Between 2008 and 2018, the number of patients with cancer increased by >25% globally (1, 2), and excisional surgery remains one of the main treatments for solid organ tumours in cancer patients (3). Particularly, colorectal cancer is the fourth deadliest cancer worldwide, with approximately 900000 cases of mortality yearly (4). Surgery is the cornerstone of many treatment options for colorectal cancer. Although it is usually aimed at healing, the removal of tumours is also a risk factor for metastasis. Tumour cells can enter the bloodstream before, during or after surgery, leading to distant organ metastasis (5). The mechanism of metastasis includes carcinomas escaping the immune system, proliferating and invading tissues. Surgery creates a tumorigenic physiological environment that may directly or indirectly affect tumour cell survival.
Multiple perioperative factors collectively contribute to a relatively immunosuppressive state, including surgical stress response and surgical inflammatory response, as well as the direct effects of aneasthetics, opioids and other perioperative drugs. Research has shown that volatile anaesthetics used in inhalation anaesthesia (IA) promote tumour metastasis, which may include direct promotion of carcinoma survival, inhibition of immune cell function and tumour cell-killing function (6–9). Propofol used in total intravenous anaesthesia (TIVA) is the most commonly used intravenous inducer, and some preclinical evidence suggests that it may have anti-tumour effects. Propofol exerts anti-tumour effects by directly regulating key ribonucleic acid pathways and signal transduction in carcinomas (10). It also has anti-inflammatory and anti-oxidant effects (11–16), preventing immune suppression during the perioperative period.
The impact of TIVA and IA on the prognosis of patients with colorectal cancer has always been controversial. Previous research results showed inconsistent trends. A retrospective analysis showed that volatile anaesthetics slightly increased the cancer recurrence rate in patients undergoing colorectal cancer surgery compared with TIVA using propofol (17). Another study (18) showed that there was no difference in overall survival (OS) or recurrence-free survival (RFS) between the two anaesthesia methods for colorectal cancer.
Based on the above controversy, this study aimed to explore the impact of TIVA and IA on the prognosis of patients with colorectal cancer after resection through meta-analysis.
MethodsProtocol and guidanceThis study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses reporting guidelines (19). This study did not require ethical approval or informed consent. The protocol for this review has been registered with PROSPERO (CRD42023453185).
Search strategyWe searched PubMed, EMBASE and the Cochrane Library electronic databases for research written in English from its establishment until 18 November 2023, with keywords including (‘Colorectal Cancer’, ‘colon’ or ‘rectal’), (‘analgesia’, ‘Anesthesia’, ‘Inhalation’ or ‘intravenous’) and (‘Desflurane’, ‘Propofol’, ‘dexmedetomidine’ or ‘Sevoflurane’). Other studies were searched for by reviewing reference lists and qualified publications of potential qualified studies. All searches were conducted independently by two authors, and differences were discussed after the search process.
Inclusion and exclusion criteriaIf the retrieved studies (1) were cohort studies, (2) investigated patients with colorectal cancer, (3) compared clinical studies on long-term all-cause mortality and recurrence after TIVA or IA and (4) provided hazard ratios (HRs) or risk ratios and their 95% confidence intervals (CIs), they were eligible for qualitative and quantitative analyses.
If study participants (1) had malignant tumours other than colorectal cancer and (2) lacked measurements of cancer recurrence or mortality, the study was excluded.
Data extraction and quality assessmentTwo reviewers independently extracted data from the included studies. This review introduced the following details: the name of the first author, year of publication, country, number of participants, tumour location, research type, intervention measures and main research indicators.
The quality of all selected studies was checked according to the Newcastle–Ottawa cohort study quality assessment scale (20). This semi-quantitative scale uses a star rating system to evaluate the quality of eight items in three fields: selection (four items, one star each), comparability (one item, up to two stars) and exposure (three items, one star each). In this meta-analysis, we classified quality as good (≥7 stars), average (4–6 stars) or poor (<4 stars). Differences between the two reviewers were resolved through discussion with the third reviewer.
Data analysisBased on the effects of TIVA and IA, the results show RFS and OS in patients with cancer. This method is based on the HR obtained from each study with a 95% CI. If HR was unreported, the odds ratio was considered equal to HR. The study selected TIVA rather than IA. If the data included in the study comprised IA rather than TIVA, then it was adjusted (calculate derivative). When there are multiple sets of useful data in the same study, only data from propensity score matching is selected for analysis. Subgroup analysis was performed based on region (Asia and Europe) and tumour location (colon, rectum and colorectum).
Statistical analysisReview manager, version 5.4 (Nordic Cochrane Center, Cochrane Collaboration, London, UK) was used for data analysis. The HR was used to measure effectiveness at a 95% CIs. I2 values were used to describe heterogeneity and were categorised into four levels: no heterogeneity (I2 < 25%), low heterogeneity (25% ≤ I2 < 50%), moderate heterogeneity (50% ≤ I2 < 75%) and high heterogeneity (I2 ≥ 75%). When the I2 value was <50%, a fixed model effect was used, whereas when it was >50%, a random model effect was used.
ResultsEligible studies and study characteristicsAfter identifying 4315 references, 1035 duplicate publications and 3205 irrelevant studies were excluded, leaving 75 potentially eligible studies (Figure 1). Finally, six cohort (17, 18, 21–24) studies conducted between 2014 and 2022 were included in the meta-analysis.
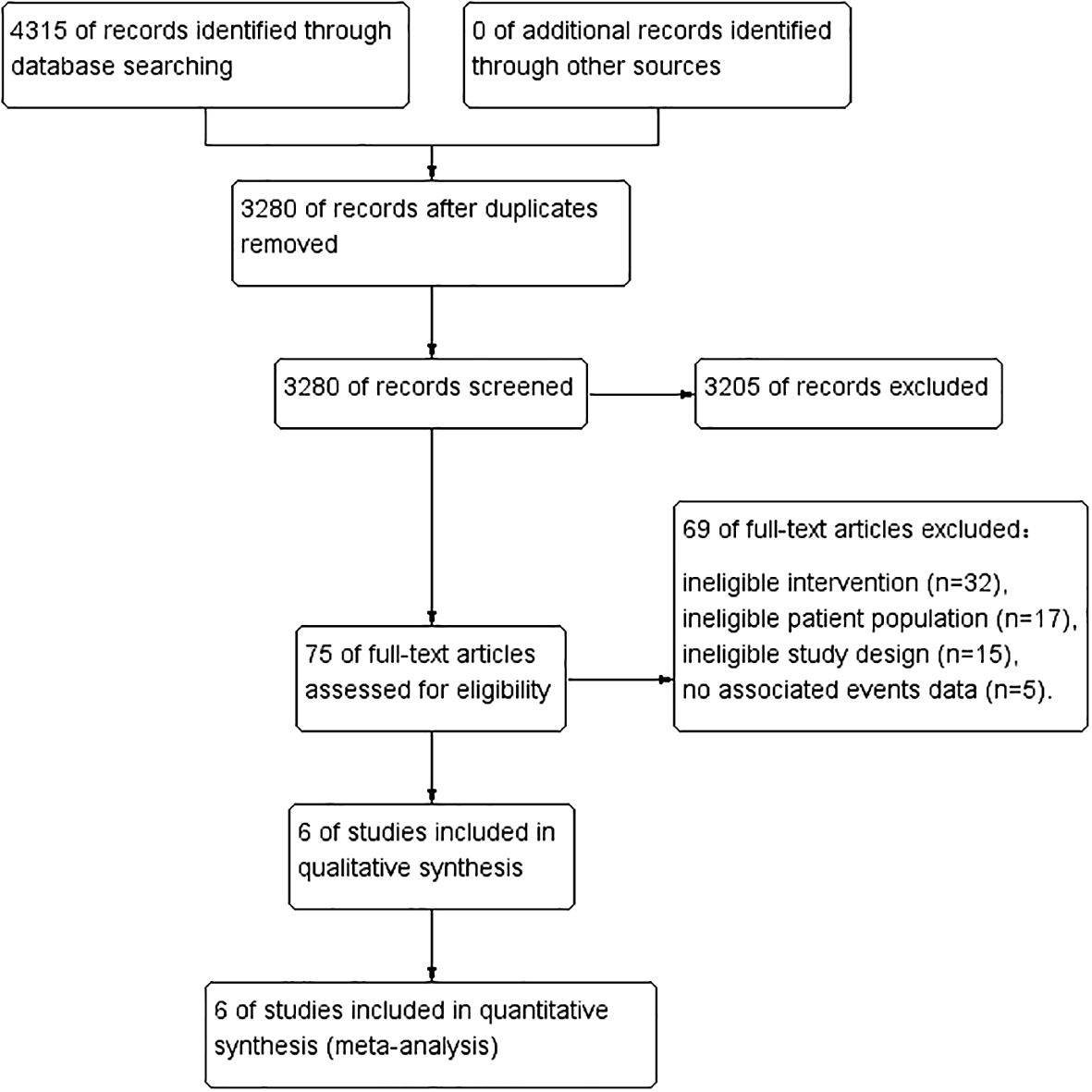
Figure 1 PRISMA flow diagram of study selection.
Table 1 lists the general characteristics of the included studies. A total of 111043 patients with cancer participated in the study, with trial sizes of 1001–88184 people. The six studies were retrospective studies using propensity matching scores. The main outcome measures are OS and RFS. Among these studies, two were from Europe and four were from Asia. According to the quality evaluation criteria, all six studies were rated as good quality.

Table 1 Characteristics of the included trials.
Recurrence-free survivalThree studies investigated the effects of TIVA and IA on the RFS rate of colorectal cancer (Figure 2). The total sample size was 99005 patients. Compared with IA, the use of TIVA was not associated with an improved RFS rate in colorectal cancer (HR, 0.99; 95% CI, 0.90–1.08; p = 0.75).
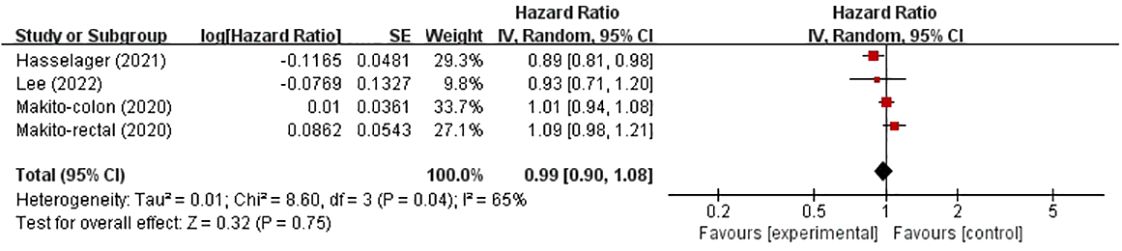
Figure 2 TIVA and IA on RFS of colorectal cancer.
Overall survivalsix studies investigated the effects of TIVA and IA on OS in colorectal cancer patients (Figure 3), involving 111043 patients. Compared with IA, TIVA improved OS (HR, 0.83; 95% CI, 0.70–0.99; p = 0.04).
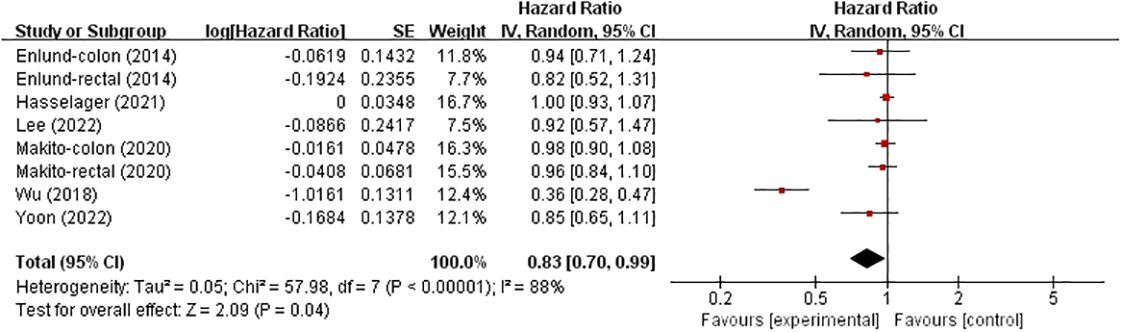
Figure 3 TIVA and IA on OS of colorectal cancer.
In these analyses, two studies analysed the colon and rectum, one analysed the colon and the remaining three analysed the colorectum. Subgroup analysis was conducted based on country and cancer location. The results showed no significant correlation between patients from Asia (HR, 0.77; 95% CI, 0.57–1.05; p = 0.09), and not between patients from Europe (HR, 0.99; 95% CI, 0.93–1.06; p = 0.83) (Figure 4). However, in the subgroup analysis of tumour location, no significant associations were found in either the colon, rectum or colorectal tumours (HR, 0.70; 95% CI, 0.38–1.28), (HR, 0.95; 95% CI, 0.83–1.08) and (HR, 0.99; 95% CI, 0.93–1.06) (Figure 5).
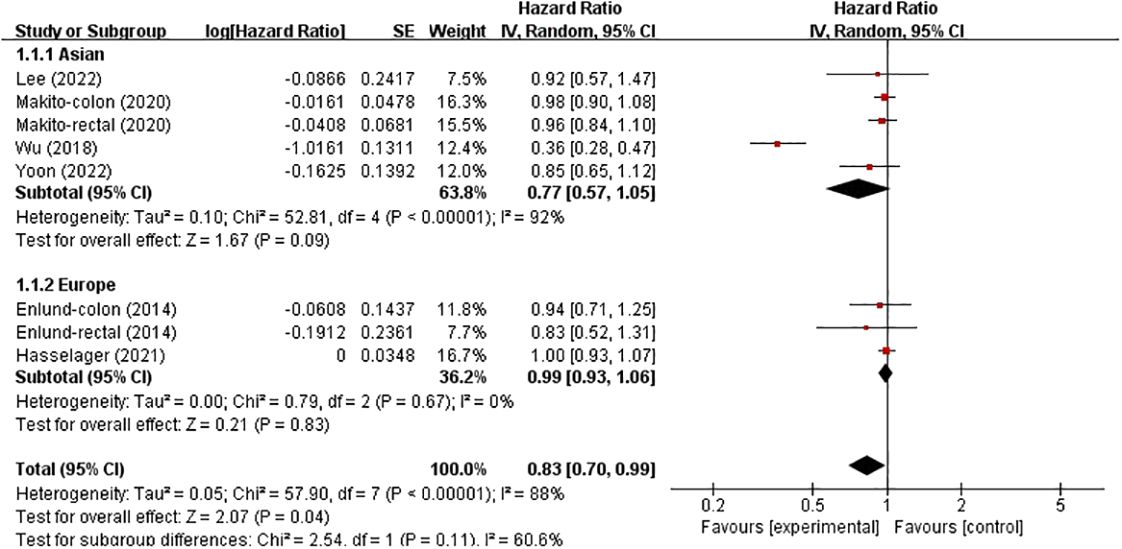
Figure 4 Subgroup analysis based on country with OS.
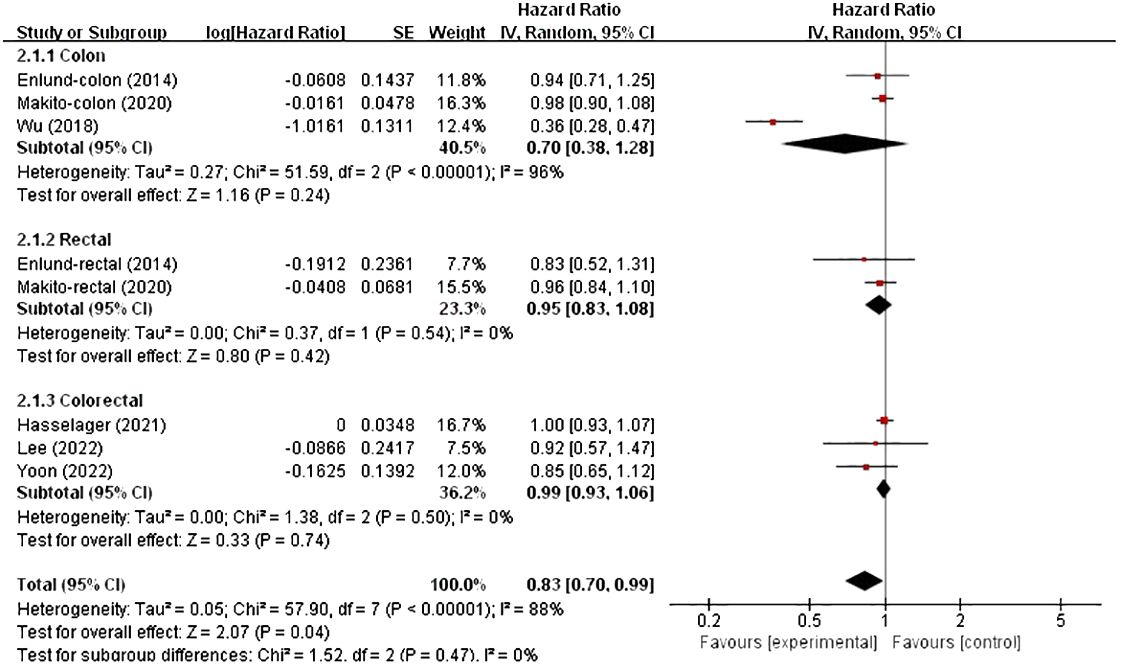
Figure 5 Subgroup analysis based on cancer location with OS.
Publication biasIn accordance with the criteria in the Cochrane Handbook for systematic reviews of interventions, publication bias was not analysed because none of the groups comprised >10 studies.
DiscussionOur meta-analysis included six retrospective studies for comparing the effects of TIVA and IA on postoperative prognosis after colorectal cancer resection. The data results processed using propensity score matching reduced the impact of selection bias; therefore, conducting a meta-analysis on these data yielded more consistent and less heterogeneous results. We found a statistically significant difference in OS between TIVA and IA for patients with colorectal cancer, but none in RFS. We conducted a subgroup analysis on OS and found no statistically significant difference between TIVA and IA in patients with colorectal cancer in Asia, and not in Europe. Regarding tumour location, no significant association was found between TIVA and IA in colon, rectum or colorectal cancer.
Propofol is the most commonly used intravenous inducer for anaesthesia maintenance. Some preclinical evidence suggests that it may have anti-tumour effects. Laboratory research has shown that propofol exerts anti-tumour effects by directly regulating key ribonucleic acid pathways and signal transduction in carcinomas (10). It also has anti-inflammatory and anti-oxidant effects, preventing immune suppression during the perioperative period. In vitro studies have confirmed that propofol has multiple anti-tumour effects in different cancer cell lines. In gastric cancer cell lines, it inhibits cell proliferation, invasion and migration (25). In non-small cell lung cancer (NSCLC), propofol interferes with HIF1A upregulation, thereby reducing carcinoma migration and invasion (26). In a study of breast cancer cell lines, propofol reduced the expression of neuroepithelial transformation gene 1, which promotes adenocarcinoma migration in vitro (27).
Laboratory studies have shown that the mechanisms by which volatile anaesthetics promote tumour metastasis may include the direct promotion of carcinoma survival and inhibition of immune cell and tumour cell-killing functions. However, the molecular mechanism remains unclear, and the evidence for different inhaled drugs and different cancer cell lines is contradictory. Volatile anaesthetics also have pro-inflammatory effects (28). They may upregulate hypoxia-inducible factor (HIF) and protect carcinomas during the perioperative period (29).
The results of clinical research comparing intravenous and inhaled drugs are inconsistent. Regarding the survival rate, a meta-analysis in 2019 included 6 studies, with >7800 patients with breast cancer, oesophageal cancer or NSCLC undergoing surgery. The results revealed that the RFS of TIVA users was higher than that of IA users (summary HR 0.78, 95% CI 0.65–0.94) (30). Regarding circulating tumour cells, a randomised trial included 210 patients undergoing breast cancer surgery. The results showed that the number of circulating tumour cells after surgery was similar among patients treated with sevoflurane and propofol (31). Regarding immune cells, a randomised trial found a similar proportion of NK cells, helper T cells and cytotoxic T cells in postoperative circulation among 153 patients who underwent colorectal cancer resection under sevoflurane- and propofol-induced anaesthesia (32). Regarding tumour regulatory factors, in vivo studies have not clarified the effects of intravenous and inhaled drugs on these factors. A small study evaluated the expression of oncogenes in patients undergoing head and neck cancer resection and found a significant increase in HIF1A expression among users of volatile anaesthetics (33).
Regardless of the exact mechanism, the choice of TIVA or VA is a potential modifiable factor in the management of colorectal cancer, and our meta-analysis results indicate that TIVA is associated with lower postoperative mortality. Further prospective clinical trials are required to elucidate the role of anaesthetics in cancer prognosis.
In the assessment of bias risk, we noticed the control of confounding factors with the most prominent bias risk. Many studies have not fully considered confounding factors such as patient comorbidities or tumour grading. For any group wishing to conduct further research on this topic, these issues need to be considered. Furthermore, most studies are retrospective and lack prospective randomised controlled trials.
Finally, although our meta-analysis established a possible association, it inferred no causal relationship nor explained potential mechanisms. We believe that further prospective clinical trials are required to elucidate the molecular mechanisms underlying the role of anaesthetics in cancer prognosis.
In conclusion, we conducted a meta-analysis using six studies, which included 111043 patients, and the results showed an association between TIVA and postoperative mortality in cancer surgery, but its impact on RFS remains inconclusive.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributionsSX: Conceptualization, Data curation, Formal analysis, Software, Writing – original draft, Writing – review & editing. YZ: Data curation, Formal analysis, Software, Writing – original draft. WW: Conceptualization, Funding acquisition, Resources, Writing – original draft, Writing – review & editing. YL: Data curation, Formal analysis, Writing – original draft. LY: Data curation, Software, Writing – original draft.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding was received for the construction of key clinical specialties in Futian District, Shenzhen.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol. (2018) 4(11):1553–68. doi: 10.1001/jamaoncol.2018.2706
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) 136(5):E359–86. doi: 10.1002/ijc.29210
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Murphy O, Forget P, Ma D, Buggy DJ. Tumour excisional surgery, anaesthetic-analgesic techniques, and oncologic outcomes: a narrative review. Br J Anaesth. (2023) 131:989–1001. doi: 10.1016/j.bja.2023.07.027
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Engilbertsson H, Aaltonen KE, Björnsson S, Kristmundsson T, Patschan O, Rydén L, et al. Transurethral bladder tumor resection can cause seeding of cancer cells into the bloodstream. J Urol. (2015) 193(1):53–7. doi: 10.1016/j.juro.2014.06.083
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Benzonana LL, Perry NJ, Watts HR, Yang B, Perry IA, Coombes C, et al. Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and Malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology. (2013) 119(3):593–605. doi: 10.1097/ALN.0b013e31829e47fd
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Buckley A, McQuaid S, Johnson P, Buggy DJ. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: a pilot study. Br J Anaesth. (2014) 113 Suppl 1:i56–62. doi: 10.1093/bja/aeu200
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Desmond F, McCormack J, Mulligan N, Stokes M, Buggy DJ. Effect of anaesthetic technique on immune cell infiltration in breast cancer: a follow-up pilot analysis of a prospective, randomised, investigator-masked study. Anticancer Res. (2015) 35:1311–9.
PubMed Abstract | Google Scholar
9. Ecimovic P, McHugh B, Murray D, Doran P, Buggy DJ. Effects of sevoflurane on breast cancer cell function in vitro. Anticancer Res. (2013) 33:4255–60.
PubMed Abstract | Google Scholar
10. Jiang S, Liu Y, Huang L, Zhang F, Kang R. Effects of propofol on cancer development and chemotherapy: Potential mechanisms. Eur J Pharmacol. (2018) 831:46–51. doi: 10.1016/j.ejphar.2018.04.009
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Jaura AI, Flood G, Gallagher HC, Buggy DJ. Differential effects of serum from patients administered distinct anaesthetic techniques on apoptosis in breast cancer cells in vitro: a pilot study. Br J Anaesth. (2014) 113 Suppl 1:i63–7. doi: 10.1093/bja/aet581
PubMed Abstract | CrossRef Full Text | Google Scholar
13. Chen Y, Liang M, Zhu Y, Zhou D. [The effect of propofol and sevoflurane on the perioperative immunity in patients under laparoscopic radical resection of colorectal cancer]. Zhonghua Yi Xue Za Zhi. (2015) 95:3440.
PubMed Abstract | Google Scholar
14. Baki ED, Aldemir M, Kokulu S, Koca HB, Ela Y, Sıvacı RG, et al. Comparison of the effects of desflurane and propofol anesthesia on the inflammatory response and s100β protein during coronary artery bypass grafting. Inflammation. (2013) 36(6):1327–33. doi: 10.1007/s10753-013-9671-6
PubMed Abstract | CrossRef Full Text | Google Scholar
15. Liu S, Gu X, Zhu L, Wu G, Zhou H, Song Y, et al. Effects of propofol and sevoflurane on perioperative immune response in patients undergoing laparoscopic radical hysterectomy for cervical cancer. Med (Baltimore). (2016) 95(49):e5479. doi: 10.1097/MD.0000000000005479
CrossRef Full Text | Google Scholar
16. Liu TC. Influence of propofol, isoflurane and enflurance on levels of serum interleukin-8 and interleukin-10 in cancer patients. Asian Pac J Cancer Prev. (2014) 15:6703–7. doi: 10.7314/apjcp.2014.15.16.6703
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Hasselager RP, Hallas J, Gögenur I. Inhalation or total intravenous anaesthesia and recurrence after colorectal cancer surgery: a propensity score matched Danish registry-based study. Br J Anaesth. (2021) 126:921–30. doi: 10.1016/j.bja.2020.11.019
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Lee S, Pyo DH, Sim WS, Lee WY, Park M. Early and long-term outcomes after propofol-and sevoflurane-based anesthesia in colorectal cancer surgery: A retrospective study. J Clin Med. (2022) 11:2648. doi: 10.3390/jcm11092648
PubMed Abstract | CrossRef Full Text | Google Scholar
19. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PloS Med. (2009) 6(7):e1000100. doi: 10.1371/journal.pmed.1000100
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Enlund M, Berglund A, Andreasson K, Cicek C, Enlund A, Bergkvist L. The choice of anaesthetic–sevoflurane or propofol–and outcome from cancer surgery: a retrospective analysis. Ups J Med Sci. (2014) 119:251–61. doi: 10.3109/03009734.2014.922649
PubMed Abstract | CrossRef Full Text | Google Scholar
22. Wu ZF, Lee MS, Wong CS, Lu CH, Huang YS, Lin KT, et al. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in colon cancer surgery. Anesthesiology. (2018) 129(5):932–41. doi: 10.1097/ALN.0000000000002357
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Makito K, Matsui H, Fushimi K, Yasunaga H. Volatile versus total intravenous anesthesia for cancer prognosis in patients having digestive cancer surgery. Anesthesiology. (2020) 133:764–73. doi: 10.1097/ALN.0000000000003440
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Yoon S, Jung SY, Kim MS, Yoon D, Cho Y, Jeon Y. Impact of Propofol-based Total Intravenous Anesthesia Versus Inhalation Anesthesia on Long-term Survival after Cancer Surgery in a Nationwide Cohort. Ann Surg. (2023) 278(6):1024–31. doi: 10.1097/SLA.0000000000005568
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Yang C, Gao J, Yan N, Wu B, Ren Y, Li H, et al. Propofol inhibits the growth and survival of gastric cancer cells in vitro through the upregulation of ING3. Oncol Rep. (2017) 37(1):587–93. doi: 10.3892/or.2016.5218
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Yang N, Liang Y, Yang P, Ji F. Propofol suppresses LPS-induced nuclear accumulation of HIF-1α and tumor aggressiveness in non-small cell lung cancer. Oncol Rep. (2017) 37:2611–9. doi: 10.3892/or.2017.5514
PubMed Abstract | CrossRef Full Text | Google Scholar
27. Ecimovic P, Murray D, Doran P, Buggy DJ. Propofol and bupivacaine in breast cancer cell function in vitro - role of the NET1 gene. Anticancer Res. (2014) 34:1321–31.
PubMed Abstract | Google Scholar
29. Tavare AN, Perry NJ, Benzonana LL, Takata M, Ma D. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer. (2012) 130:1237–50. doi: 10.1002/ijc.26448
PubMed Abstract | CrossRef Full Text | Google Scholar
30. Yap A, Lopez-Olivo MA, Dubowitz J, Hiller J, Riedel B, Global Onco-Anesthesia Research Collaboration Group. Anesthetic technique and cancer outcomes: a meta-analysis of total intravenous versus volatile anesthesia [published correction appears in Can J Anaesth. 2019 Aug;66(8):1007-1008]. Can J Anaesth. (2019) 66:546–61. doi: 10.1007/s12630-019-01330-x
PubMed Abstract | CrossRef Full Text | Google Scholar
31. Hovaguimian F, Braun J, Z’graggen BR, Schläpfer M, Dumrese C, Ewald C, et al. Anesthesia and circulating tumor cells in primary breast cancer patients: A randomized controlled trial. Anesthesiology. (2020) 133(3):548–58. doi: 10.1097/ALN.0000000000003409
PubMed Abstract | CrossRef Full Text | Google Scholar
32. Oh CS, Park HJ, Piao L, Sohn KM, Koh SE, Hwang DY, et al. Expression profiles of immune cells after propofol or sevoflurane anesthesia for colorectal cancer surgery: a prospective double-blind randomized trial. Anesthesiology. (2022) 136(3):448–58. doi: 10.1097/ALN.0000000000004119
PubMed Abstract | CrossRef Full Text | Google Scholar
33. Ferrell JK, Cattano D, Brown RE, Patel CB, Karni RJ. The effects of anesthesia on the morphoproteomic expression of head and neck squamous cell carcinoma: a pilot study. Transl Res. (2015) 166:674–82. doi: 10.1016/j.trsl.2015.09.001
留言 (0)