three groups was also observed.
Compared with ERC, less surgical manipulation might be the evidence of shorter operative time, less intraoperative blood loss, shorter hospital stays, faster return to regular diet, and shorter time to first flatus for SC. ERC is associated with a higher occurrence of postoperative ileus than LC, higher postoperative mortality, and shorter time to first flatus after surgery. In terms of lymph node harvest, the greater number of lymph nodes retrieved in ERC is reasonable, as a larger extent of surgical resection is performed in case of ERC than in case of LC and SC, while the greater number of vessels that required ligation for ERC is also considered one of the possible reasons. However, the number of lymph nodes harvested in LC was greater than that harvested in SC, which might be speculated to be because the tumor location and the level of invasion differ and lymph node distribution varies between individuals. As we understand, the bidirectional blood supply of the splenic flexure colon from the middle colic artery (MCA) and left colic artery (LCA) is complicated. Nearly 30% of patients also have the accessory MCA derived from the celiac trunk (23, 24). According to a study conducted by Fukuoka et al. (24), the blood supply of the splenic flexure colon is highly individualized and is thereby subdivided into 6 categories, among which the LCA is the most prominent artery to supply the splenic flexure colon. Surprisingly, there was also no blood supply to the splenic flexure colon in nearly 25% of patients. As a result, the subsequent treatment plan based on the unique blood supply should be taken into consideration. Despite the lack of relevant literature resources, we may boldly suspect that the oncological outcome is also correlated with individual anatomical variations. However, the statistical support is inadequate, therefore, further detailed studies should be carried out to facilitate the optimal surgical technique selection by surgeons, which is also in the scope of personalized medicine (25).
The 5-year survival rate for lymph node-negative patients is 70%-80% compared to 30%-60% for lymph node-positive patients (26). Hence, a precise surgical tactic of blood vessel ligation and lymph node management should be taken into consideration to minimize the occurrence of hematological and lymphatic dissemination. It has been suggested to use indocyanine green (ICG) to navigate lymph node dissection. Meanwhile, near-infrared imaging (NIR) for clear vessel visualization may also contribute to precise vessel ligation (23).
Postoperative anastomotic leakage remains a serious complication that is more commonly seen during emergency operations than during elective operations. Logically, post ileo-colonic anastomosis leakage tends to be prominent due to the difference in the diameter of the colon and ileum (27, 28). However, it is interesting to determine that the three groups of anastomoses did not show any significant differences. Different positions of the tumor and the experience of the surgeon might lead to such outcomes, but further consideration and evaluation are still of clinical value. However, the bulk of the researchers did not include emergency scenarios in their data analysis despite the fact that ERC is the preferred procedure in an emergency setting with intestinal obstruction (29). In our study, only 287 cases among 5918 cases underwent emergency operations, making up only 4.85% of the total cases. The preoperatively extensive assessment of surgical technique selection is insufficient for emergency surgery when compared to the elective surgery of SFC. As a result, it can have an impact on the primary outcome and lead to complications, which might ultimately result in bias. The thorough exploration and examination of the three independent surgical procedures based on the emergency condition, however, requires high-quality and satisfactory prospective studies and randomized clinical trials (RCTs) due to the dearth of RCTs specifically focused on the three approaches during emergency surgery.
In addition to the 5-year survival rate as the most important indicator, lymph node harvest and anastomosis leakage with detailed anastomosis type selection should also be deeply studied as the primary endpoints. Developing novel surgical approaches for facilitating surgical resection and enhancing long-term survival is still meaningful. Based on our study, the advantages and shortcomings of the three surgical techniques are still under-investigated. The determination of any one of them for surgical intervention of splenic flexure colon cancer seems reasonable, and consensus has still not been reached. Because limited amounts and qualities of samples are included in our study, the conclusion we made is still robust and well documented. Currently, the observational studies available are not sufficient to provide more encouraging findings, thus additional clinical trials incorporating a meticulous comparison of ERC, SC, and LC with solid statistical support are anticipated.
Moreover, type 2 error, publication bias and reporting bias could not be avoided, as the sample sizes were inadequate, which requires further estimation.
5 ConclusionIn observational studies, SC appeared to have a more favorable outcome intraoperatively and postoperatively, but ERC harvested more lymph nodes. There was no obvious difference among the three surgical modalities in long-term survival, and considering that the included were all retrospective studies, more randomized controlled studies are needed to confirm this conclusion.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributionsYC, MH, KC, ZL, DK, TK, XC, PP, SE, AK conceptualized the study. YC, MH, KC, and ZL performed the literature analysis and wrote different parts of the original manuscript draft. DK, TK, and XC Processed data. PP, SE, and AK revised, edited, and extended the final draft. All authors have reviewed and approved the manuscript before submission.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19(3):329–59. doi: 10.6004/jnccn.2021.0012
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Vogel JD, Felder SI, Bhama AR, Hawkins AT, Langenfeld SJ, Shaffer VO, et al. The american society of colon and rectal surgeons clinical practice guidelines for the management of colon cancer. Dis Colon Rectum. (2022) 65:148–77. doi: 10.1097/DCR.0000000000002323
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Nakagoe T, Sawa T, Tsuji T, Jibiki M, Nanashima A, Yamaguchi H, et al. Carcinoma of the splenic flexure: multivariate analysis of predictive factors for clinicopathological characteristics and outcome after surgery. J Gastroenterol. (2000) 35:528–35. doi: 10.1007/s005350070076
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Murono K, Miyake H, Hojo D, Nozawa H, Kawai K, Hata K, et al. Vascular anatomy of the splenic flexure, focusing on the accessory middle colic artery and vein. Colorectal Dis. (2020) 22:392–8. doi: 10.1111/codi.14886
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Andersen BT, Stimec BV, Edwin B, Kazaryan AM, Maziarz PJ, Ignjatovic D. ReiRe-interpreting mesenteric vascular anatomy on 3D virtual and/or physical models: positioning the middle colic artery bifurcation and its relevance to surgeons operating colon cancer. Surg Endosc. (2022) 36:100–8. doi: 10.1007/s00464-020-08242-8
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Pang AJ, Marinescu D, Morin N, Vasilevsky CA, Boutros M. Segmental resection of splenic flexure colon cancers provides an adequate lymph node harvest and is a safe operative approach - an analysis of the ACS-NSQIP database. Surg Endosc. (2022) 36:5652–9. doi: 10.1007/s00464-021-08926-9
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10:1–11. doi: 10.1136/bmj.n71
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (2000).
10. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley & Sons (2019).
11. de'Angelis N, Espin E, Ris F, Landi F, Le Roy B, Coccolini F, et al. Emergency surgery for splenic flexure cancer: results of the SFC Study Group database. World J Emerg Surg. (2021) 16(1):20. doi: 10.1186/s13017-021-00365-0
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Degiuli M, Reddavid R, Ricceri F, Di Candido F, Ortenzi M, Elmore U, et al. Segmental colonic resection is a safe and effective treatment option for colon cancer of the splenic flexure: A nationwide retrospective study of the italian society of surgical oncology-colorectal cancer network collaborative group. Dis Colon Rectum. (2020) 63(10):1372–82. doi: 10.1097/DCR.0000000000001743
PubMed Abstract | CrossRef Full Text | Google Scholar
13. Bademci R, Bollo J, Martinez Sanchez C, Hernadez P, Targarona EM. Is segmental colon resection an alternative treatment for splenic flexure cancer? J Laparoendosc Adv Surg Tech A. (2019) 29:621–6. doi: 10.1089/lap.2019.0041
PubMed Abstract | CrossRef Full Text | Google Scholar
14. El-Hendawy EI, Amin MF, Fahmy AM, Alattar AZ, Elshorbagy S, Harb OA. A comparison between different management surgical approaches in the treatment of splenic flexure colon cancer. J Coloproctology. (2022) 42:047–53. doi: 10.1055/s-0041-1740201
CrossRef Full Text | Google Scholar
15. de’Angelis N, Martínez-Pérez A, Winter DC, Landi F, Vitali GC, Le Roy B, et al. Extended right colectomy, left colectomy, or segmental left colectomy for splenic flexure carcinomas: a European multicenter propensity score matching analysis. Surg Endosc. (2021) 35:661–72. doi: 10.1007/s00464-020-07431-9
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Martín Arévalo J, Moro-Valdezate D, García-Botello SA, Pla-Martí V, Garcés-Albir M, Pérez Santiago L, et al. Propensity score analysis of postoperative and oncological outcomes after surgical treatment for splenic flexure colon cancer. Int J Colorectal Dis. (2018) 33:1201–13. doi: 10.1007/s00384-018-3063-1
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Odermatt M, Siddiqi N, Johns R, Miskovic D, Khan O, Khan J, et al. Short- and long-term outcomes for patients with splenic flexure tumours treated by left versus extended right colectomy are comparable: a retrospective analysis. Surg Today. (2014) 44:2045–51. doi: 10.1007/s00595-013-0803-2
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Rega D, Pace U, Scala D, Chiodini P, Granata V, Fares Bucci A, et al. Treatment of splenic flexure colon cancer: a comparison of three different surgical procedures: Experience of a high volume cancer center. Sci Rep. (2019) 9:10953. doi: 10.1038/s41598-019-47548-z
PubMed Abstract | CrossRef Full Text | Google Scholar
19. Kim MJ, Kim JH, Lee YS, Kye BH, Cho HM, Kim HJ, et al. Short- and long-term outcomes of laparoscopic segmental left colectomy for splenic flexure colon cancer: comparison with propensity score matching. Ann Surg Treat Res. (2021) 101:274–80. doi: 10.4174/astr.2021.101.5.274
PubMed Abstract | CrossRef Full Text | Google Scholar
20. de'Angelis N, Hain E, Disabato M, Cordun C, Carra MC, Azoulay D, et al. Laparoscopic extended right colectomy versus laparoscopic left colectomy for carcinoma of the splenic flexure: a matched case-control study. Int J Colorectal Dis. (2016) 31:623–30. doi: 10.1007/s00384-015-2469-2
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Gravante G, Elshaer M, Parker R, Mogekwu AC, Drake B, Aboelkassem A, et al. Extended right hemicolectomy and left hemicolectomy for colorectal cancers between the distal transverse and proximal descending colon. Ann R Coll Surg Engl. (2016) 98:303–7. doi: 10.1308/rcsann.2016.0112
留言 (0)