Toward the end of the 20th century, we raised the prospect that even mild to modest levels of “insulin resistance” (IR)1, the principal driving force behind type 2 diabetes mellitus (T2DM) and the ubiquitous metabolic syndrome (MetS),[1-3] might play a significant adverse role in overall health and the “natural” biological aging phenomenon even in average fit, nondiabetic individuals.[4-8] Our purpose is to “determine if the slight intensification of IR and/or its associated metabolic risk factors over the lifespan of most nondiabetics is great enough to reduce general health status and accelerate aging patholog y”[4-8] and to ascertain if the presence of diagnosed metabolic disorders, for example, T2DM and/or MetS, can assure enough potency of IR to produce significant, adverse aging-related trends.
The mild to moderate presence of IR and its accompanying risk factors have been suggested to hasten aging in aging nondiabetics over the lifespan.[9-11] Evidence in volunteers that the appearance of “survivor bias” in the elderly can be linked to a weakened IR status is also somewhat reassuring of such a possible happening.[9-14] 2
The goal is to analyze a novel version of the aging process (age-related renal estimated glomerular filtration rate [eGFR] decline in addition to chronological age)[15-22] and employ another novel but less popular surrogate for IR (ratio triglyceride/high-density lipoprotein [HDL]-cholesterol in addition to fasting blood glucose [FBG]).[23-26] The utilization of lifestyle improvements in dietary and exercise regimens, as well as proper use of natural dietary supplements and pharmaceuticals, enhance insulin sensitivity and mitigate manifestations related to MetS[27-30] peH.
MATERIAL AND METHODS Clinical materialThe clinical materials for this study came from Integrative Health Technologies, Inc., a Clinical Research Organization in San Antonio, Texas, USA. The Longitudinal Database of Medical Biomarkers includes multiple dual energy X-ray total body scans (DEXAs) and thousands of fasting blood chemistry measurements. The data were obtained from study participants representing all 50 States. All measures were taken to ensure that the subjects involved in the present study were essentially “normal volunteers” after historical health matters were considered. In short, the volunteers were relatively healthy, nondiabetic individuals. Many values in this single-center and observational cohort study were expected to fall in commonly accepted “normal” ranges. Linear correlations became key means to assess trends in developments related to health matters; that is, slope-based rather than threshold analyses are employed largely here.[9,10]
Baseline data were accumulated from male and female subjects (age 25–87 years). The material was collected from tests conducted from February 2014 to July 2019 on purportedly “nondiabetic, healthy subjects” who had agreed to participate after they received a Consent Form and were requested to review the form with their healthcare provider to confirm that they had no medical conditions that would preclude their involvement. Information was used only from subjects with a circulating FBG level in the nondiabetic range (125 mg/dL or less). Individual data employed for analyses were limited to values within three standard deviations of the group mean. This created very little elimination of material, and data from 1049 subjects were still left to evaluate material from volunteers who granted written permission to use the redacted data in future analyses. “Resting” blood pressures were obtained after study participants had remained prone for ~15 min while completing their DEXA testing. All study participants executed an informed consent form in compliance with the Helsinki Declaration and were allowed to participate by the independent Institutional Solutions Review Board (IRB) (http://ohrp.cit.nih.gov/search/IrbDtl.aspx). Quest diagnostics conducted all blood testing after study participants had fasted for at least ten hours. Quest also provided the glomerular filtration rate (GFR) readings estimated from circulating creatinine values.[31] These are listed as “eGFR.” To measure the strength and direction of the relationship between correlations, the Pearson correlation coefficient ® was used to calculate the significance level (P).
Time intervals consideredThe first period consists of the developmental stage, where individuals are growing from childhood and mature into adulthood. This time interval is devoted largely to growth and development, which was specified to last through the age of 24 years. The second period begins with the initiation of the characteristic decline in the renal GFR.[22] This mechanism (declining GFR) may be intrinsically related in some manner to the general aging phenomenon preceding final declining health and eventual death.[9,10] This life cycle aging period in the data interpretation presented arbitrarily begins at 25 years of age and lasts until 70 years. During this longest designated period of the arbitrary lifecycle, many risk factors pertaining to MetS and the aging process continue to progress. The functioning of a variety of risk factors such as blood pressure, a multitude of dyslipidemias, and glucose-insulin perturbations is taking place and providing a continuum of metabolic risks linked to the aging process despite not usually reaching levels consistent with any disease diagnoses – especially for our purpose, diabetes mellitus.
For a more precise approximation, the timing is sometimes split in the period that we have labeled in the past “Continuum of Risks” (CR).[9,10] This temporal interval is at times opportunely divided here further into an earlier (CR early, 25–50 years) period and a later (CR late, 51–70 years) period.
The 3rd time sequence (Aging Paradox [AP]) is set here to begin at 71 years and end at the oldest age level, 87 years.[9,10,14] This last temporal stage contains an unanticipated paradox that often defies reason when compared to the preceding CR period – marked improvements in many aspects of the glucose-insulin system relating to MetS.
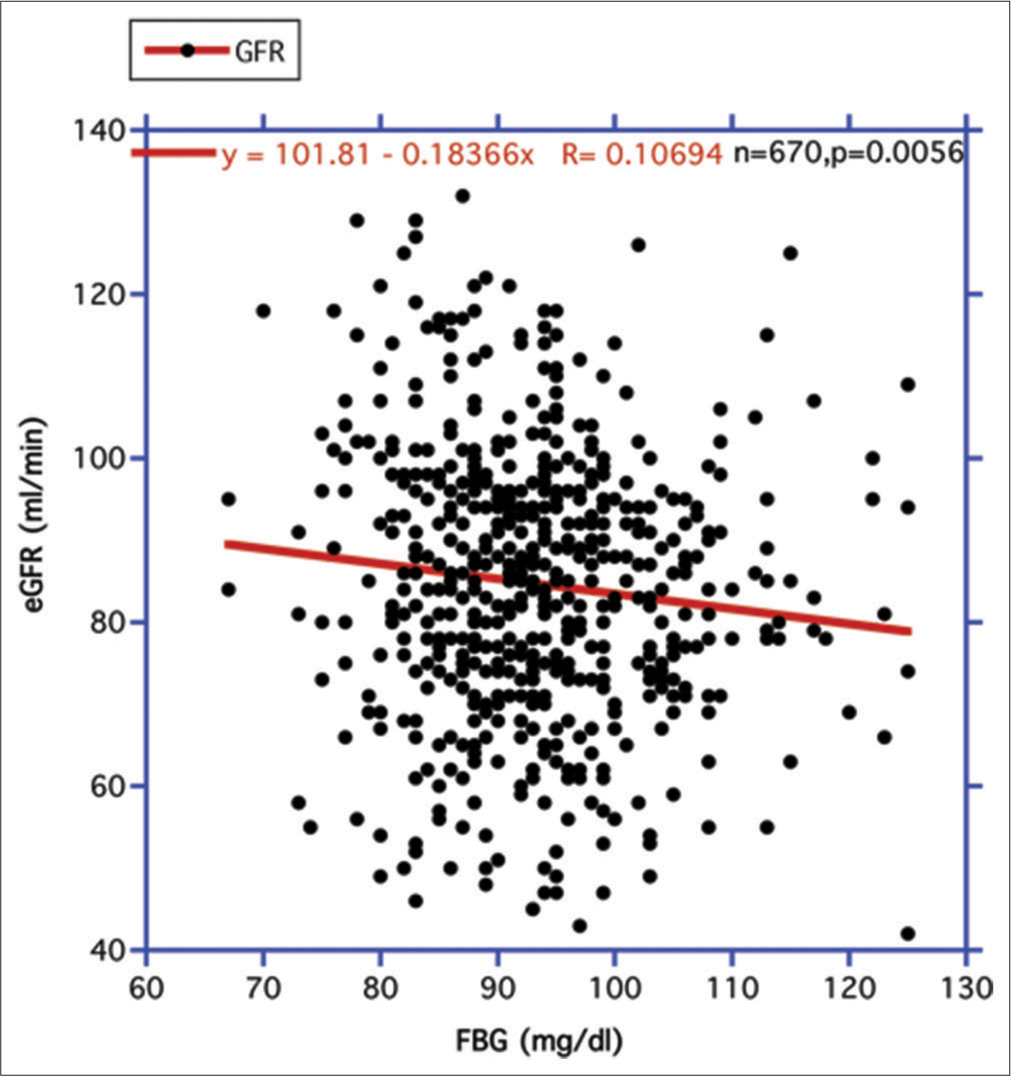
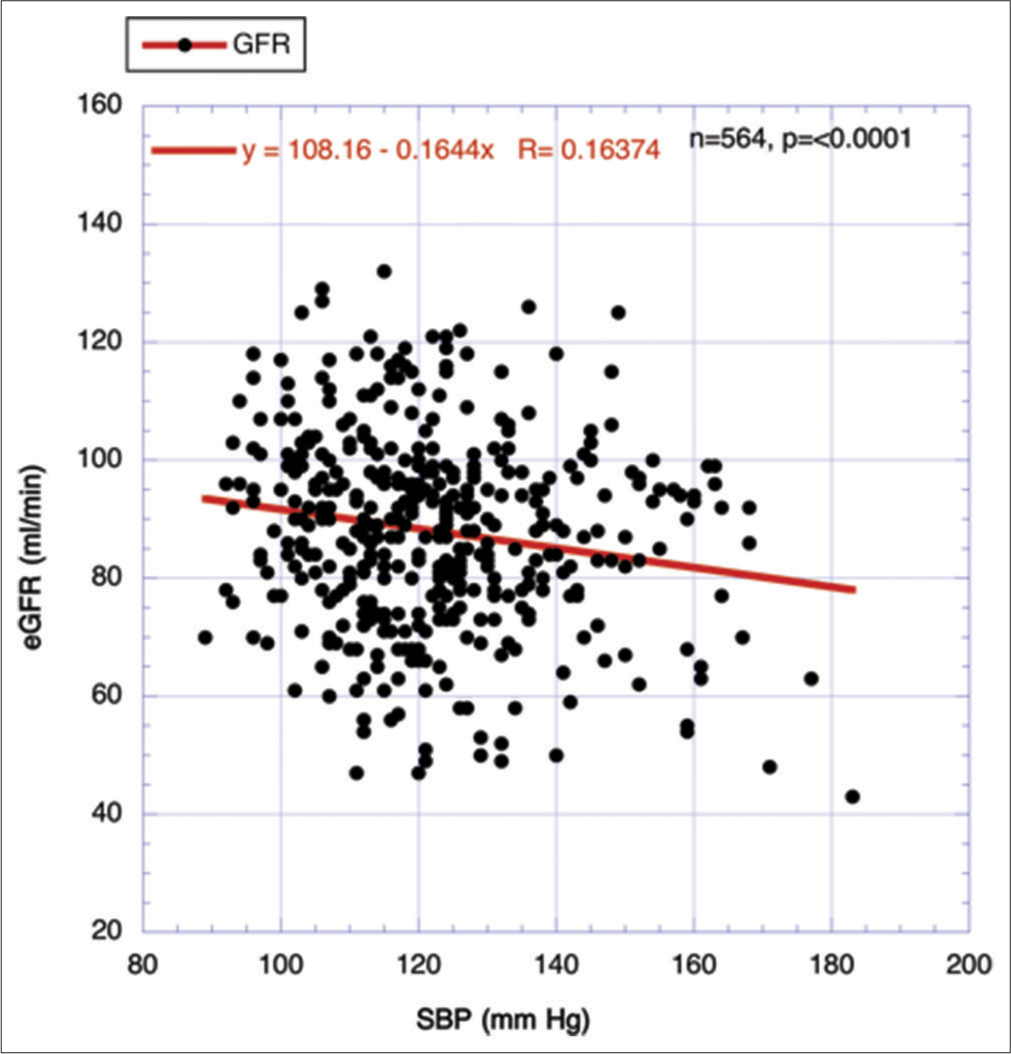
RESULTS Focusing on the association of declining human eGFR over the lifespan with the IR surrogate FBG and the level of systolic blood pressure (SBP)Two major metabolic driving forces behind age-related GFR decline are circulating FBG levels, which can be used as a surrogate for the strength of IR, and secondarily, elevations of SBP.[18,20,22]Figure 1 displays the relationship between FBG and eGFR. A statistically significant negative correlation is revealed. Accordingly, when FBG rises, eGFR decreases. In contrast, when fat mass (FM) replaces FBG as the independent variable, no such statistically significant relationship is evident positively or negatively (R = 0.024778, n = 670, P = 0.522) (figure not shown). In Figure 2, the elevation in SBP is highly and significantly correlated with the decline of the eGFR over the combined CR period, as previously reported.[18]
Export to PPT
Export to PPT
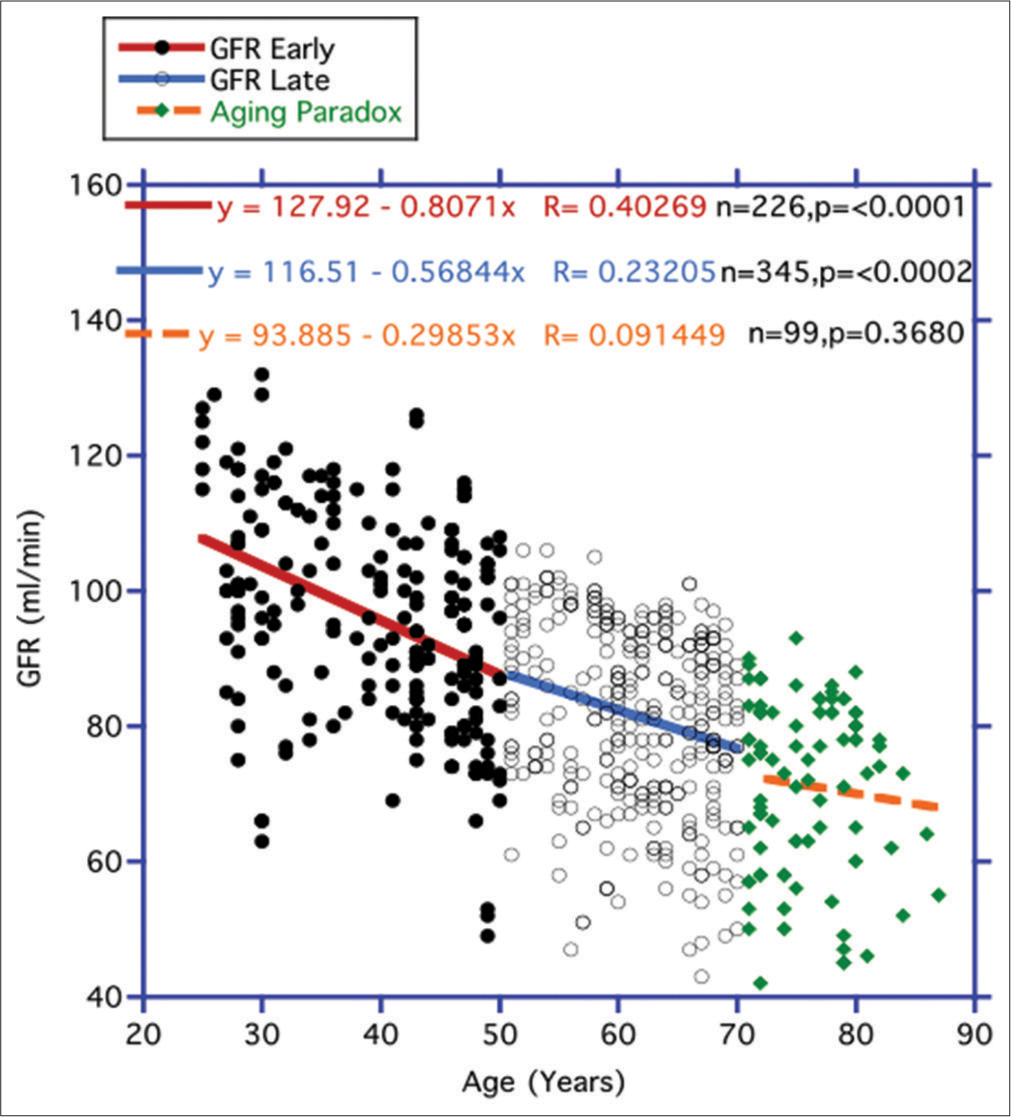
Comparing the progression of eGFR and FBG over the arbitrary lifespanUnlike previous findings,[21] we did not uncover an ever-increasing rate of eGFR descent in Figure 3 over the lifespan in the two consecutive CRs periods black dots/red line for early (25–50 years) and open dots/blue line for later (51– 70 years) periods respectively. There seemed to be a lessening of the decline over the later temporal interim compared to the earlier CR period. In any case, there was no eventual increase in the rate of descent with age between these first two timed separations. During the period referred to as the AP (71–87) years portrayed here by the green diamonds orange broken line), the decline was even less. Unlike the preceding two designated CR periods, a statistically significant negative correlation was not established for this temporal interval (P = 0.3680).
Export to PPT
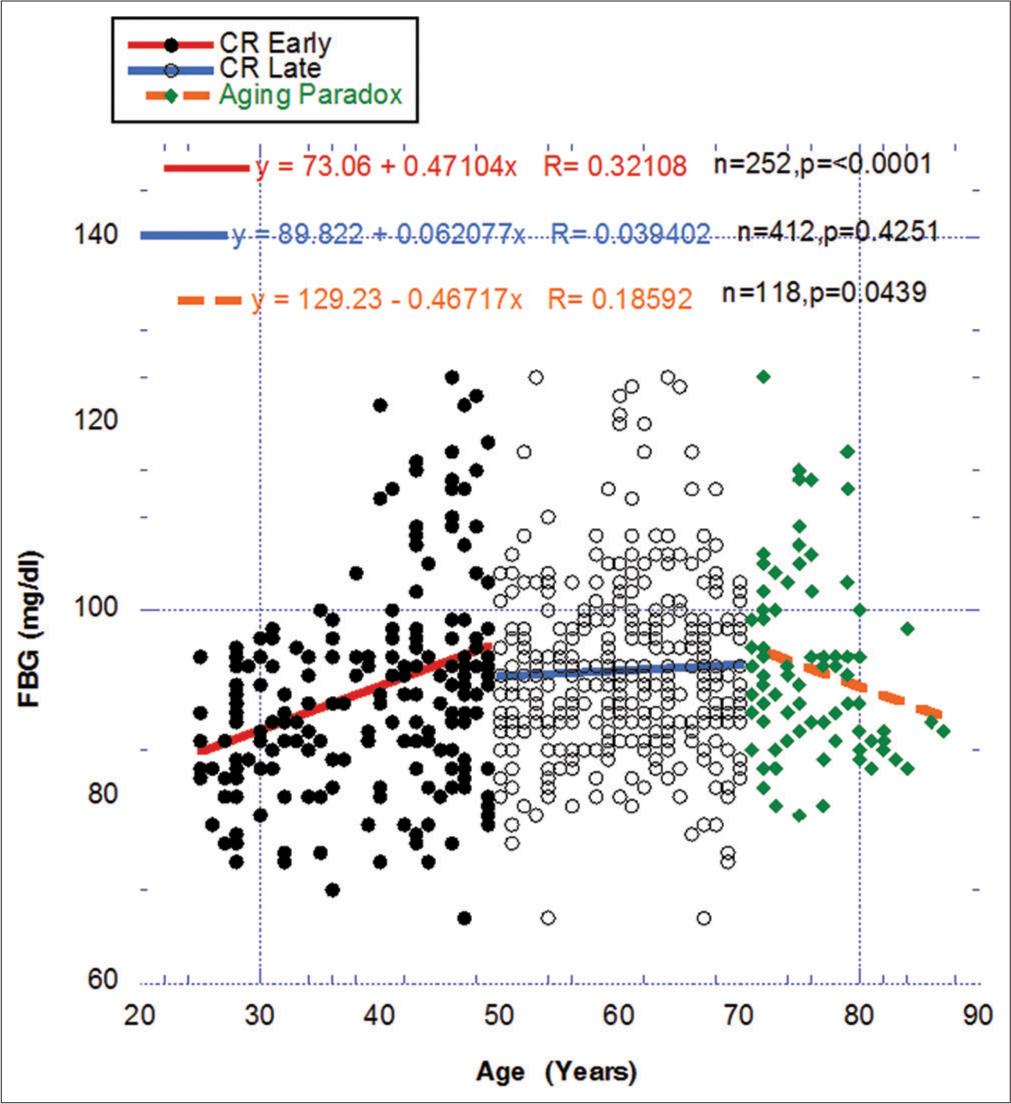
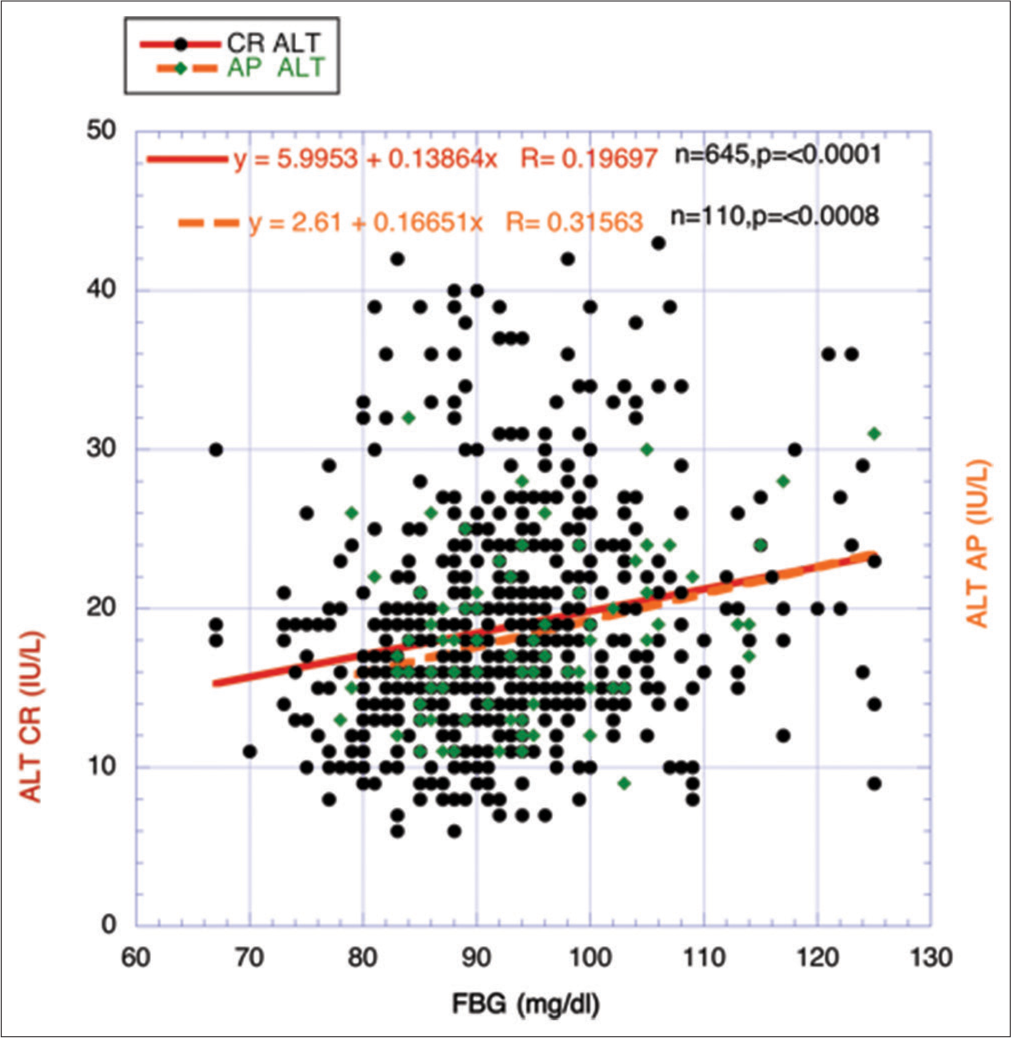
For comparative purposes with the previous figure, Figure 4 portrays a similar temporal relationship between FBG, a surrogate for IR, over the three designated periods between ages 25 and 87. Even with a more precise definition of the overall CR period, the early rise and eventual maintenance in FBG over the early and late CR periods coincides with the gradual reduction in eGFR in Figure 3. In contrast, the modification to a less significant descent of eGFR in the AP period Figure 3 matching the statistically significant changing negative direction of FBG also in the AP period Figure 4 strengthens the possibility of a meaningful relationship between IR and the declining eGFR. Does this represent in this figure an overall metabolic alteration, that is, improvement in healthful metabolism in the AP period compared to the CR period?
Export to PPT
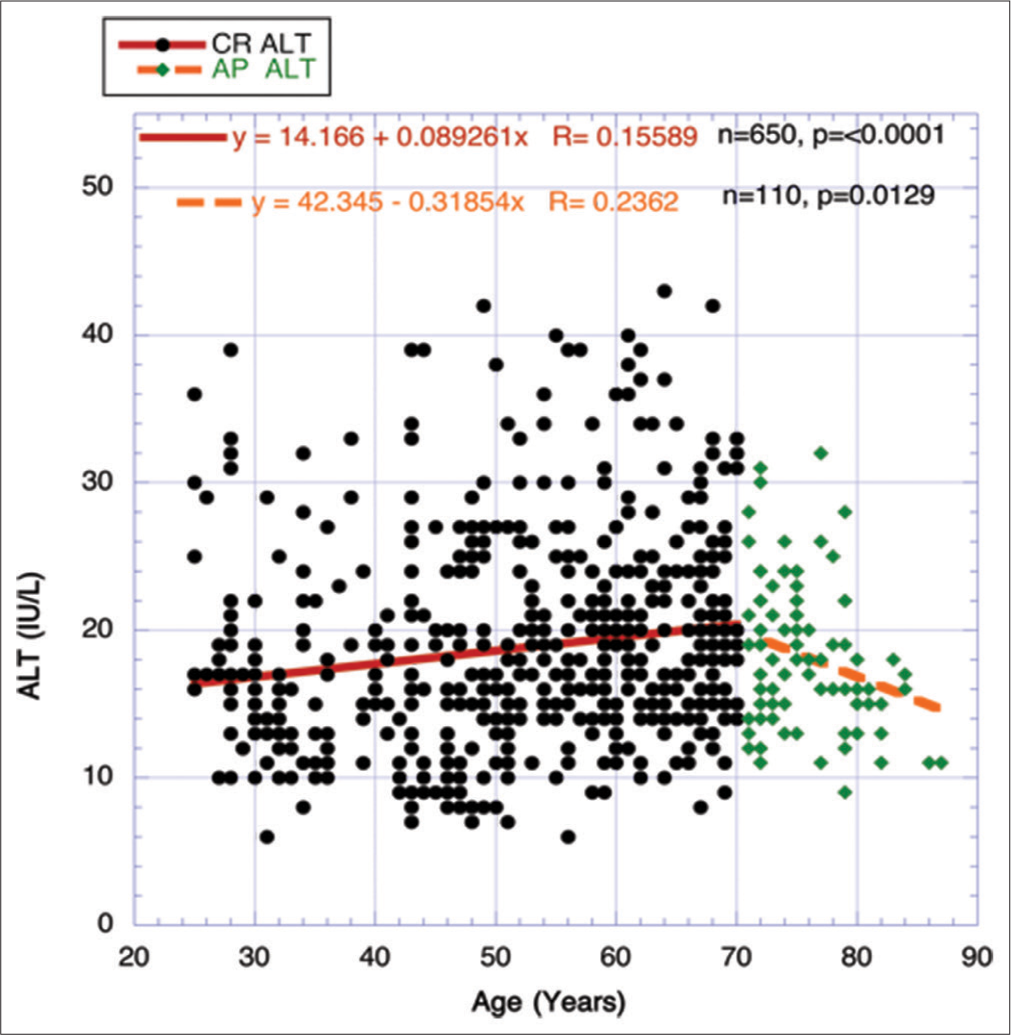
Figure 5 compares the separate correlations between the whole CRs period and the AP period using FBG as the independent variable and one selected aspect of the MetS cadre – alanine aminotransferase (ALT) as the dependent variable. The CR period shows significant upward mobility for ALT, while the AP period reveals a significant negative descent. The very close correlations in Figure 6 between the two CR and AP periods in ALT slopes suggest no real change in IR, and the hepatic enzyme activity relationships in those periods explain the occurrence of the paradox through changing major metabolic parameters. This strengthens the survivor bias theory considerably.
Export to PPT
Export to PPT
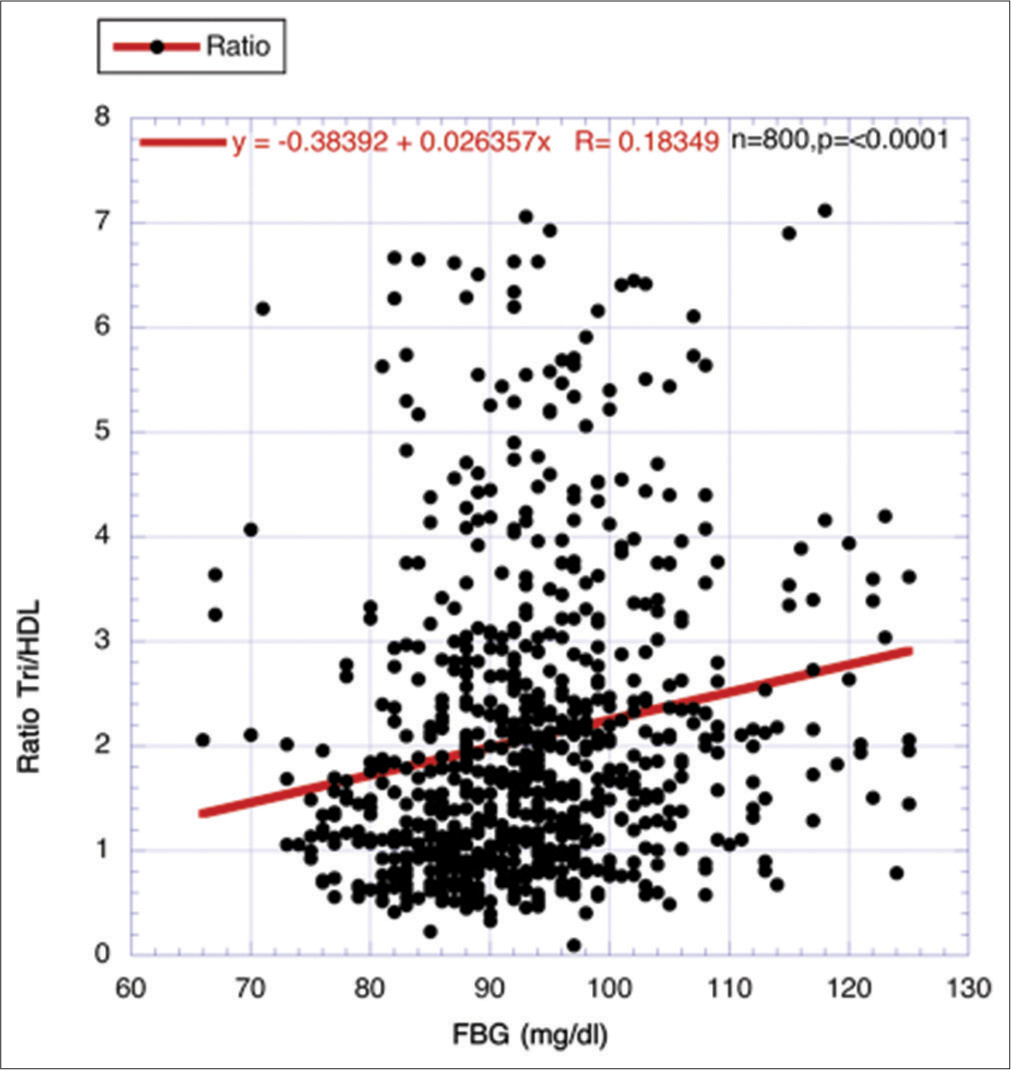
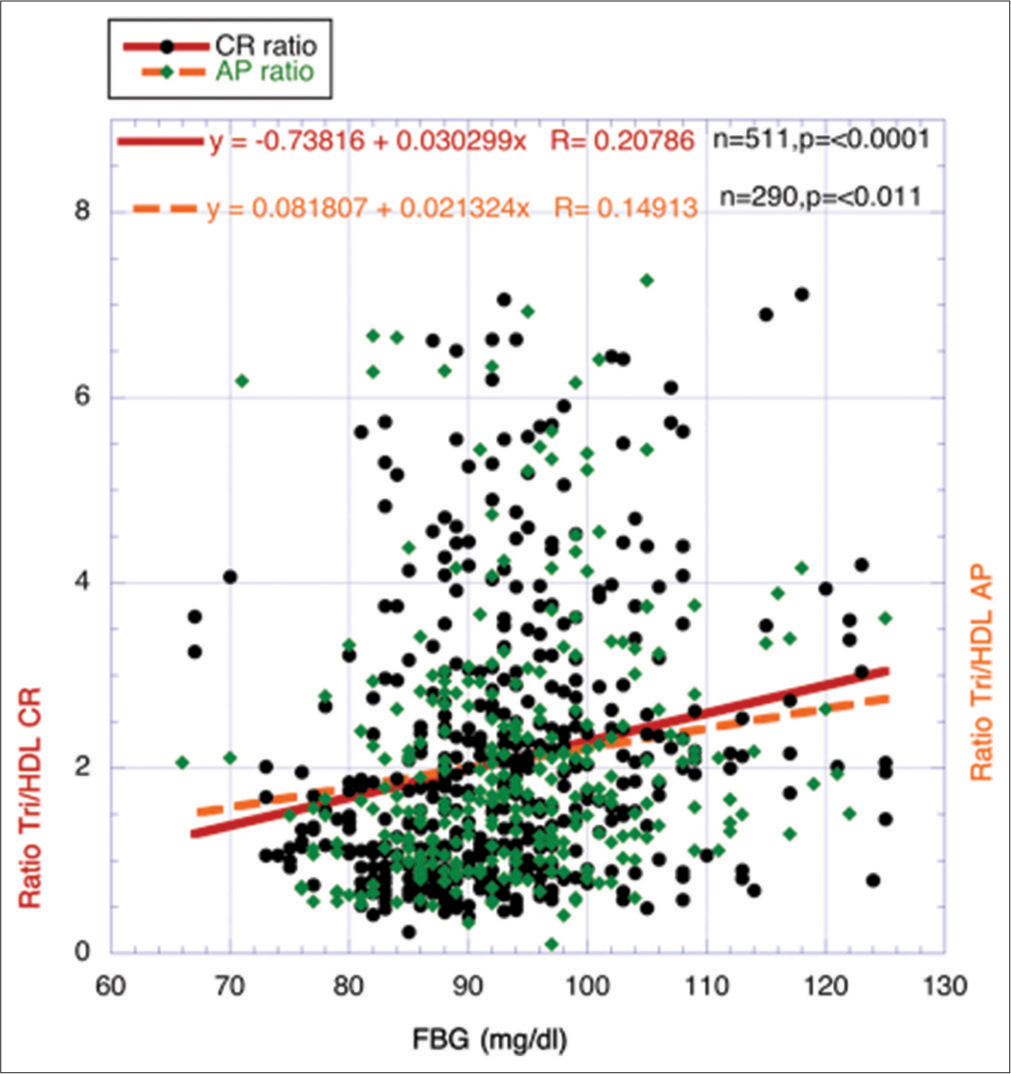
Corroboration through another surrogate for IR, that is, ratio of Tri/HDL-cholesterol, correlating with characteristic descent of eGFR during agingThe ratio between Tri and HDL-cholesterol has been used effectively as a surrogate for IR, and different investigators have previously found a strong relationship between the strength of IR and the ratio.[22-26] Data from 800 volunteers (ages 25–87 years), a highly statistically significant positive correlation, was found between FBG and the ratio Tri/HDL-cholesterol (P < 0.0001) [Figure 7].
Export to PPT
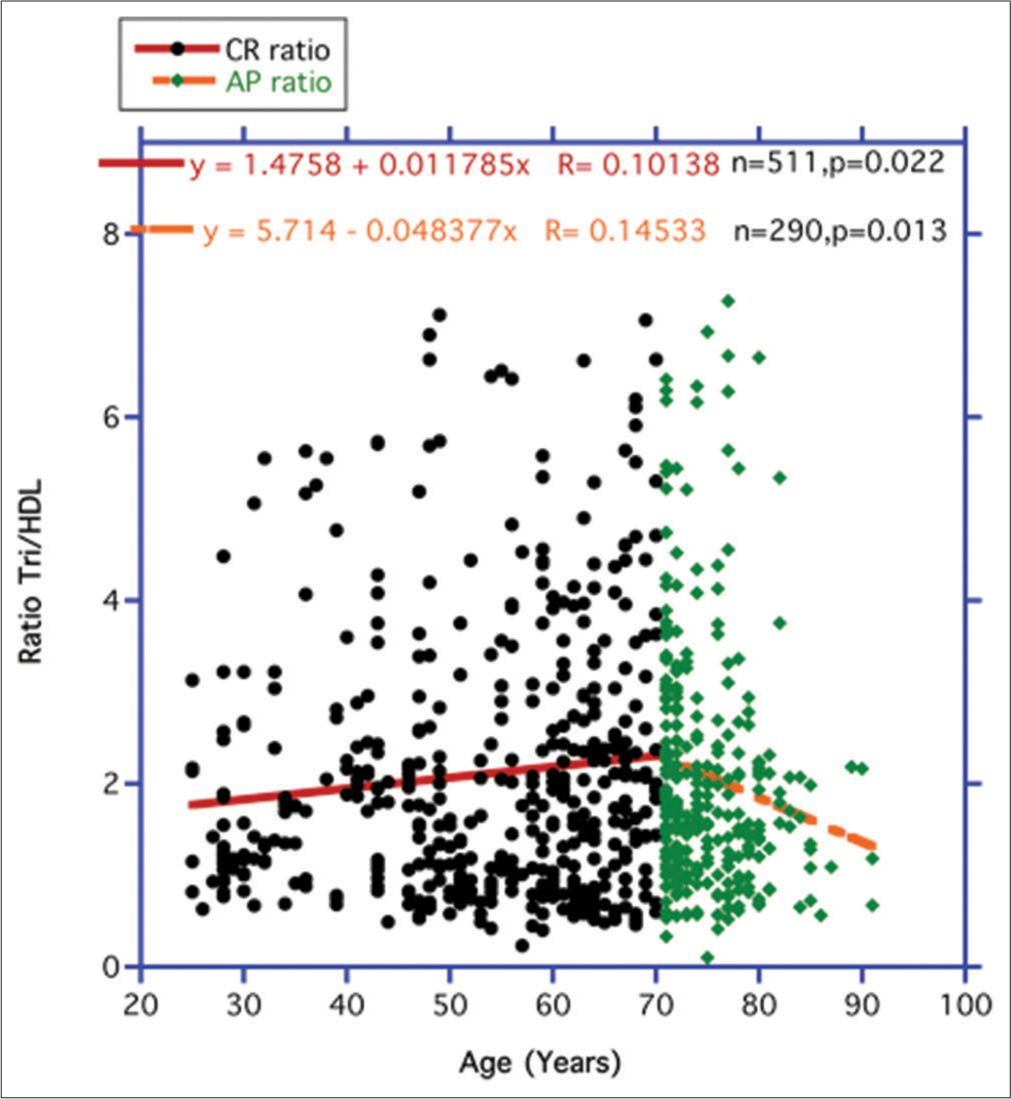
In Figure 8, the independent variable FBG was replaced with the ratio Tri/HDL-cholesterol as a surrogate for IR. Over the entire CR period in Figure 8 (25–70 years), the strength of IR represented by the ratio increases significantly, but during the AP period (71–87 years), IR shows a significant downward decrease like that shown for FBG and ALT in Figures 4 and 5.
Export to PPT
Figure 9 once more resembles the previous outcome for ALT in Figure 6; that is, no significant metabolic changes take place in the correlation slopes between FBG and Tri/HDL from the two temporal periods to account for any significant metabolic changes between these two independent variables during the CR and AP Periods.
Export to PPT
Comparison of eGFR with various components of MetSDuring the long CR period that comprises 46 years (ages 25–70 years), several statistically significant associations appear when correlating eGFR as the independent variable with various components of MetS [Table 1]. The slopes using the declining eGFR as the independent variable were significantly negative with passing time for FBG, SBP, low-density lipoprotein-cholesterol, Tri, non-HDL-cholesterol, and ALT.3 FM and HDL-cholesterol revealed no statistically significant linkages. A previous suggestion has been strongly accepted that IR, represented here mainly by circulating FBG levels, not so much FM, drives most elements comprising MetS.[28]
Table 1: Correlating parameters linked to the metabolic syndrome throughout the active continuum of risks period with the declining eGFR in human volunteers.
Dependent variable N Slope R P FBG (mg/dL) 571 −0.057 0.09464 0.023722 SBP (mmHg) 549 −0.130 0.13337 0.001737 Fat mass (pounds) 565 +0.041 0.02284 0.587978 Triglyceride (mg/dL) 578 −0.317 0.08692 0.036696 HDL-cholesterol (mg/dL) 564 −0.026 0.02638 0.531834 LDL-cholesterol 526 −0.280 0.14904 0.000361 Non-HDL-cholesterol 532 −0.306 0.14145 0.001070 ALT (IU/L) 567 −0.055 0.12816 0.003401 DISCUSSION Historical recognition of the insulin-resistant-driven MetSReaven, in the 1980s, emphasized the close connection between several adverse detrimental metabolic events such as corpulence, glucose-insulin aberrations, lipid abnormalities, and elevated blood pressure.[1,28] Even more exceptional, Reaven promoted the fundamental concept that intensified IR, a most prominent glucose-insulin abnormality, is the primary driving force behind the other accompanying harmful components of MetS. The associated metabolic interactions predominate in the elderly also proved to be valuable critical information, particularly regarding the current presentation.[1,11,28]
Ironically, the specific recognition of the perturbed glucose-insulin interplay (IR) as a major driving force behind other metabolic aberrations had not been generally recognized earlier on despite its evident importance then and now. This key perception, as pointed out by Morley and Sinclair, only blossomed at the beginning of the 1920s.[32,33] Conceivably, much confusion may have arisen based on nomenclature difficulties because this syndrome has had several differing labels over the years. The grouping of hyperinsulinemia, hypertriglyceridemia, and hypertension was initially promoted as “Syndrome X.”[1] This metabolic collection was retitled “insulin resistance syndrome” to emphasize the importance of events that produce and amplify adverse health elements.[34] Nevertheless, a term attributed to Haller in 1977 predominates today, and the clustering and linking of the characteristic aspects, along with many new additions, are now universally established and recognized as the “Metabolic Syndrome.”[35]
Defining the MetSThe generally accepted form of MetS comprises many familiar health risk factors that are solidly linked to the development of cardiovascular,[1,3,24,28,36,37] hepatic,[9,38-40] and renal infirmities.[41-46] The diagnosis of MetS is IR/ T2DM; overweight/obesity; dyslipidemias (specifically hypertriglyceridemia and low HDL-cholesterol levels); and elevated SBP/hypertension readings.[1-3,47] The effect of MetS can include coagulation difficulties,[48,49] amplified general inflammation,[28,50] and/or fatty infiltration of the liver (Non-alcoholic fatty liver disease, NAFLD).[38-40] Indeed, the intertwined triad of MetS, T2DM, and NAFLD closely share many pathophysiologic characteristics.[40]
Informative background dealing with the role that IR and linked metabolic components have on healthy well-being and aging in nondiabetic individualsA close correlation exists between IR and various metabolic risk factors found in our purported abated version of MetS.[51,52] Employment of circulating concentrations of FBG, insulin, and/or HbA1C as the independent variables representing IR in cross-sectional investigations shows strong links with various components of MetS when using correlative assessments.[32,33] It is essential to accentuate that the present material employed originated from relatively healthy volunteers (no conclusive evidence of diabetes) receiving baseline evaluations to eliminate actual diagnosed T2DM before entering a variety of clinical studies. In these volunteers, risk factors associated with MetS usually intensify gradually with time passage. Generally it is observed between chronological ages between 25 and 70 years.[9,10] As mentioned in the Methods Section, we labeled this temporal phase “Continuum of Risks,” a period whereby the metabolic maladies that correlate with glucose-insulin perturbations usually adjust unfavorably but, for the most part, rarely exceed or fall outside ranges that would diagnose established pathological diseases. Ironically, much further along the age scale in our cross-sectional analyses, the potencies of some of these risk factors surprisingly appear to revert significantly in an opposite, beneficial direction.[9,10,53]
Because separating and then comparing data from the CR and AP periods does not reveal any significant variances in the glucose-insulin relationships and their connection to other components of MetS, the favorable direction of experiences in AP is largely attributed to “Survivor Bias” rather than counteracting metabolic modifications.[9,14] The meaning behind this is that those volunteers possessing the more promising profile of risk factors over time can contribute actively to ongoing clinical studies, whereas participants with poorer profiles may not participate as volunteers in later life due to medical debilities or even death4.
In solid corroboration with this interpretation, De Boer et al. have been able to show in one of their studies that older adults with the highest insulin sensitivity index (ISI) and/ or circulating fasting insulin levels were the most prone to increased risk of death.[36] ISI was calculated from fasting and 2-h post-load insulin and glucose concentrations. Correspondingly, the level of FBG, our routine surrogate for IR in nondiabetic volunteers, has long been noted to be a risk factor for cardiovascular death.[37] Finally, data from a few studies dealing with centenarians confirm the importance of maintaining ideal glucose-insulin regulation in prolonging lifespan.[54-56] Healthwise, the average values of the following markers changed in a favorable direction from the CR period when evaluated in the later AP period in our volunteers: body weight, FM, and levels of FBG, Tri, and ALT enzyme.[14]
Providing substantiating confirmation is accomplished by two separate prominent changes in the protocol: first, by examining in detail a relatively fresh surrogate for IR, the ratio Tri/HDL cholesterol [23-26], and second, by comparing the effects of IR along with its “MetS risk factors” on the age-related decline in GFR, we believe to be a well-recognized model of age-related senescence.[21] These additional findings based on different markers would be compared with the former surrogates and CRs and Paradox periods in the original chronological aging studies[15-22] [Figures 3, 4, 9].
Newer surrogate for IR – Tri/HDL cholesterol ratioIR primarily infers a less than adequate metabolic functioning of insulin, resulting in a poorer uptake of glucose by muscle and fat tissue as well as improper excess release of glucose by the liver.[57,58] The first novel adjustment in our protocol uses the ratio Tri/HDL cholesterol to replace FBG as the primary variable. The ratio should represent some peripheral inhibition of insulin action through the muscle and fat tissue as well as the hepatic release of glucose in its assessment. In contrast, FBG as a surrogate largely represents just the latter.[36,55] Despite these acknowledged differences, they both accomplish the same purpose, appearing to be reasonable first approximations.
Declining GFR during characteristic agingRegarding the second major adjustment in the protocol, a series of cross-sectional studies mixed in with a few longitudinal ones reveals that the renal GFR of humans characteristically declines in a gradual but steady manner over the lifespan.[15-21] While the precise direct ca
留言 (0)