Global cancer burden is increasing with the cancer burden in India being projected at 26.7 million in 2021, and this is likely to reach 29.8 million by 2025.[1] Cancer treatment has evolved over the years with increased cancer survivorship,[2] but this also translates to more aggressive treatment of cancer patients. Even at the end of life, often the aggressive cancer therapy continues, resulting in many patients with progressive cancer dying in intensive care.[3] A recent prospective evaluation advocates the provision of palliative care (PC) in the intensive care unit (ICU) for the high symptom burden in intensive care.[4]
In the last decade, there has been an increasing PC penetration into cancer centres in India.[5] PC is essential for terminally sick cancer patients in ICU for alleviation of symptom distress, clear communication, and to ensure family support and continuity of care of patients with the treatment aligned to family preferences based on the discussion on goals of care.[6] There is evidence to suggest that PC in ICU improves survival and decreases ICU length of stay.[7] Published Indian guidelines for end-of-life care are available[8] and point toward many benefits of PC, especially the improved quality of life[9] However, PC integration in Indian ICUs is not always available. Despite having an outpatient PC and inpatient referral for PC, we did not have any referral from the ICU for PC, so the availability of different models of integration of PC in the ICU[10] was not relevant to our institution. To make PC available in intensive care, we decided to participate in a quality improvement (QI) program as a part of the National Cancer Grid educational initiative.[11] The A3 problem-solving method was used. The seven components of the A3 included background state, current state, aims and objectives, analysis of root cause results, and follow-up. Our Specific, Measurable, Achievable, Realistic, and Timely (SMART) goal was to initiate PC referrals from ICU from 0% in November 2019 to 50% by May 2020 in a Cancer Institute in South India.
MATERIAL AND METHODSWe used the A3 method of problem-solving.[12] We formed a group consisting of PC physicians, medical and surgical oncologists, and psycho-oncologists. We had weekly team meetings to explore the background cause for a lack of PC referrals from the ICU. As this was a QI program, we could get a waiver for the institutional review board clearance. We informed our hospital administrative head about the project and obtained permission. Our institute has an integrated department of anaesthesia and PC, and the PC team took the lead in the QI process.
PlanThe main outcome that we were targeting was the initiation of referral for PC from intensive care. We aimed to make PC services available for sick patients in the ICU who were on best supportive care from a baseline of 0–50% by May 2020. To understand the background, we collected data on the deaths of patients in the ICU for the preceding 6 months. Only patients who were being treated with palliative intent and were in ICU for over 48 hours were collected. Patients in acute care whose treatment was deemed curative were excluded from the audit. Of the 46 deaths in that period, 22 patients had been on the best supportive care but had not received any PC intervention.
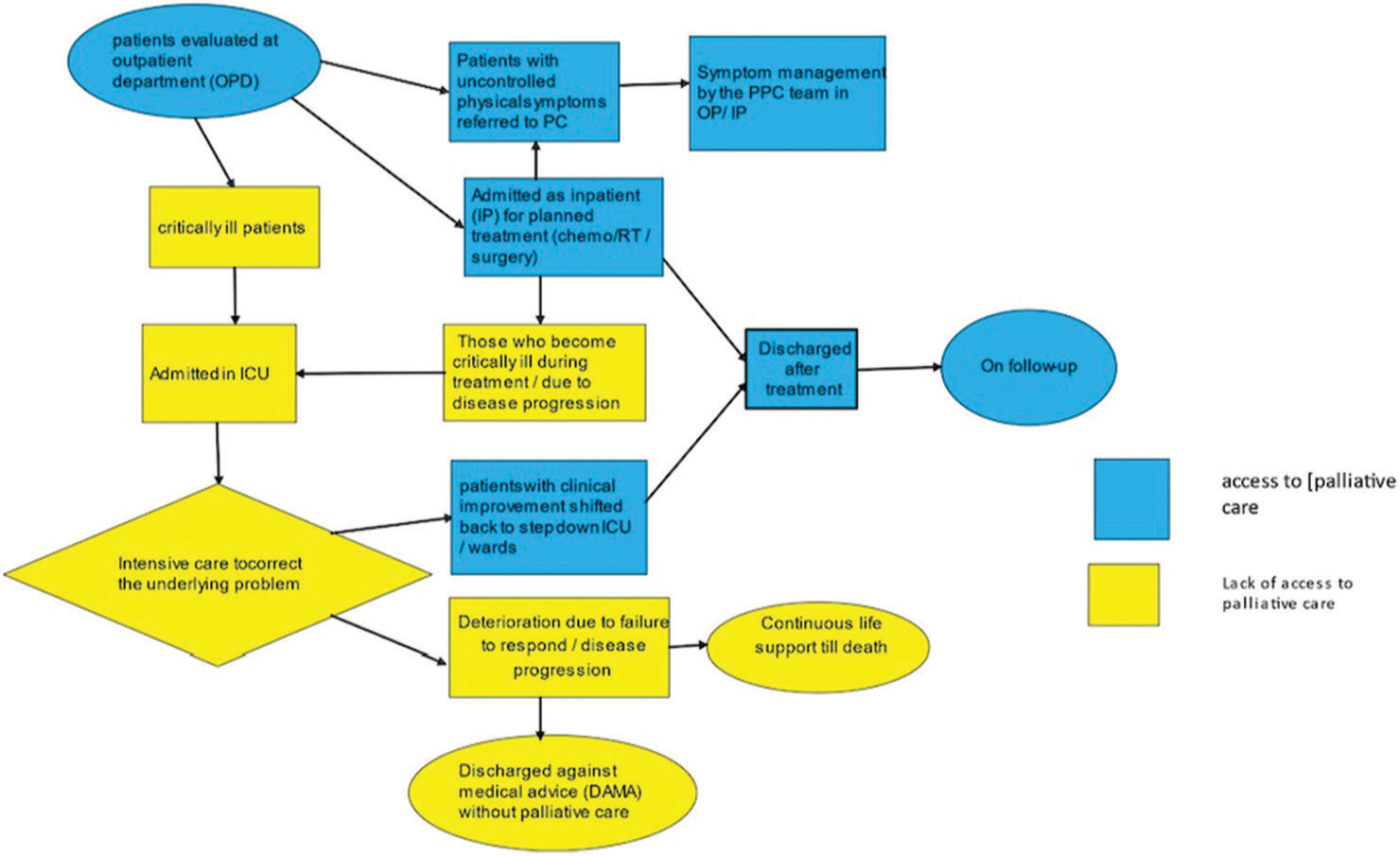
A Gemba walk to understand patient flow into the ICU[13] was carried out, and we found that the inflow of patients into the ICU was either from wards once patients became sick or were directly admitted into the ICU from the emergency. The outflow of patients from the ICU occurred when patients became better and were shifted to the ward, became sicker and died, or were sent against medical advice on explaining the prognosis and likely poor outcome [Figure 1].
Export to PPT
We used the five why method to understand the cause for the absence of PC referral.[14] We thought of the possibility of lack of knowledge of PC amongst stakeholders, so we conducted a survey amongst the oncologists to explore their knowledge about PC in ICU and to find out if treating oncologists thought PC would be useful and the possible reasons for lack of referral. We shared the survey through e-mail with all treating oncologists, the queries in the survey explored the knowledge of PC amongst the oncologists and their feedback on the reason for a lack of referral The survey showed that there were many causes for non-referral including: (1) absence of an objective tool to assess PC needs in ICU, (2) discharge of patients to home or ward but without PC referral, (3) lack of access of PC data in electronic case records in ICU – a patient on outpatient PC treatment if admitted to ICU, the treating oncologist was unaware of the patient visits to PC outpatient department (OPD) earlier. (4) Caregivers of patients not being comfortable with the term ‘palliative care’.
A group meeting with all treating oncologists was conducted to explore their concerns and barriers to PC referral from the ICU. We also educated them about the need for referral not only when the patient was sick in the ICU and when treatment was considered futile but also when discharging patients home, even if against medical advice discharge against medical advice (DAMA).
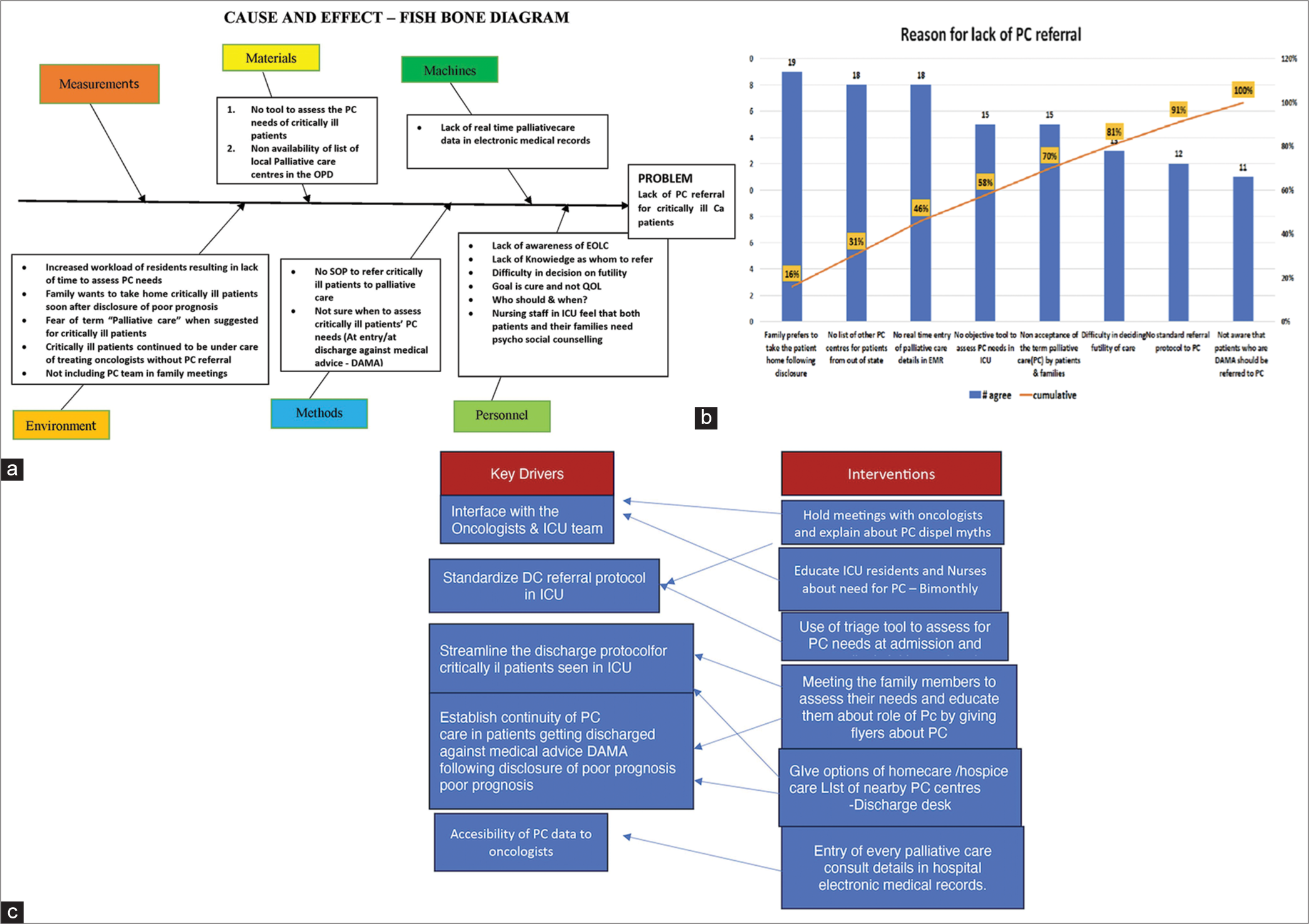
We placed the causes gleaned from the survey into different heads and constructed a fishbone diagram so that interventions could be planned [Figure 2a]. Next, we made a Pareto diagram to prioritise the key drivers so that we could choose an early intervention.
Export to PPT
The Pareto [Figure 2b] showed that (1) there was no tool to routinely assess PC needs in ICU, (2) caregivers fear the term ‘palliative care’, (3) patients deemed to be too sick for further care were being discharged home without PC referral, (4) discharge desk did not have a list of PC centres in the state and (5) ICU did not have access to OPD PC data entry. We tried to prioritise the Pareto to look for interventions that would be of high benefit without too much effort with the help of a two-by-two table. Our key drivers and interventions are in [Figure 2c].
DoWe prepared a basic PC trigger tool and modified it based on the opinions of nursing staff, residents, and oncologists and finally tailored a tool to fit our needs [Table 1]. The tool had 10 questions with a score of 1 per query. Any score of over 1 meant the resident should initiate a PC consultation. We first conducted an educational session for the staff in the ICU on how to use the trigger tool and initiate PC referral, following which we encouraged the resident to use the tool on all ICU patients at least once daily. We also requested our administration to send a circular on the need to use the tool in the ICU. We shared the list of PC centres with the discharge desk so that they could share it with families during DAMA; this would help patients going home to access PC close to their place of residence. We asked our hospital information system to be updated such that outpatient PC notes were accessible for viewing in intensive care.
Table 1: Trigger tool to initiate PC referral.
S. No. Explanation Score 1. Difficult to control physical symptoms such as pain and breathlessness 1 2. ICU length of stay more than 7 days 1 3. Patient on ventilator support for more than 5 days 1 4. Prolonged multiorgan failure (more than 3 days) 1 5. Major acute neurological insult (post-CPR, encephalopathy, major stroke, etc.) 1 6. More than one ICU admission during the same hospital stay 1 7. Oncologist request for PC 1 8. Family request for PC 1 9. Answer No to ‘surprise question’ (would you be surprised if this patient died in the next 6 months 1 10. Disease progression during treatment or unresponsive to treatment (Futile) 1 CheckThe first meeting with all stakeholders was in December 2019, and we could make the electronic data available to oncologists by January 1st week 2020. Our PC referral was 10–15% after this intervention. After the PC trigger tool was introduced, the referrals started to go up reaching over 60% by the middle of March. The effect of the pandemic and subsequent lockdown meant that only curative cases were taken up for oncological treatment and all palliative intent patients were not getting admitted as per the institute policy. While our percentage of referrals went up, the total number of cases in the ICU came down.
ActWe wanted to ensure continued PC referral from the ICU and for this started education of new postgraduates and staff nurses who were posted in the ICU. We tried to schedule the training to coincide with the new staff ’s arrival in the ICU. As decided regular audit on the use of the trigger tool was carried out. We audited the referrals in December 2021 and found that many patients were getting directly admitted to wards after triaging in the emergency or in outpatient without getting admitted to ICU.
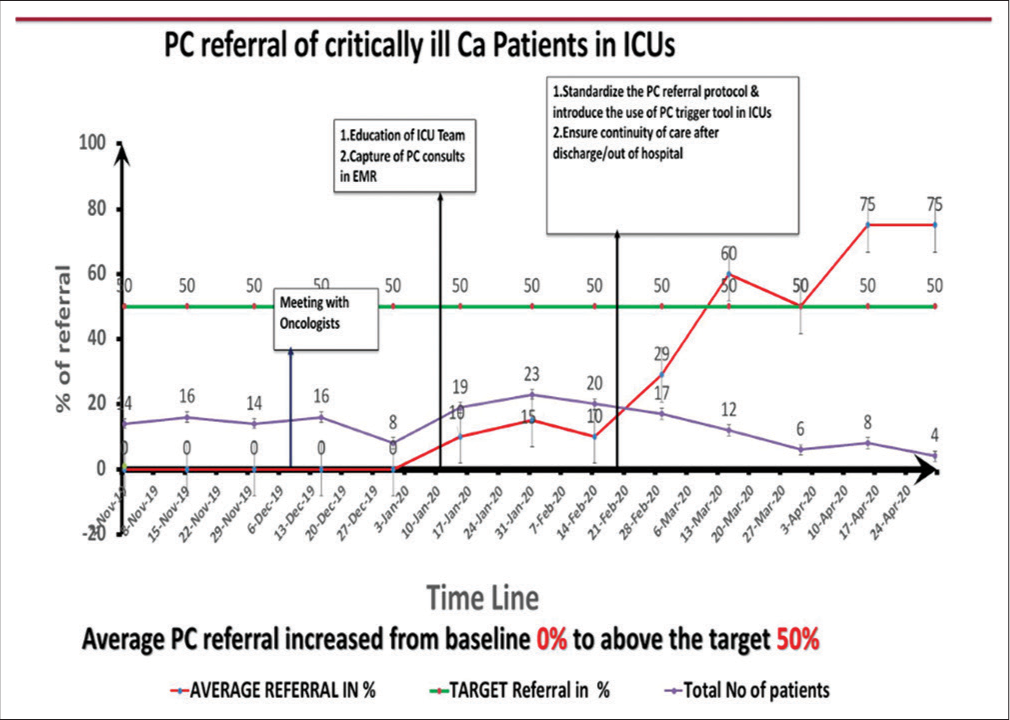
RESULTSThe results are depicted in the run chart [Figure 3]. It shows that PC referral in November 2019 was nil when 14–16 patients were in ICU. The referrals increased first to 15% once data of PC were available in the ICU but started climbing to 60% and later to 75% by the middle of March. The average number of patients in intensive care for whom PC referral could be triggered ranged between 14 and 23 (our denominator) between mid-December to February. With the onset of the COVID pandemic, our denominator of the number of patients likely to need PC in ICU decreased to around 6 by April 2020. A recent audit in December 2021 found that, while the PC referral from the ICU was not increasing, patients considered futile were shifted to inpatient wards from the ICU, and then PC referrals were initiated from the ward (6 patients in November and December 2021). We also found that 4 patients were directly admitted to wards; these would normally have been admitted to ICU; PC referral for these patients, however, was initiated from the ward.
Export to PPT
DISCUSSIONOur primary objective of initiating PC referral from the ICU was achieved by following the QI pathway. Quality healthcare is timely, effective, patient centred, safe, and equitable. An interventional study from Israel where ICU nurses were educated on intensive care to increase PC in the ICU did not lead to improved PC in the ICU[15] in comparison to our QI process. QI is a change that improves the processes, clinical structure, and outcomes and is expected to happen at the organisation level. A single intervention like the Israel study can only address one component, for an organisational change to occur a systematic process of finding key drivers and then applying interventions depending on the likely impact in a systematic manner is essential to achieve results. The structured change that we wanted to bring about was making PC accessible to patients in the ICU who were not in the ambit of curative treatment. Introducing the PC trigger tool and training the staff on its use resulted in a slow increase in PC referral from the ICU reaching our goal of 50%. The advisory board on improving PC in the ICU describes screening criteria or triggers,[16] as an important mechanism to engage physicians, we too found using the trigger tool to be an important and fruitful intervention.
One of the feedback items pointed to the aversion of the caregivers towards the term ‘Palliative care’, but changing this was beyond the scope of our QI project, hence we did not intervene in this. However, the term serious health-related suffering as has been coined by the Lancet Commission may be an option.[17]
We examined our QI process by the Donabedian model[18] to analyse the outcome, balancing, and process measures. We found that we had reached the outcome measure of initiating PC for sick patients in ICU, and we feel that regular use of the PC trigger tool is a process measure but realise that this may not be sufficient over a period of time and repeated education and training on using the trigger will be needed and hence we propose regular training of new residents and staff and quarterly audit of the use of the tool to ensure sustenance. Our balancing measure will be that the availability of PC in the ICU should not be a deterrent to the routine therapeutic options of critically ill cancer patients, but this will be easy to achieve as oncologists are in charge of our ICU and they decide when further disease directed treatment will be futile. There are studies that implicate oncologists in delayed PC referral,[19] but on the contrary, our oncologists were receptive and willing to initiate PC referral from ICU. Oncologists not accepting therapeutic failure are also cited as a reason for the lack of referral.[20] Lack of education in PC is considered one of the barriers amongst physicians in managing end-of-life patients;[21] however, as we mandate that all oncologists undergo a basic course in PC at our institute, this was not a deterrent for us. Our nurses were the leaders in this process as they saw the effect of PC on patients and the benefit to patients and caregivers with the initiation of PC referral. An unexpected outcome was that we found many of the patients needing PC were directly admitted to wards and referral to PC was sent from the wards bypassing ICU admission This outcome achieved the objective of PC referral for critically ill cancer patients while avoiding unnecessary ICU admission. This we feel is an unexpected outcome beyond expectation. A recent review on choosing wisely in India[22] clearly advocates against ICU admission for metastatic cancer patients.
A major limitation of the initiative was the impact of COVID-19 and the associated restrictions on in-patient admissions, causing a decreasing effect of our intervention as our institute created guidelines to admit only curative intent patients from April 2020, this can be seen in the run chart as a decrease in total number of patients from 17 to 6; however, our percentage of referral was still over 50%. One other limitation was the need to remind the staff to administer the tool as their priorities changed with the pandemic – with the staff having to follow many pandemic-related guidelines. We are not sure of the generalisability of the tool as we are yet to evaluate if our trigger tool can be used in other hospitals to initiate PC referrals from the ICU.
CONCLUSIONInitiation of PC referral from the ICU could be implemented by a structured QI process by the introduction of PC trigger tool and continued availability of PC could be ensured for patients getting discharged by ensuring that details of nearby PC centres are shared by the discharge desk.
留言 (0)