Chagas disease, caused by the protozoan Trypanosoma cruzi, was discovered over a century ago and remains a serious public health concern worldwide (1). According to the World Health Organization (WHO), the disease affects approximately 6–7 million people, mainly in Latin America, with 75 million people at risk of infection and more than 10,000 deaths per year (2, 3).
T. cruzi is a flagellate digenetic protozoan belonging to the order Kinetoplastida and family Trypanosomatidae (1). This species is heterogeneous and consists of several subpopulations that circulate in diverse environments, presenting great genetic diversity (4, 5). The analysis of a large panel of clones derived from wild-type isolates confirmed that the genome size can vary up to 48% between T. cruzi strains, and this difference is significant for populations of the same species (6). Hybridization events have contributed to current T. cruzi population structures and distinct genetic subgroups’ evolution of the parasite (4, 7).
These T. cruzi subgroups have been studied with different molecular tools over the years and have received distinct designations, including zymodemes (8–10), schizodemes (11), biodemes (12, 13), clones (14), groups (15), lineages (16), clades (17), and discrete typing units (DTUs) (18). In a Satellite Meeting in 1999, the standardization of T. cruzi strain nomenclature was discussed to adopt a standard classification that taxonomists and the general community of researchers would use. An expert committee reviewed the subdivision of the T. cruzi strains considering biological, biochemical, and molecular parameters to classify the species into two principal groups: T. cruzi I and T. cruzi II. The apparent hybrid strain was named T. cruzi (19).
However, with the advances in molecular biology and knowledge of parasite biology, this classification showed limitations due to the great dispersion of characteristics between members of the same T. cruzi genetic group (20). In the last decades, several molecular approaches have been used to elucidate the population structure of T. cruzi and have revealed a variable number of relevant subgroups in T. cruzi species (21–27). These data suggest that investigations employing the smaller T. cruzi subdivisions present more specific correlations between genetic parasite and biological, clinical, and epidemiological properties of the Chagas disease (Table 1). Consequently, 10 years after the Satellite Meeting, the scientific community has advanced knowledge of the diversity of T. cruzi. Thus, the current classification consensus proposes six genetic groups or DTUs, referred to as TcI to TcVI (38), related to previous classifications according to different markers. A DTU restricted to bats (Tcbat) was also considered (5). Studies based on multiple molecular markers support the definitive classification of Tcbat as a seventh DTU closely related to TcI, mainly found in bats (39, 40) and registered in Colombia, Panama, Chile, Equator, and Brazil (36, 41, 42). TcI and TcII represent ancestral T. cruzi lineages, which, from hybridization events, give rise to TcIII and TcIV, respectively (43, 44). In contrast, Freitas et al. (2006) (26) suggested the existence of three ancestral T. cruzi lineages (TcI, TcII, and TcIII). Nonetheless, genetic analyses showed TcI and TcII traits in TcIV and TcIII isolates, supporting the first model (7, 43, 45). TcV and TcVI are considered genetic hybrid groups formed from TcII and TcIII, which are widely circulated in the domestic cycle in South American countries (46–49). However, more recently, they have been found to infect dogs in the United States (50).
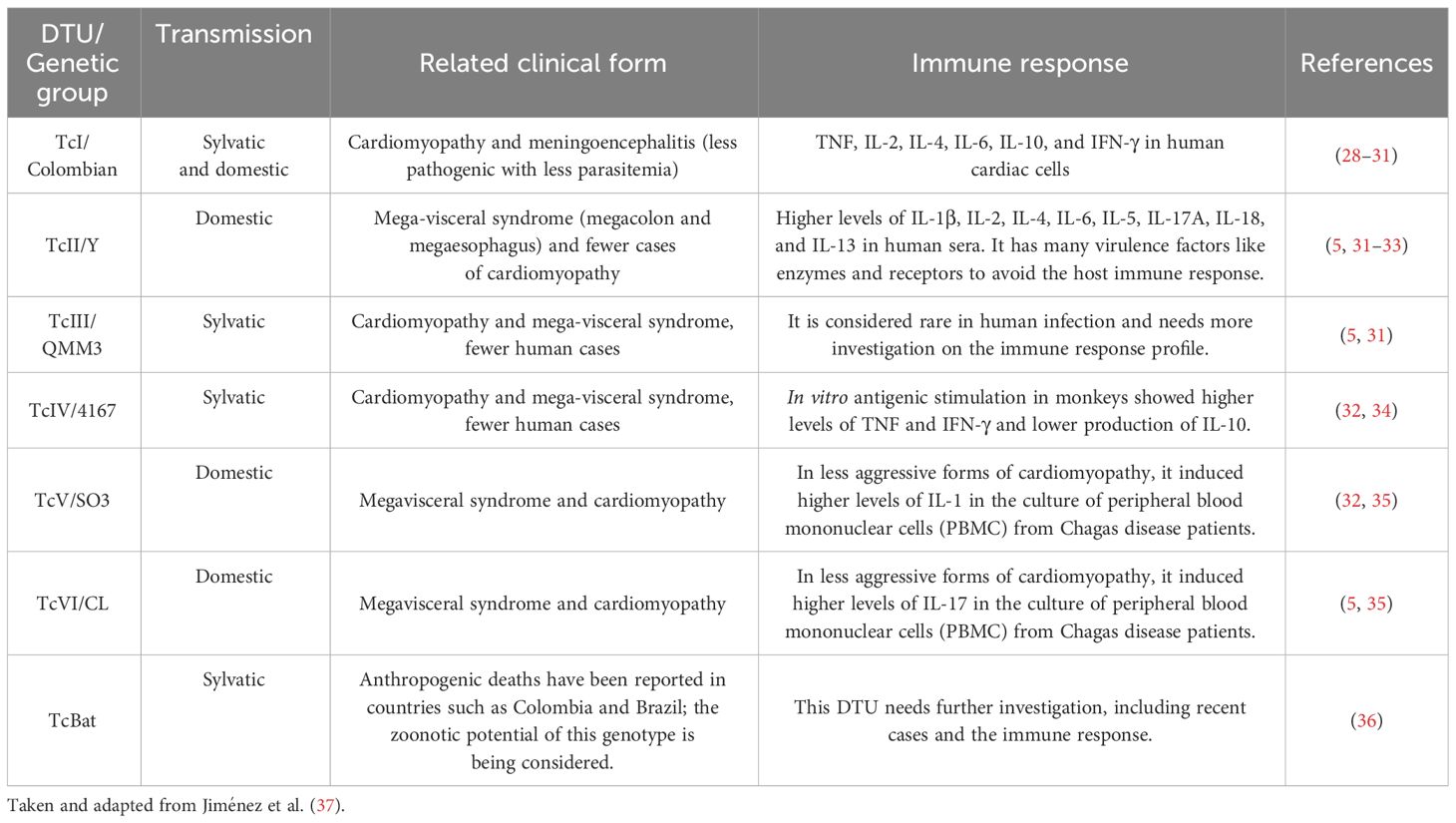
Table 1 Association between Trypanosoma cruzi DTUs, related clinical form, and immune response.
Several studies have shown that the heterogeneity of T. cruzi species directly affects the diversity of clinical manifestations, the control, the laboratorial diagnosis performance, and the susceptibility to treatment in the Chagas disease (Table 1). Thus, this review aims to describe how T. cruzi genetic diversity influences the biology of the parasite and/or clinical parameters in humans.
2 Epidemiology and DTU geographic distributionChagas disease is endemic to 21 countries and is considered a neglected disease by the WHO (51–53). In some regions, especially in countries in the Southern Cone of America, the acute phase of the disease had a clear reduction due to the success of vector and blood bank control. Owing to the migration of individuals with Chagas disease and the possibility of transmission by other routes, such as blood transfusion, congenital, and organ transplants in individuals chronically infected with T. cruzi (54, 55), the infection has also acquired importance in non-endemic areas of Chagas disease (56, 57).
An outstanding characteristic of T. cruzi is its significant plasticity, characterized by genetic variability and intense intraspecific phenotypic diversity. Regarding geographic dispersion, evident differences in T. cruzi DTU distribution were observed in distinct areas. For example, all DTUs were identified in South America, while TcI, TcII, and TcIV were identified in North America, and only TcI and TcIV were detected in the hosts and vectors from Central America (42, 49). TcI is the most ubiquitous DTU found in the wild cycle throughout all endemic areas. In the domestic cycle of the T. cruzi infection, it was widely prevalent in patients from North and Central America, Colombia, and Venezuela (7, 42, 58–60); some cases have also been identified in Chile and Argentina (21, 42, 61). The TcI has been frequently associated with outbreaks of oral transmission in the Northern Amazon (39, 62), but not in other South American regions, where TcII, TcV, and TcVI are more related to chronic infection, regardless of the clinical manifestation of Chagas disease (63–65).
TcII is the main DTU detected in Brazilian patients from the Central and Southeastern regions, where there were also registers of TcVI as a secondary DTU (66, 67). TcII is abundant in Chile and Colombia and less frequent in other regions (21, 42, 68).
TcIII and TcVI were predominantly associated with sylvatic cycles of the T. cruzi infection. TcIII was detected in sylvatic hosts from Northeastern Venezuela to Argentina, but infection in dogs and humans has been reported in Colombia (69, 70) and Argentinean Chaco (71–73). TcIV is a relevant agent in Chagas disease in Venezuela (58). It has also been reported in oral outbreaks in the Brazilian Amazon region (39, 62, 74).
TcV and TcVI were observed predominantly in the Southern Cone greater Gran Chaco (Peru, Bolivia, Northern Chile, and Argentina), Paraguay, and Uruguay. There are some registers of TcV in the extreme South of Brazil and Paraguay (5, 73). Some cases of human T. cruzi infection with TcV and/or TcVI have been reported further north in Ecuador (75) and Colombia (41, 76). In Bolivia, all DTUs were identified as circulating in humans except TcIII (42), with a predominance of TcI and TcV.
In addition, to the ecogeographic diversity, the literature demonstrated variations in biological and biochemical characteristics between T. cruzi subpopulations. In 1909, Chagas already described the morphological differences in the blood forms of the parasite. Generally, the stout forms of T. cruzi remain in the bloodstream and are unable to invade host cells rapidly, while the slender forms invade cells and start the intracellular cycle more quickly. The variation in parasitemia and tissue tropism correlates with the morphology of the T. cruzi forms because different strains have variable numbers of each form (77, 78). Miles et al. (1981) (58) described the first evidence that chronic Chagas disease manifestation could differ according to strain type and geographical location. In Venezuela, patients rarely develop mega-syndromes, while central and eastern Brazil have common cardiac and mega-syndrome manifestations (58). Although considerable advances have been made in the understanding of the physiopathogenesis of Chagas disease, no prognostic factors still allow a conclusive definition of patients infected with T. cruzi who develop symptomatic clinical forms. This multifactorial process involves host, parasite and environmental features, so a broad approach must be considered to better understand the factors that influence the physiopathogenesis of Chagas disease (79–81). Considering the T. cruzi genetic diversity, the current data corroborate Miles’ observations since the digestive forms are associated with infections where TcII, TcV, and TcIV DTUs were identified, while indeterminate forms and cardiac forms could be related to different DTUs with greater or lesser severity depending on the geographic regions (73, 82, 83). Differences in the capacity of T. cruzi for cell penetration, infectivity, differential induction of the immune response, tissue tropism, and response to treatment have already been demonstrated (84–86), which could confer differential capacity for adaptation to the host, influencing the transmission, response to the treatment, and the course of the infection (5, 87). Several studies have also demonstrated that distinct T. cruzi strains could trigger different immune responses and be influenced by geographical location (31, 88–93).
In addition, T. cruzi mixed infections and reinfections can alter the natural course of infection compared to individual populations, giving the parasite new properties and hampering correlations (13, 89, 94–96).
3 Infectivity and virulence of T. cruzi DTUsThe first criteria for subdivision of T. cruzi were based on parameters such as infectivity and virulence in mice infected with distinct parasite strains (12, 97). Andrade and Magalhães (1997) (97) characterized Type I as strains with a prevalence of thin forms and macrophage tropism that multiply quickly, developing high parasitemia and consequently leading to the death of Swiss mice in the first days after infections (7th–12th days). Type II T. cruzi strains have a frequency of wide forms and myocardial tropism that multiply slowly and present irregular peaks of parasitemia between the 12th and 20th day after infection. Moreover, the authors considered Type III as a T. cruzi strain with a prevalence of wide forms and skeletal musculature tropism, which multiply slowly and present late parasitemia peaks (20th–30th days), as well as low mortality rates in Swiss mice.
The correlation between the genetic variability of T. cruzi and its biological properties (60) has been scientifically sustained and corroborated in several studies with strains and clonal stocks of the parasite in vitro (98–100), experimental mouse infections (84, 100–102), and vectors (103–106). In general, these studies demonstrated that TcI presented higher multiplication and survival in acellular and cellular cultures than TcII when the biological behavior of clones (13, 98, 99) and strains of T. cruzi was evaluated (97, 107, 108). Revollo et al. (1998) (99) and Nogueira-Paiva et al. (2015) (108) used the “Vero” cells (cells isolated from African Green monkey kidney obtained from the Laboratory of Parasitology at the Pasteur Institute in Paris) to show differences in multiplication and survival when comparing the growth of different DTUs of T. cruzi in vitro, whereas Andrade and Magalhães (1997) (97), Rimoldi et al. (2012) (107) and Andrade et al. (2010) (13) used different mice organs and cells to demonstrate the distinct biological behavior of T. cruzi genotypes. Oliveira et al. (2017) (100) showed different results regarding the biological behavior in vitro of parasites isolated from patients with chronic Chagas disease. These authors verified that one sample of TcII demonstrated more significant growth in acellular culture than TcI and TcVI, and two other samples of TcII showed similar growth. The authors also revealed that the infectivity of “Vero” cells after 72 h was similar for all DTUs. However, one sample of TcII DTU yielded a higher number of amastigotes per cell.
The genetic variability of T. cruzi can influence parasite infectivity, reproduction, and differentiation in vectors (103, 104, 109, 110). Previous studies have demonstrated that clones of TcI are better able to infect and complete the life cycle in the Triatoma infestans vector than TcII clones, indicating that the DTUs do not exhibit the same vectorial transmissibility (103, 104). De Abreu et al. (2022) (111) showed differences in vector susceptibility to infection and competence when two vector species were infected with TcI, TcII, and TcIV strains from Amazon (AM) and Paraná (PR) in single and mixed T. cruzi infections. It is verified that Rhodnius robustus showed vector competence for TcIV (AM) and TcI (AM) + TcIV (AM), and Rhodnius pictipes showed vector competence for TcI (AM) + TcIV (AM) and TcI (AM) + TcII (PR).
The biological behavior of T. cruzi DTUs such as parasitological parameters, infectivity, and virulence was also assayed in the vertebrate hosts (84, 90, 91, 97, 100, 112–121). Lana et al. (2000) (113) revealed that 19/20 (TcI), 32 (TcII), and 39 (TcV) clonal T. cruzi genotypes exhibited high, intermediate, and low maximum parasitemia peaks, respectively, indicating that the clonal genotypes differed significantly in their infectivity in BALB/c mice. Toledo et al. (2002) (84) verified the biological behavior of a broader panel of T. cruzi clonal stocks. They demonstrated differences in parasitemia, infectivity, mortality, tissue parasitism, and inflammatory processes during the acute and chronic phases of experimental T. cruzi infection in BALB/c mice. The authors concluded that closely related clonal T. cruzi genotypes, 19 vs. 20 (TcI) and 32 (TcII) vs. 39 (TcV), showed, in general, fewer differences among themselves than distantly related groups, 19 or 20 (TcI) vs. 32 (TcII) or 39 (TcV). Some studies have shown that TcI has a high ability to infect experimental BALB/c (85) and Swiss mice (97, 112), developing larger parasitemia than other T. cruzi genetic groups (85, 97, 112). However, it has been demonstrated that samples of TcI isolated from patients with chronic Chagas disease may also present low levels of parasitemia in Swiss mice (100, 118). Sales-Campos et al. (2015) (121) verified that strains belonging to TcI exhibited low levels of parasitemia in BALB/c mice. T. cruzi stocks belonging to TcI and TcIV from the Brazilian Amazon diverged in terms of biological and medical properties in Swiss mice, while TcIV displayed higher virulence and parasitemia parameter values, and TcI determined the most severe inflammatory process and higher frequency of tissue parasitism (117). These infectivity and virulence alterations in TcI may be associated with intra-DTU differences. In contrast, TcII isolated from T. cruzi-infected humans exhibits high infectivity and virulence in experimental Swiss mice (90, 100, 114, 116). Lisboa et al. (2007) (122) studied sylvatic T. cruzi stocks and verified higher parasitemia in Swiss mice infected with TcII than with TcI. Significant biological heterogeneity was also observed in T. cruzi TcII isolates derived from L. rosalia from the Atlantic Forest, unlike the TcII isolates derived from marsupials, which present similar profiles. Together, these data confirm the high level of intraspecific divergence in T. cruzi and that the behavior of isolates can vary according to geographic and host origin (85, 117, 122, 123).
Standard T. cruzi strains, representative of the major genotypes of the parasite, have also been used to evaluate the biological behavior of DTUs in experimental animals (91, 124). Magalhães et al. (2015) (119) showed that Colombiana (TcI) and Y (TcII) strains lead to the differential activation of human monocytes and T cells, which might influence disease progression. Corroborating this work, Duz et al. (2014) (91) indicated that dogs infected with the Colombiana strain (TcI) reached the parasitemia peak later than animals infected with the Y strain (TcII) and that the Y strain (TcII) triggered a more intense immune response during the acute phase of infection in dogs. This is consistent with the Colombiana strain (TcI) in experimental models that induced a milder infection than the Y strain (TcII).
Studies using other strains isolated from T. cruzi DTUs, such as TcIII, TcV, and TcVI, have also verified the infectivity and virulence of these genetic groups in C57BL/6J mice (115, 120). Ragone et al. (2015) (120) showed that TcVI induces high parasitemia, severe histological damage in the heart, and moderate lesions in C57BL/6J mice skeletal muscle. On the other hand, TcIII presents less parasitemia than TcVI and mild-to-moderate histological damage in the heart and skeletal muscle. Lastly, TcV induced subpatent parasitemia and mild lesions in the analyzed tissues.
In natural infections with T. cruzi, it is crucial to consider that mixed infections with multiple DTUs are common and can lead to changes in the biological properties of the parasite as well as the clinical course of Chagas disease (94, 95, 105, 113, 125–127). Some studies have demonstrated that T. cruzi clones with less ability to develop in invertebrate and vertebrate hosts during monoclonal infections can multiply in mixed infections (94, 104, 105, 113, 128). It has been demonstrated in vivo that the tissue tropism of one T. cruzi DTU could change in the presence of other DTUs (124, 129). Moreover, the histopathological damage and intensity of the inflammatory process resulting from these mixed T. cruzi infections also present remarkable variations (89, 130).
4 Immune response and cell interactionThe immune response against Chagas disease has been investigated for many years and can generate protective or pathogenic events. The host’s genetic background, in addition to the specific characteristics of the parasite, seems to play a relevant role in influencing the outcome of the disease (131). The murine model of Chagas disease has been well established and effectively reflects the key features of human T. cruzi infection. Notably, mice’s immune response and cytokine expression levels during the acute and chronic phases closely resemble those observed in human Chagas disease (132). Ferreira et al. (2018) (132) assessed aspects of the acute and chronic phases of T. cruzi infection using G (TcI) and CL (TcVI) strains in two distinct mouse lineages (C57BL/6 and BALB/c). These findings revealed that the CL strain established infection more rapidly than the G strain. Concurrently, the immune response in BALB/c mice was initiated earlier than that in C57BL/6 mice. In the acute phase, all animals responded to T. cruzi infection by elevated serum concentrations of cytokines, with BALB/c mice demonstrating a more regulated immune response than C57BL/6 mice. In the chronic phase, C57BL/6 mice continued to exhibit exacerbated cytokine and chemokine responses. Despite differences in T. cruzi tropism among parasite strains, host background can significantly influence immune responses throughout infection (132).
Previous studies have demonstrated variations in the features of T. cruzi infection based on the mouse lineage and parasite strain used in the infection (133–135). In a study conducted by Sanoja et al. (2013) (134), a comparative analysis of CD4+ T-cell subset dynamics was performed after infection with the Y strain during the acute and chronic phases in BALB/c and C57BL/6 mice. The findings revealed that C57BL/6 mice exhibited heightened levels of CD4+ T-cell infiltration and expression of Th1 cytokines in the heart, coupled with the presence of Treg cells. In contrast, BALB/c mice showed a higher heart parasite burden, lower heart CD4+ T-cell infiltration, and reduced levels of Th1 and inflammatory cytokines, but with an increased presence of Th17 cells. In the chronic phase, BALB/c mice continued to exhibit higher parasite burdens than C57BL/6 mice, along with elevated levels of interferon (IFN)-γ, tumor necrosis factor (TNF), interleukin (IL)-10, and transforming growth factor (TGF)-β (134). Domingues et al. (2020) (135) also explored the influence of host genetics on histopathological and immunological aspects following infection with the SC2005 T. cruzi strain (isolated from a patient with Chagas disease) in BALB/c and A mice. They observed that BALB/c mice displayed higher parasitemia and mortality rates than A mice. Despite both lineages demonstrating a resistant immune profile, characterized by an increase in CD8+ T cells in the heart, liver, and blood; an increase in CD19+ B cells in the liver; and high levels of proinflammatory cytokines, the response to infection occurred later in BALB/c mice. Consequently, A mice were less susceptible to T. cruzi infection. The host genetic background can influence the early development of a cytotoxic cellular response profile, crucial in developing a less severe manifestation of Chagas disease (135). Other studies also support the significance of host genetic background in the clinical manifestations of chronic Chagas disease (136, 137). Frade et al. (2013) (136) analyzed CCR5, CCL2, and MAL/TIRAP genes in patients with chronic chagasic cardiomyopathy. Their findings demonstrated that the CCL2rs2530797A/A and TIRAPrs8177376A/A genotypes were associated with increased susceptibility, whereas the CCR5rs3176763C/C genotype was associated with cardiomyopathy protection (136).
The entry of T. cruzi into cells is a multifactorial process (138). Recent studies have indicated that many molecules interacting with specific cell receptors are expressed on the surface of T. cruzi, allowing the invasion of several cell types and the modulation of cell metabolism to enable its survival (139). Among the proteins involved in this process are GPI-anchored mucins, glycophosphatidylinositol, transialidase, and cruzipain, which are involved in the differential capacity of cell adhesion of T. cruzi strains and evasion of the immune response (140, 141).
The expression levels and glycosylation pattern diversity of mucin-associated surface proteins (MASPs) can vary among different T. cruzi strains, influencing the parasite’s virulence and immune response. MASP is the second-largest multigene family in T. cruzi and comprises approximately 1,300 genes. MASP proteins are characterized by a highly variable and repetitive central region comprising peptides shared among all MASP members. The pronounced polymorphism within this family and its surface location on infective forms of T. cruzi imply that MASP actively participates in host–parasite interaction mechanisms. Initial proteomic findings related to the MASP family suggested potentially low expression levels, with only four proteins identified in the trypomastigote stage and one in the epimastigote stage. One hypothesis posits that MASPs may be predominantly expressed during the intermediate stages of the parasite’s life cycle or undergo post-translational modifications, such as distinct N-glycosylation (142). Subsequent research on these genes has revealed protein expression in epimastigotes, trypomastigotes, and amastigotes of T. cruzi. Moreover, MASPs could be secreted during the early stages of amastigote genesis in vitro at pH 5.0 (143, 144). The expression profiles of these proteins may vary throughout infection, with different strains exhibiting differential expression of various MASP family members (145). Notably, immature MASP proteins and their C-terminal portion have been identified in trypomastigotes’ exosomes, showing immunogenic properties (146, 147). Recent studies have demonstrated that host antibodies against MASPs may exhibit strain-specific recognition patterns. Understanding the variation in surface-expressed molecules and diversity of MASPs among different T. cruzi strains is crucial for unraveling the complexity of Chagas disease and developing targeted interventions, including vaccines and treatments. A few years ago, a synthetic peptide derived from T. cruzi MASP was tested as a vaccine candidate against Chagas disease (148). This vaccine candidate induced an 86% survival rate in the immunized group after challenge with a highly lethal dose of trypomastigotes. Immunized animals also exhibited the lowest parasite load in the heart, liver, and spleen compared with control animals, as demonstrated by real-time polymerase chain reaction (PCR) (148). Ongoing research endeavors continue to unveil the intricacies of T. cruzi diversity and its implications in disease pathogenesis (148).
Gp90 has been described as a molecule with an antiphagocytic effect in mammalian stages of T. cruzi (149) and is considered a negative modulator of infection (150). Gp82 and gp35/50 are also implicated in cell invasion and are expressed on the surface of metacyclic trypomastigotes of T. cruzi. The mucin-like gp35/50 is resistant to protease digestion and responsible for protecting the parasite from destruction by the oral route (150). Gp83 is a ligand employed by the parasite to attach to and enter phagocytic and non-phagocytic cells (140, 151). All were expressed on the surface of metacyclic trypomastigotes.
T. cruzi has been reported to activate Toll-like receptor 2 (TLR2), TLR4, and TLR9 (152, 153), and this innate recognition mechanism can influence cell activation and infectivity (Figure 1). TLRs are crucial components of the innate immune system, recognizing various pathogen-associated molecular patterns (PAMPs) and initiating immune responses (152–156). These receptors are important transmembrane proteins that confer a certain degree of specificity to the innate immune system cells. This innate recognition mechanism can influence cell activation and infectivity (Figure 1). Each TLR initiates downstream signaling that culminates in activating signaling pathways that regulate the expression of cytokines, chemokines, and interferons (153). Cell surface TLRs mainly recognize microbial membrane components such as lipids, lipoproteins, and proteins; intracellular TLRs recognize nucleic acids derived from bacteria and viruses and self-nucleic acids in disease conditions such as autoimmunity (155). TLR2, TLR4, and TLR9 are specifically mentioned in the context of T. cruzi infection. TLR2 recognizes various PAMPs, including lipoproteins and glycolipids (152–154, 156, 157). Studies have shown that TLR2 can be activated by parasite-derived molecules, contributing to the host immune response (153). TLR4 is primarily associated with bacterial infections for recognizing lipopolysaccharide (LPS), and some studies suggest its involvement in parasitic infections as well. The activation may occur through the recognition of parasite components (153). TLR9 recognizes bacterial and viral DNA that is rich in unmethylated CpG-DNA motifs; in the case of T. cruzi, the activation may be related to the presence of parasitic DNA, and additional studies attributed the production of proinflammatory cytokines (TNF and IL-12) to the cooperative activation of TLR2 and TLR9 (152, 157). This recognition can lead to the induction of pro-inflammatory responses and the initiation of an effective immune reaction against the parasite.
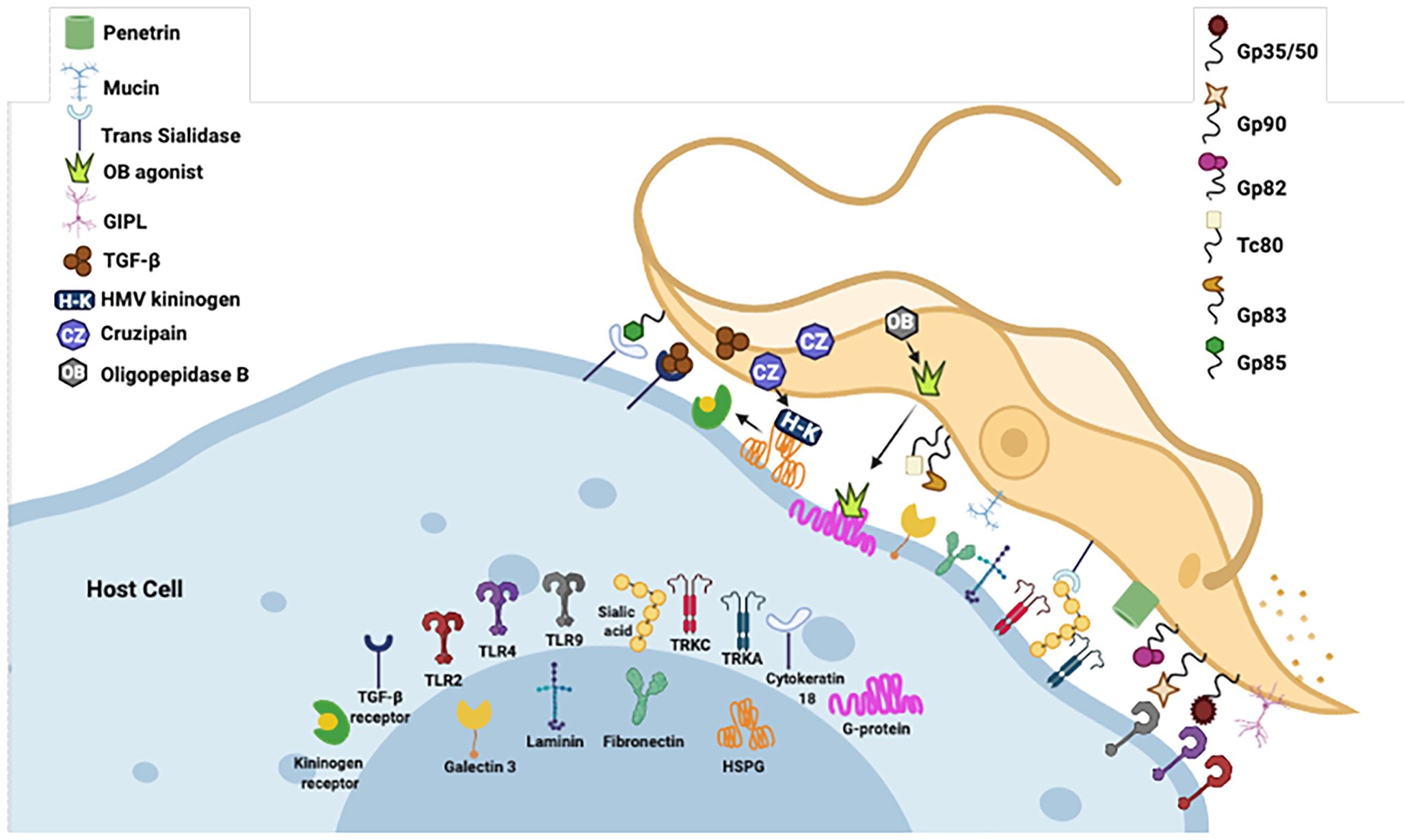
Figure 1 Examples of molecules expressed on the surface of T. cruzi that interact with specific cell receptors. Gp, glycoprotein; TLR, Toll-type receptors; MVs, microvesicles; LPS, lipopolysaccharide; PAMP, pathogen-associated molecular patterns; ssDNA, single-stranded DNA. Figure created by the author on the biorender.com platform, inspired by an Oswaldo Cruz Foundation website publication.
Although the adaptive immune response has been considered for many years to be the most relevant in building the balance between protective and pathogenic events during chronic infection of Chagas disease, several studies have demonstrated the importance of the innate immune response as a significant factor in this issue, mainly in the process (154). During the acute phase, cells of the innate immune system play a pivotal role in controlling the infection by suppressing parasite replication. The classic mechanism proposed is that T. cruzi initiates a cascade of events that trigger the synthesis of IL-12 by macrophages, which is a crucial mediator of IFN-γ production through Th1 and NK activation (158, 159) (Figure 2). IFN-γ plays a crucial role during T. cruzi infection by increasing the production of IL-12, TNF-α, and nitric oxide (NO) by macrophages. The NO is cytotoxic to intracellular microorganisms and exerts trypanocidal activity, inhibiting the development of T. cruzi (160–162) (Figure 2). Moreover, IFN-γ stimulates T-cytotoxic lymphocyte activation, a central mechanism for systemic and antigen-specific protection against T. cruzi intracellular infection (163, 164) (Figure 2).
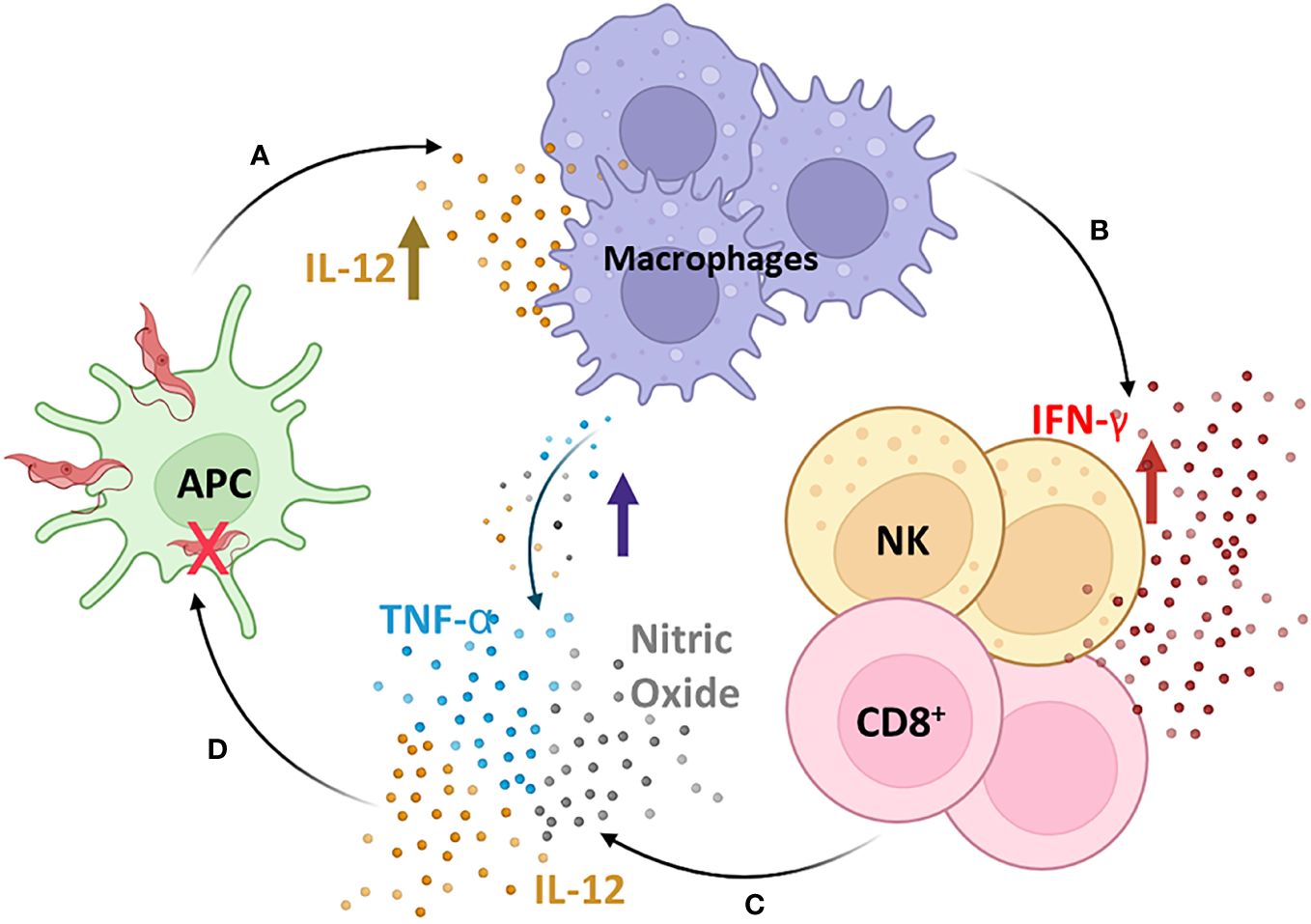
Figure 2 The cascade of induction of the innate immune response. T. cruzi initiates a cascade of events that trigger the synthesis of IL-12 by macrophages (A), which is a crucial mediator of IFN-γ production through Th1 and Natural Killer (NK) activation (B). IFN-γ plays a crucial role during T. cruzi infection by increasing the production of IL-12, TNF-α, and nitric oxide (NO) by macrophages (C), which are cytotoxic to intracellular microorganisms and exert trypanocidal activity, inhibiting the development of T. cruzi (D). Moreover, IFN-γ stimulates T-cytotoxic lymphocyte activation, a central mechanism for systemic and antigen-specific protection against T. cruzi intracellular infection. APC, antigen-presenting cells; NK, natural killer cells; CD8+, CD8+ T lymphocytes. Figure created by the author on the biorender.com platform, inspired by an Oswaldo Cruz Foundation website publication.
One of the first lines of defense is the complement system, which consists of more than 35 plasma and cell surface receptors/regulators. After interaction with the pathogen, it can be activated by three pathways of the protease cascade: classical (CP), alternative (AP), and lectin (LP), resulting in inflammation, opsonization (phagocytosis), and lysis of pathogens (Figure 3) (165–167). Although these pathways differ in the initial steps of their respective cascades, all three converge to produce a C3 convertase and then a C5 convertase, forming a membrane attack complex (MAC) and subsequent pathogen lysis (167). The main mechanisms involved in the ability and capacity of trypomastigotes to avoid complement action are as follows: T. cruzi calreticulin (TcCRT), which is a 45-kDa calcium-binding protein, primarily located in the endoplasmic reticulum (ER) and expressed in infective trypomastigotes. After infection, TcCRT translocates to the emerging area of the flagellum on the plasma membrane surface (168–170). This protein can bind to host mannose-binding lectin (MBL) and interacts with ficolins, preventing C4 conversion to C4b and C1q and interfering in the activation of the CP and LP (170–172). TcCRT has also been shown to internalize parasites into mammalian cells (171). Another mechanism is T. cruzi Complement Regulatory Protein (TcCRP): the protein TcCRP, also called Gp160, is a 160-kDa glycoprotein anchored into trypomastigote membranes that can bind to C3b and C4b, preventing the assembly of proteolytically active C3 convertase, thus inhibiting the formation of CP and AP complement C3 convertase (168, 170, 173). The T. cruzi complement C2 receptor inhibition trispanning (TcCRIT) is a 32-kDa transmembrane protein mainly expressed in trypomastigotes. TcCRIT has amino acid sequence homology with the C4 beta-chain, the binding site of C2. Thus, it blocks C2 cleavage by C1s or MASP-2 into C2a and prevents C3 convertase formation, thus regulating the activation of the LP and CP (174–177) mediating cell lysis, allowing survival and cell invasion by the parasite. The glycoprotein 58/68 (Gp58/68) is a glycoprotein of 58 kDa (non-reduced) and 68 kDa (reduced) (178), expressed on the parasite surface or released by trypomastigotes. It is a part of the fibronectin/collagen receptor of T. cruzi and plays an important role in the interaction of T. cruzi with mammalian cells (178, 179). This protein was able to inhibit the formation of cell-bound and fluid-phase C3 convertases (178) to allow the parasite to evade AP complement activation by preventing the initial association of factor B (FB) with surface-fixed C3b (167, 178). The trypomastigote decay-accelerating factor (T-DAF) is an 87- to 93-kDa glycoprotein expressed on the surface of metacyclic trypomastigotes of T. cruzi. T-DAF mimics the activity of the complement regulatory protein DAF and regulates the activation of the AP, CP, and probably the LP of the complement by interfering with the assembly efficiency of C3 convertases (180, 181), which is essential for the escape of T. cruzi from complement activation and lytic effects (167).
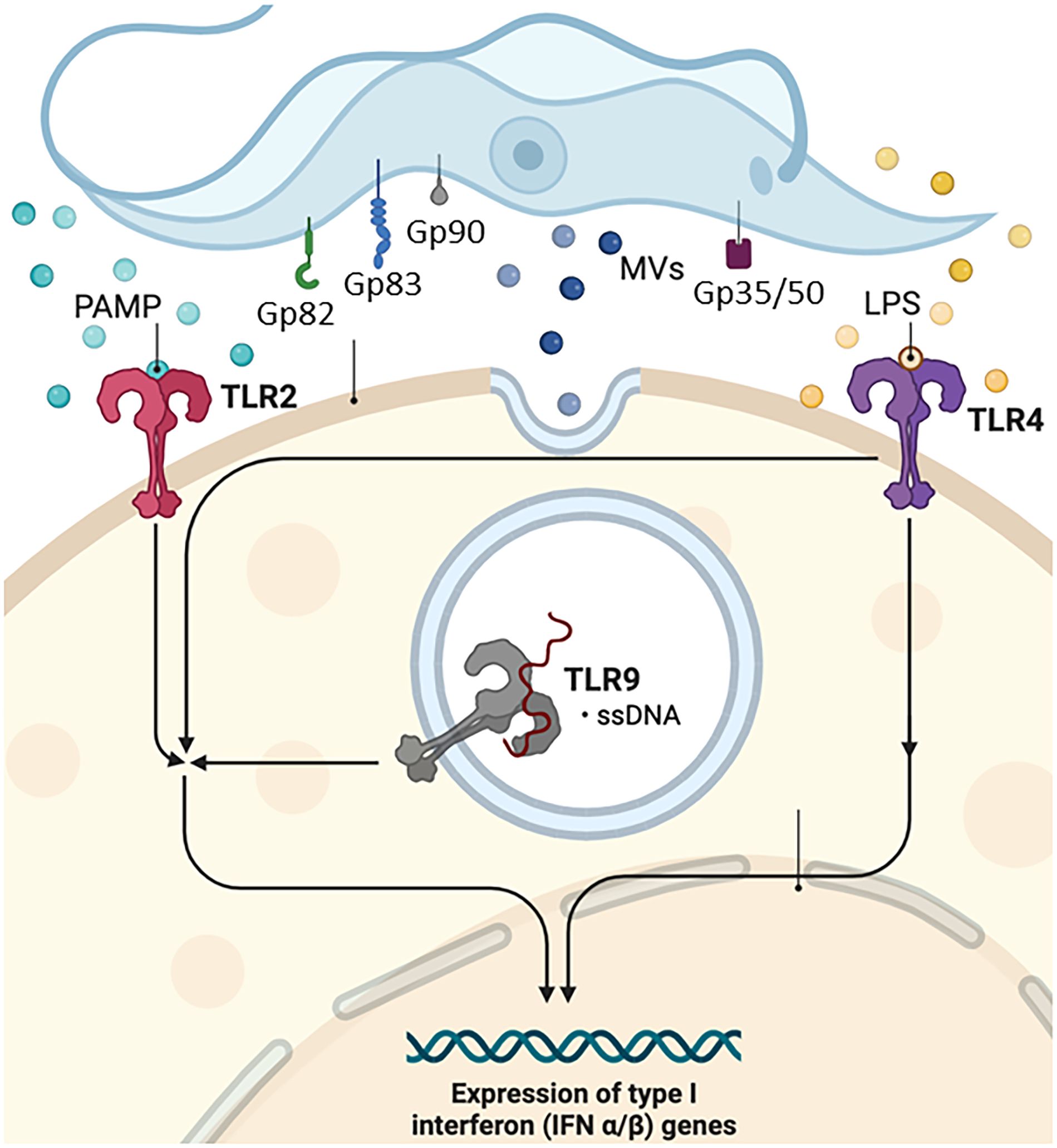
Figure 3 Complement system. Gp, glycoprotein; Tc, T. cruzi molecule; TLR, Toll-type receptors; MAC, complement membrane attack complex; MBL, mannose-binding lectin; MVs, microvesicles; T-DAF, Trypanosoma-decay accelerating factor. Figure created by the author on the biorender.com platform, inspired by an Oswaldo Cruz Foundation website publication.
Extracellular vesicles, whether microvesicles (MVs) or exosomes, shed by pathogens, transfer virulence factors and biomolecules to host cells, thereby altering the host’s susceptibility to infection (177) (Figure 1). Cestari et al. (2012) (176) and Wyllie and Ramirez (2017) (177) described a mechanism of complement immune evasion by T. cruzi through the induction of membrane-derived vesicles from host cells by metacyclic trypomastigote forms and the consequent secretion of these MVs. MVs are 100- to 1,000-nm molecules produced by outward budding and fission of the plasma membrane, released by cell blood, immune system, epithelial, and endothelial tissues, among others. They are an integral part of the intracellular microenvironment and act as regulators of cell-to-cell communication (182, 183). The complement system is a fundamental immune response component, recognizing and eliminating pathogens. T. cruzi may evade immediate immune responses by inhibiting the complement system, promoting its survival in the host environment (177). The infective form of the parasite induces the release of MVs from immune cells in a calcium-dependent manner at the beginning of infection. These MVs bind LP (177) and CP C3-convertase complexes on the surface of T. cruzi inhibiting complement-mediated lysis and favoring invasion and infection of host cells (176). Interestingly, MVs derived from different parasite strains (TcI and TcII) did not alter complement resistance and the invasion process (177). These studies provide valuable insights into the complex interplay between the parasite and host cell-derived MVs, establish a communication network that allows it to manipulate host cell functions, modulate immune responses, and potentially escape immune surveillance, contributing to the chronicity of T. cruzi infection and the establishment of persistent parasitemia (176).
According to Quijano-Hernandez and Dumonteil (2011) (184), the protective immune response against T. cruzi requires the activation of a Th1 immune profile with stimulation of T-cytotoxic cells and B-lymphocytes to secrete parasite-specific immunoglobulins (185). Furthermore, the control of parasite infection spread has also been associated with NK cells (186). Sathler-Avelar et al. (2006) (187) suggested that NK cells could protect against early T. cruzi infection, comparing the cytokine profile of patients with Chagas disease with healthy uninfected children. Vitelli-Avelar et al. (2005) (188) analyzed the frequency of NK cells in the peripheral blood of patients with Chagas disease, both of which were particularly high in indeterminate patients. These authors suggested that increased circulating NK cells may protect the host from morbidity during the chronic phase.
It has been proposed that T. cruzi DTU promotes diverse host immune responses and affects disease progression (Table 1). Similarly, anti-inflammatory and pro-inflammatory cytokines may play a pivotal role during infection and the differential regulation of cytokine synthesis in developing the Chagas cardiomyopathy or indeterminate clinical status (31, 188–190). It has been described that the lower expression of IL-10 is associated with the development of chagasic cardiomyopathy (190), and a higher expression was observed in patients with the indeterminate form (188–191), suggesting that IL-10 contributes significantly to parasite control without severe damage, regulating immune response during chronic Chagas disease. Magalhães et al. (2015) (119) showed that while TcI DTU induced IL-10 production and higher monocyte activation, TcII DTU led to lower monocyte activation but a higher inflammatory profile. TGF-β was demonstrated to play a role in regulation during the acute phase of Chagas disease, whereas it was shown to be a potent inhibitor of the effects of macrophage-activating cytokines, such as IFN-γ (192). IFN-γ and TNF-α, inflammatory cytokines, and cytotoxic cells have been correlated with tissue damage and the severity of chronic Chagas disease (190, 193–195) in cardiac patients, suggesting a role for pro-inflammatory monocytes in developing this disease. Guedes et al. (2012) (35) showed that higher levels of IL-17 were associated with less aggressive chagasic cardiomyopathy.
Distinct T. cruzi DTUs may exhibit variations in parasitemia, immune responses, and tissue pathology in their hosts (89–91, 93). Moreover, these parameters may differ in co-infections compared to T. cruzi mono-infections (89). In a study comparing the biological behavior of TcII and TcVI DTUs conducted by Rodrigues et al. (2010) (89), it was demonstrated that TcII presented reduced parasitemia in mice compared to TcVI. TcVI induced higher parasitemia, increased the systemic release of pro-inflammatory mediators, and induced higher mortality during the acute phase of the disease. Co-infection with TcII and TcVI results in intermediate parasitemia, heart parasitism, and mortality. This finding correlated with a decrease in inflammatory cells, suggesting that the T. cruzi co-infected animals exhibited a less intense inflammatory reaction in the heart. These data indicate that T. cruzi co-infection can trigger both protective inflammatory immunity and regulatory immune mechanisms that attenuate the damage caused by inflammation, consequently reducing disease severity (89). However, when the biological behaviors of TcII and TcIV were compared in infected mice, TcIV showed lower virulence and fewer inflammatory processes than TcII. This includes significantly lower parasitemia levels and a lower frequency of organs with inflammatory processes (90). Magalhães et al. (2019) (93) observed that TcIV induced a regulated profile in human monocytes, whereas TcV induced an inflammatory profile. Differences in the host’s cellular immune response were noted considering the different DTUs and phases of T. cruzi infection (91). In an experimental model using dogs, Duz et al. (2014) (91) proposed that, in the acute phase, TcI could go unnoticed by peripheral blood mononuclear cells, enabling faster parasitization of target organs. In the chronic phase, TcI presents characteristics of inflammation, with higher levels of IL-4 observed in the peripheral blood. TcII DTU triggered the production of IFN-γ and IL-4 in the acute phase, and significant heart inflammation and fibrosis were observed in the chronic phase. Differences in humoral immune response were also associated with different DTUs during acute and chronic T. cruzi infection in mice. TcI is more efficient in overexpressing specific antiparasitic IgG subclasses (IgG, IgG1, IgG2a, and IgG2b) than TcII and TcV (88). This finding was recently corroborated by Silveira-Lemos et al. (2021) (81), who demonstrated that distinct T. cruzi genotypes influence the phenotypic and functional features of the host immune response.
The host genetic background plays an important role in the course of infection, such as the genetic variability among the six different DTUs of T. cruzi that interact differently with the host and their eco-epidemiological effects. The immune response is decisive throughout the entire process. The cytokine profiles switch between anti-inflammatory and pro-inflammatory cytokines, which may be relevant in determining chronic patients’ clinical presentation and disease outcomes. These results show that the progression of human Chagas disease from asymptomatic to severe forms is related to a lack of adequate immune modulation.
5 Pathogenesis and DTUThe clinical course of Chagas disease is polymorphic, ranging from asymptomatic to severe chronic cardiovascular or gastrointestinal involvement. After parasite transmission, trypomastigote forms of T. cruzi can invade different cell types, including macrophages, muscle cells, and fibroblasts. Parasitic amplification into the cytosol results in intense tissue parasitism and blood parasitemia during the acute phase. The symptoms in this phase are generally mild, except in immunosuppressed patients and children who may develop cardiomyopathy and encephalomyelitis (196, 197). After the acute phase, parasitemia decreases in the chronic phase of the disease, starting with the asymptomatic or indeterminate form, where no clinical symptoms are observed. Most individuals remain in this phase of infection throughout the rest of their lives. Approximately 30% of these patients develop chronic phase disease with cardiac and/or gastrointestinal symptoms. This involvement may be severe and a determinant of the morbidity of the disease (32, 198, 199).
Cardiomyopathy is one of the most important clinical changes in patients with Chagas disease in the chronic phase due to the high frequency with which it develops (in 20%–30% of infected individuals), in addition to its severity, morbidity, and mortality (200). The classic pathology of chronic Chagas cardiomyopathy includes myocarditis accompanied by myocytolysis, myofiber hypertrophy, and interstitial fibrosis (201). In general, only focal areas of inflammation are found in the hearts of patients with chronic Chagas disease. Inflammatory infiltrates mainly comprise T cells, macrophages, eosinophils, plasma cells, and mast cells (200). Fibrosis in chronic Chagas disease exhibits a unique distribution pattern, often surrounding and involving individual myocardial fibers. This feature distinguishes it from the fibrosis patterns in idiopathic dilated cardiomyopathy (202, 203). Moreover, fibrosis in Chagas disease can also be characterized by intrafascicular deposition of collagen (204, 205), which causes disorganization and isolation of cardiomyocytes and contributes to the electrocardiographic changes observed in a canine model and in humans (206). The host innate and adaptive immune responses may contribute to cardiac damage and increase the risk of heart failure through the induction of inflammation, fibrosis, and oxidative stress injury, leading to myofibril rupture, cardiomyocyte necrosis, microvascular dysfunction, autonomic dysfunction, cardiac hypertrophy, and fibrosis (200). Duz et al. (2014) (91) evaluated the immune response and cardiac lesions in dogs infected with Colombian (TcI) and Y (TcII) strains of T. cruzi. The authors demonstrated that in the chronic phase, the inflammation triggered by the Y strain was balanced by tissue rearrangement and fibrosis, whereas infection with the Colombian strain showed more significant inflammation and a lower degree of cardiac fibrosis. Ferrer et al. (2014) (207) analyzed the impact of different T. cruzi isolates obtained from Argentine patients (TcI, TcV, or TcVI) on cardiac tissue from T. cruzi-infected CF1 mice and showed that the degree of myocarditis ranged from minimal to mild for TcV and TcVI to moderate to intense for TcI. T. cruzi DTUs define the pattern of inflammatory mediators in heart tissue and may contribute to the magnitude of cardiac pathogenesis (208).
Controversies surround the question of the relative contribution of autoimmunity and the presence of the parasite to the development of chronic T. cruzi infection. Persistent inflammation and divergence between low parasite load in the tissue and the severity of the lesions observed during the chronic phase support this autoimmune etiology (209, 210). The autoimmunity hypothesis suggests that tissue damage leads to an immune reaction against self-proteins. This theory has been considered a key mechanism to explain the tissue damage observed in the chronic phase of the Chagas disease, even in the absence of parasites in the affected tissues.
However, despite the wide acceptance of autoimmunity in the etiology of Chagas disease, several studies have suggested a strong association between parasite load and disease severity (211–214). Even if anti-self-immune responses are induced in these models of Chagas disease, these responses are insufficient to generate disease without local parasitic infection. The direct pathogenic role of the parasite has gained strong support from very sensitive new immunohistochemical techniques and applications of PCR, which show a strict correlation between the presence of the parasite and tissue lesions (210, 215, 216).
The circulation of distinct genotypes of T. cruzi within infected triatomines suggests complex interactions among various parasites, leading to diverse infection patterns (125, 217). This genetic diversity may account for regional variations in the incidence of the cardiac and/or digestive forms of the disease. However, the distinct clinical manifestations of Chagas disease can arise from factors beyond the genetic diversity of T. cruzi, such as the interaction between the parasite and the host (60, 125, 190, 218).
The varied symptoms of Chagas disease likely result from differences in the immune response among individuals, which effectively control parasitic levels and limit organ damage, while inefficient responses fail to control parasite proliferation, resulting in persistent inflammation and severe clinical manifestations (219). Parasites selectively form multiclonal populations in different individuals, giving rise to stable lineages. According to the “clonal histiotrophic model” of Chagas disease, the polymorphism of biological factors allows distinct T. cruzi clones within a lineage to exhibit tropism for various tissues, which also contributes significantly to the clinical outcome of the disease (220, 221). Classic studies have demonstrated that the Y strain (TcII) is associated with high parasitism of the spleen, liver, and bone marrow in addition to increased virulence in the acute phase of the infection, whereas the Colombian strain (TcI) presents cardiomyotropism and pathogenicity in the chronic phase (78, 222, 223). The T. cruzi clone Sylvio-X10/4, belonging to the TcI DTU, also produces myocarditis in C3H/He mice, similar to that observed in human chronic Chagas disease caused by the Colombian strain (TcI) (224). On the other hand, different groups have pointed out that the Y strain (TcII) produces inflammation at the cardiac level in mouse models (225), and that infection with the CL (TcVI) strain results in high parasitism of countless cell types, indicating that the T. cruzi strain is pan-infective and does not affect only the heart tissue (226). In a canine model, the Y strain (TcII) triggers a more robust immune response during the acute phase of infection than the Colombian strain (TcI) (127). Colombian infection (TcI) shows characteristics of inflammation during the chronic phase. It is possible that this strain can escape the host’s acute immune response and parasitize the organs faster, while the Y strain (TcII) triggers a specific immune response during the acute phase, helping to control myocardial lesions in the early chronic phase (91). A more recent study realized by Reis Machado et al. (2014) (92) shows that the animals infected or reinfected with the Colombian strain (TcI) had a significantly higher expression of IFN-γ, both in the cardiac tissue and in spleen cells when compared to groups infected with the Y strain (TcII).
De Diego et al. (1998) (227) found qualitative and quantitative histopathological differences in studying T. cruzi genotypes belonging to different DTUs. Genotype 39 (TcV) was detected in infected mice’s skeletal muscle, spleen, liver, and heart. In genotype 20 (TcI), heart inflammatory foci were small. In genotype 19 (TcI), skeletal and cardiac muscle fibers were the most parasitized, demonstrating that differences can occur between T. cruzi strains in the same subgroup (206). In rats, infection with JG (TcII) and CL Brener (TcVI) revealed differential tissue tropism among the strains. While the JG strain (TcII) occurs in the cardiac muscle, CL-Brener (TcVI) is detectable in skeletal muscles and other organs (129). In a study of Colombian patients, TcII was detected in the heart, causing chronic chagasic cardiomyopathy (CCC). In contrast, TcI was found in the muscular layer of the esophageal tissue, along with lymphocytic infiltrates and interstitial fibrosis (126). In D’Avila et al. (2009) (228), the authors did not find a correlation between the genetic profiles of T. cruzi isolates and different clinical forms of Chagas disease. Likewise, Del Puerto et al. (2010) (64) analyzed blood samples from 306 Bolivian patients with Chagas disease in the chronic phase and found no associations between T. cruzi DTUs and the clinical manifestation of the Chagas disease. The differences between the findings indicate that histopathological studies can produce different results according to genetic variations between T. cruzi strains and the experimental model or type of clinical study performed.
It is well known that T. cruzi can change virulence and tissular tropism over time to maintain its infectious properties, depending on factors from the host (229, 230). Many groups have not successfully correlated the parasite’s genetic variability with the disease’s clinical characteristics since most studies on T. cruzi isolates and the parasite’s growth in laboratory animals or in vitro cultures have a high chance of clonal selection. Often, parasite populations in the heart, skeletal muscle, liver, or spleen differ from those found in the host’s blood (231). The presence of different T. cruzi DTUs with different tropisms in the same host originated from distinct populations of parasites in diverse tissues, even when infected with a particular strain. Some studies have d
留言 (0)