Peripheral T-cell lymphomas (PTCL) are a heterogeneous group of mature aggressive T-cell non-Hodgkin’s lymphomas. They carry a worse prognosis for most subtypes compared with their B-cell counterparts.1 Despite the recent approval of newer therapies, the response rate and duration of clinical benefit with standard therapies is short and the outlook for patients with relapsed/refractory (RR) PTCL remains poor.2 Therefore, there is a definite need for novel effective therapies in PTCL.
We and others have shown that the tumor microenvironment (TME) in PTCL is profoundly immunosuppressive.3 4 Although the TME in lymphoid malignancies is made of a mixture of various inflammatory cells, it remains ineffective against the malignant cells. Program death ligands (PD-L) 1 and 2, expressed on antigen presenting cells, activated T cells, and even on tumor cells,5 interact with program death receptor 1 (PD-1) on T cells, leading to an inhibitory signal with subsequent T cell function exhaustion and anergy, providing lymphoma cells a mechanism to evade immune surveillance.5 Furthermore, interactions between PD-1 and its ligands are even more unusual in PTCL in that both the receptor and ligands can be expressed on the malignant T-cell.6 While it is reasonable to target the PD-1/PD-L axis in the TME in an attempt to improve outcomes in PTCL, there is a potential for malignant T cells to be activated and this could be associated with inferior outcomes.
While the use of anti-PD-1 blocking antibodies has shown remarkable efficacy particularly in relapsed Hodgkin lymphoma,7 only a small number of patients with PTCL have been treated with checkpoint blockade. Nivolumab is a fully human IgG4 monoclonal antibody which blocks PD-1 receptor thereby regulating this immune checkpoint. We therefore conducted an investigator-initiated phase 2 prospective study of single-agent nivolumab for RR PTCL and report the results of the pre-specified interim analysis.
Patients and methodsThis was a phase II single arm study conducted prospectively to assess response rates and safety of single agent nivolumab in patients with RR PTCL.
Patient selectionPatients, 18 years and older, with a diagnosis of RR PTCL including PTCL—not otherwise specified (NOS), anaplastic large cell lymphoma (ALCL)-anaplastic lymphoma kinase (ALK) negative or positive, angioimmunoblastic T-cell lymphoma (AITL), enteropathy associated T-cell lymphoma (EATL), and hepatosplenic gamma delta T-cell lymphoma (HSGDTCL), extranodal natural killer (NK)/T-cell lymphoma, nasal type, blastic NK-cell lymphoma, transformed mycosis fungoides, and T/NK-cell lymphoma, unclassifiable were eligible for enrollment. To be eligible, subjects were required to have: at least one lesion that was >15 mm in the longest diameter on cross-sectional imaging and measurable in two perpendicular dimensions per CT (spiral CT); failed an autologous stem cell transplant (ASCT) or failed frontline chemotherapy in subjects who declined or were not ASCT candidates; an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of ≤2; appropriate hematologic, liver, and kidney parameters.
The following patients were excluded: prior therapy with anti-PD-1, anti-PD-L1, anti-PD-L2, anti-CD137, or anti-cytotoxic T-lymphocyte-associated protein (CTLA-4) antibodies; prior allogeneic stem cell transplant; known central nervous system involvement; a known diagnosis of interstitial lung disease or autoimmune disease; pregnant or nursing women.
TreatmentNivolumab was given at a flat dose of 240 mg intravenously every 2 weeks for eight cycles and then 480 mg intravenously every 4 weeks starting cycle 9.
OutcomesThe primary objective was to assess the overall response rate (ORR) defined as proportion of subjects achieving either a partial response (PR) or complete response (CR) within 12 cycles of treatment. Secondary objectives were to assess safety and tolerability of nivolumab in PTCL and to assess progression-free survival (PFS), duration of response (DOR) and overall survival (OS). Positron emission tomography (PET) scans in conjunction with CT scans were done at baseline in all patients. Response was assessed with CT scans after cycles 4, 8, and 12 of therapy, then every 16 weeks thereafter. Clinical response was assessed based on the Revised Lugano Response Criteria for Malignant Lymphoma.8 9 To account for the pseudoprogression phenomenon described with immunotherapies, a subject whose radiologic disease assessment was indicative of progression was allowed, at the discretion of the investigator, to continue treatment pending a repeat scan, if all the following criteria were met: absence of symptomatic or clinical progression; stable PS; adequate tolerance to nivolumab; and treatment beyond progression did not delay an intervention felt to be in the patient’s best interest. If the second confirmatory scan indicated unequivocal disease progression, then the patient was deemed to have progression of disease (PD). Adverse events (AEs) were recorded based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) V.4.0. Hyperprogressive disease (HPD) was defined as time-to-treatment failure (TTF) of equal or less than 1 month of therapy with symptomatic progression.
Statistical MethodsThe pre-planned sample size was 35 evaluable patients, assuming that an ORR of 10% or less would be considered negative and an ORR of 30% or greater would warrant further study. A Simon optimal design, with an interim analysis, was used to assess efficacy. The interim analysis decision was that the study would be considered ineffective if less than 2 of the first 12 evaluable patients did not achieve a CR or PR. This design had a 90% power with a one-sided 10% level test. The Duffy and Santner method was utilized to determine CIs for the ORR. Kaplan-Meier methods were used to assess PFS, OS, and DOR.
PFS was defined as the time from registration to relapse or death due to any cause. DOR was defined for patients who achieved a CR or PR as the date at which the patient’s objective status was first noted to be a CR or PR to the earliest date relapse was documented. OS was defined as the time from registration to death due to any cause.
The trial was registered at www.clinicaltrial.gov and was sponsored by Bristol Myers Squibb.
Correlative studiesImmunohistochemistryTo define predictors of clinical response to PD-1 blockade in patients with PTCL, we compared PD-1/PD-L1 expression by immunohistochemistry (IHC) in patients who achieved a response to nivolumab, in those without a response and in patients with HPD. Paraffin embedded tissue of pre-treatment samples were deparaffinized in xylene. After cleared through graded ethanol series, endogenous peroxidase was quenched by incubation in 50% methanol/H2O2. The sections were pretreated 30 min with 50 mM EDTA, pH 8.0 using a steamer and cooled for an additional 5 min. The staining was performed using anti-PD-1 (Abcam, 1:50) or anti-PD-L1 (Abcam, 1:50). The sections were stained with hematoxylin and rinsed well in tap water. The slides were observed and captured images with MOTIC EasyScan.
RNA preparationPeripheral blood mononuclear cells (PBMNCs) from RR patients with PTCL were collected at Mayo Clinic. They were vortexed continuously at maximum speed for 10 s in HTG lysis buffer and heated to 95°C for 20 min. Then, the samples were cooled for 10 min at room temperature (RT) and stored at −80°C. At HTG Molecular Diagnostics (Tucson, Arizona, USA), cells were spun down, resuspended and denatured at 95°C for 15–20 min. They were proceeded to thaw at RT and added proteinase K for 3 hours at 50°C. Using HTG standard protocols, each sequenced sample achieved the minimum recommended read depth. Samples were grouped as untreated, treated, responder, or non-responder. Then, the messenger RNA profiling data from the Precision Immuno-Oncology Panel were processed and reported using the HTG EdgeSeq software (https://www.htgmolecular.com/systems/chemistry).
HTG EdgeSeq assay:We also assessed the gene expression profile of PBMNC using the HTG EdgeSeq immune-oncology gene expression panel (HTG Molecular Diagnostics Arizona, USA), and comparisons were made among responders and non-responders. This panel comprises probes targeting 1392 genes implicated in the host immune response to cancer and couples quantitative nuclease protection (qNPA) with next-generation sequencing (NGS) to measure gene expression in frozen samples without RNA extraction. Briefly, the samples were lysed in HTG’s lysis buffer, followed by the introduction of gene-specific DNA nuclease protection probes (NPP). After allowing the NPPs to hybridize to their target RNAs, which can be both soluble or cross-linked in the biological matrix, S1 nuclease was added to remove excess unhybridized NPPs and RNAs, leaving behind only NPPs hybridized to their target RNAs. Thus, a stoichiometric conversion of the target RNA to the NPPs was achieved, producing a virtual 1:1 ratio of NPP to RNA. The qNPA steps were automated on the HTG EdgeSeq processor, which was followed by PCR to add sequencing adaptors and tags. The labeled samples were pooled, cleaned, and sequenced on an NGS platform using standard protocols. Data from the NGS instrument were processed and reported by the HTG EdgeSeq parser software. Relative SD (RSD) was used to assess the level of variation in expression throughout a sample, as less variation corresponds to a smaller RSD. For each sample replicate (well), RSD was computed as: RSD = 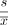
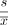 , where s and
, where s and 
 were the SD and mean of log2 (count +1), respectively.
were the SD and mean of log2 (count +1), respectively.
Twelve patients were enrolled from May 17, 2017, to May 29, 2019 (online supplemental figure 1). The median age was 65 years (range: 35–75) and half (6/12) of the patients were men. The ECOG PS was 0–1 in most patients (91%; 11/12). Half (6/12) had AITL, 25% (3/12) had PTCL-NOS, and one patient each had ALCL-ALK negative, EATL and HSGDTCL. All patients had an advanced stage (Ann Arbor stage III–IV), and 92% (11/12) had extranodal disease. Fifty per cent (6/12) of the patients had received a prior ASCT. The median number of prior lines of therapies was 2 (range 1–6) (table 1). Three out of four patients had only one line of therapy prior to nivolumab and patients 1–3 had primary refractory disease to initial chemotherapy regimen (table 2). Patient 5 was disease free for 9 months post autoSCT then had rapid disease progression on nivolumab when his disease recurred. The only patient who received two lines of chemotherapy (patient 1), he received only one cycle of the second line chemotherapy regimen (table 2).
Table 1Patient characteristics
Table 2Patients with hyperprogression characteristics
Clinical outcomesThe ORR was 33% (4/12) (95% CI: 12.3% to 63.7%) with two CR seen in patients with ALCL-ALK negative and AITL; two PR were observed in PTCL-NOS and EATL. The median PFS for all 12 patients was however of a short duration at 2.7 months (95% CI: 1.5 to NE) (figure 1A); and the median OS was 6.7 months (95% CI: 3.4 to NE) (figure 1B). The median DOR was also short at 3.6 months (95% CI: 1.9 to NE) (figure 2). Two patients remain alive at time of last follow-up (AITL and ALK-ALCL each).

 Figure 1
Figure 1 ─ Progression-free survival (A) and overall survival (B) at interim analysis. Kaplan-Meier analysis was used to estimate time to event. NE, no event; OS, overall survival; PFS, progression-free survival.

 Figure 2
Figure 2 ─ Response and duration of response of patients treated with nivolumab. The colored bars represent time on nivolumab. CR, complete response; PR, partial response; PD, progression of disease.
Safety and tolerabilityObserved grade 3 and higher AEs were as follows: non-hematologic in 42% (5/12), while hematologic AEs were seen in 25% (3/12) of the patients (figure 3). Eighty-three per cent (10/12) of patients came off therapy due to disease progression, one due to AEs and one due to rapid decline of their clinical condition.

 Figure 3
Figure 3 A swimmer’s plot illustrates frequency and grade of adverse events. Each bar represents an event. AITL, angioimmunoblastic T-cell lymphoma; HSGDTCL, hepatosplenic gamma delta T-cell lymphoma.
Treatment failure within the first 2 months (TTF <2 months) occurred in 50% of the patients (6/12; five of whom had AITL), with 33% (4/12; three with AITL, one with HSGDTL) progressing within the first month of therapy (TTF <1 month) (patients 1, 2, 3, 5) (table 2). These patients were considered to have HPD.
A closer look at the AITL cohort with HPD (patients 1, 2, and 3), all three of these patients only received a single dose of nivolumab with subsequent fulminant progression within 2 weeks of receiving nivolumab. One patient (patients #5) with HSGDTCL had rapid spleen enlargement within the first month of therapy (table 2; figure 4).

 Figure 4
Figure 4 Positron emission tomography-CT and CT images before and after initiation of nivolumab illustrating rapid progression of lymphoma. AITL, angioimmunoblastic T-cell lymphoma; HSGDTCL, hepatosplenic gamma delta T-cell lymphoma.
Due to multiple cases of HPD, as well as the moderate ORR and short DOR with nivolumab treatment, a decision was made to halt the study even though the trial officially met interim analysis criteria. Data were frozen as of September 10, 2020, for this manuscript.
Correlative analysesTo gain further understanding of potential predictors of response or non-response to nivolumab and predictors of HPD occurrence in this patient population, we performed IHC for expression of PD-1 and PD-L1. Although the number of samples were limited, we compared PD-1/PD-L1 expression in pre-treatment tumor samples in a patient who achieved response to nivolumab, in a non-responding patient and in two patients with HPD (Case #1 and 2). There was no difference in PD-1 expression, which was strongly and uniformly expressed in all cases, while PD-L1 was not expressed in any of the samples tested (figure 5).

 Figure 5
Figure 5 The tumor cells are diffusely and strongly positive for program death receptor 1 by immunohistochemistry (×50) in a patient with AITL and hyperprogression (A), a patient with AITL and no response (B) and a patient with peripheral T-cell lymphomas-not otherwise specified with response (C). AITL, angioimmunoblastic T-cell lymphoma.
Since Epstein-Barr Virus (EBV) can be associated with an upregulation of PD-L1, we reviewed EBV status in all patients. It was not reported in six patients, negative by IHC and/or by fluorescent in situ hybridization in four patients, and positive in one patient. Due to the small sample size and missing data, we were unable to make any correlations.
Next, using a NGS-based tumor profiling assay of genes implicated in the host cancer-fighting immune response, we compared the gene expression profile between responders (n=3) and non-responders (with or without HPD, n=4) in patients with available samples. Using samples collected prior to treatment, seven genes were upregulated in responders versus non-responders: ADRB2, SLFN11, KLRF1, CD163, CD244, KLRK1, KLRD1, while nine genes were downregulated: GAD1, C4A_C4B, CPE, IFNLR1, RDM1, USP9Y, KIT, CXCL14, FAP (figure 6). We note three upregulated genes in responders that are involved in NK cell function: Killer cell lectin like receptor K1 (KLRK1), killer cell lectin like receptor F1 (KLRF1), and killer cell lectin like receptor D1 (KLRD1). Interestingly, the checkpoint molecules (PD-1, TIM3, LAG3, TNFRSF, CD73/NT5E) as well as the non-PD-1 checkpoint molecules (HAVCR2, CTLA4, HLA-DR, CD86, HMGB1, CD274, TNFSF4) were not significantly different between those with HPD and those without, in both pre-treatment and post treatment samples. When comparing gene expression between those with HPD (n=3) and all others (including responders) (n=4), only a single gene (the transcription factor HEY1, a target of the Notch signaling pathway) was upregulated in the HPD group (174-fold increase; p=0.039), while three genes (MSH2, HLA-DQB1, KIF18B) were downregulated.

 Figure 6
Figure 6 Immune genes expression differences among responders and those without a response, before treatment with nivolumab.
DiscussionStandard therapies in the relapsed setting in PTCL have been associated with modest responses at best and many patients do not benefit from these agents. The rate of response seen with therapies used to manage RR PTCL, such as histone deacetylase inhibitors romidepsin10 and belinostat,11 and the antimetabolite pralatrexate12 is in the range of 25%–30% and is often short lived. Our study shows that PD-1 blockade with nivolumab in patients with RR PTCL has similar activity to currently used therapies with an ORR of 33%. This is consistent with a similar study using pembrolizumab, another PD-1 checkpoint blocker.13
It is of particular interest that responses to PD-1 blockade were associated with increased expression of KLRK1, KLRF1 and KLRD1 genes which are all involved in NK and T-cell function (figure 5). KLRK1 encodes for a member of the NKG2 family of C-type lectin like receptors that is upregulated in response to various stressors including DNA damage. It binds to a diverse family of ligands including MHC class I proteins leading to activation of NK and T cells. It provides both stimulatory and costimulatory innate immune responses to activated NK cells, leading to cytotoxic activity. It is also a costimulatory receptor for the T-cell receptor in CD8 positive T cells, amplifying T-cell activation.14 Similarly, KLRF1, which activates homodimeric C-type lectin-like receptor, is expressed on nearly all NK cells, stimulating their cytotoxicity and cytokine release.15
HEY1 upregulation in patients with HPD is intriguing. HEY1 encodes a nuclear protein belonging to the hairy and enhancer of split-related family of basic helix-loop-helix-type transcriptional repressors. Expression of this gene is induced by the Notch signaling pathway. NOTCH1 activating mutations were reported in 60% of T-cell acute lymphoblastic leukemia.16 Its expression was also reported in cutaneous T-cell lymphoma17 as well as nodal PTCL,18 although in the latter, NOTCH1 mutations were only seen within the tumor’s CD20 positive cells. Several mechanisms have been postulated as to the role of NOTCH1 signaling in tumorigenesis in T-cell lymphoma, including interaction with the PI3K–AKT–mTOR pathway and activation of the NF-kappa B signaling.19 In a cohort of patients with small cell lung carcinoma who had progressed on immune checkpoint blockers, there was an association between increased expression of Notch pathway genes and clinical benefit to checkpoint blockade.20 In our study, it is possible that HEY1 upregulation in HPD is a consequence of NOTCH1 signaling deregulation. A limitation in our study is that we did not have post treatment samples, and as such it is unclear how PD-1 blockade affected the NOTCH1 signaling pathway in our patients as a whole and in those with HPD in particular.
In terms of safety, although the reported AEs were typical for checkpoint blockers, it is concerning that 50% (6/12; 5/7 had AITL) of the patients in our study experienced TTF <2 months and 33% (4/12; 3/4 had AITL) within 1 month. Occurrence of HPD after initiation of immune checkpoint inhibitors (ICI) has been described in the literature in various malignancies (21–24). It is important to emphasize that HPD is distinct from pseudoprogression and progression of disease. It is rather an acceleration in the kinetics of tumor growth. Its definition however varies from study to study and currently there is no agreed on consensus definition. For instance, Champiat et al21 defined HPD as progression at the first evaluation and as twofold increase of the tumor growth rate before and after ICI therapy using response evaluation criteria in solid tumors (RECIST) criteria V.1.1. Based on this definition and in a cohort of 131 patients with various malignancies, the incidence of HPD was estimated at 9%. No association was noted with tumor burden or tumor type.21 Of note, of the seven patients with lymphoma (subtype not reported), only one (14%) had HPD. Another definition included TTF <2 months, >50% increase in tumor burden compared with pre-immunotherapy imaging, and >2 fold increase in progression pace.25 Several other definitions are reported in the literature.24 A caveat to these HPD definitions is that most require access to at least few imaging studies pre-immunotherapy and most rely on relatively complex mathematical formulas that cannot be used on day-to-day practice at bedside. Also, the existing definitions of HPD rely on target lesion measurements based on RESIST V.1.1 criteria. Some malignancies may progress with non-target lesions leading to an underestimation of this phenomenon.
It is important to highlight that most reported cases of HPD and all attempts at a definition to-date are in solid malignancies. Data describing this phenomenon in lymphoid malignancies are scarce. A report on a small series of patients (N=3) with acute T-cell leukemia/lymphoma (ATLL) treated with nivolumab within a clinical trial showed rapid disease progression, with abnormal values noted 6–29 days after initiation of nivolumab,26 with evidence of rapid expansion of the malignant ATLL clone in all three patients.27 The study was therefore terminated early. It is noteworthy to note however that no HPD cases were seen in the series reported by Barta et al where patients with RR PTCL were treated with single agent pembrolizumab, another PD-1 inhibitor.13 Pembrolizumab and nivolumab are used in clinical practice interchangeably and no studies have compared the two PD-1 blocking monoclonal antibodies side by side. It is important to keep in mind that all studies in T-cell lymphoma have a small number of patients. Although direct comparison among studies is not entirely appropriate, the ORR in Barta et al study was similar to ours at 33%. The PFS was also short at 3.2 months. The study was halted early after a preplanned interim futility analysis. Although frank HPD was not observed, the authors could not exclude a potential detrimental effect in a subset of patients. Also, nodal PTCL are heterogeneous group of diseases and it is possible that a sampling bias may have played a role in the differences observed in HPD between the two studies.
In our cohort, we defined HPD as TTF of equal or less than 1 month of therapy with symptomatic progression. Although our patients had clearly rapid progression and decline in their clinical condition shortly after initiation of nivolumab, they did not fit any of the pre-existing definitions for HPD in the literature.
Several mechanisms are postulated to explain HPD occurrence and are reviewed by Camelliti et al.28 Some alterations in both the innate and adaptive immunity can have paradoxical effects and lead to subsequent tumor growth rather than shrinkage. For instance, in the innate immune response, PD-1 blockade can induce a decrease in NK cells production of cytotoxic molecules,29 and an increase in production of immunosuppressive cytokines such as interleukin-10 by innate immune cells such as dendritic cells30 and monocytes.31 Blockade of the PD1/PD-L1 axis may lead to an upregulation of the other checkpoint receptors in tumor infiltrating lymphocytes,32 as well as an increase in production of immunosuppressive T regulatory cells (Treg).33 We did not however note an upregulation of checkpoint molecules expression in those with HDP as compared with responders such as PD-L1, TIM-3 and LAG3. Similarly, other non-PD-1 checkpoint pathways were not differentially expressed among both groups of patients. However, we did find that genes associated T-cell and NK cell function were differentially expressed in responding and non-responding patients. Due to the small sample size however, definitive conclusions cannot be drawn.
Moreover, PTCL are a peculiar entity to target via checkpoint blockade as both the tumor cells and the effector cells have expression of immune checkpoint receptors and ligands. PD-1 expression differs across T-cell lymphoma subtypes and is assessed routinely for lymphoma classification, including assignment of the T-follicular helper cell phenotype.34 Wartewig et al35 showed in an animal model that PD-1 has a tumor suppressor function in T-cell lymphoma. The authors showed that PD-1 deletion, or use of anti-PD-1 or PD-L1 antibodies accelerated clonal expansion and death in a murine T-cell lymphoma model. We have shown that PD-1 and its ligands are expressed both on the immune cells in the TME and on the cancer cells in PTCL and cutaneous T-cell lymphoma (CTCL).6 36 Based on these observations, there is a theoretical possibility that checkpoint blockade may lead to potential tumor growth in T-cell non-Hodgkin lymphoma subtypes. In our patient cohort, we note a higher occurrence of HPD in patients with AITL, and this subtype is known for its highly inflammatory and immunosuppressive TME. Moreover, AITL is a T-cell lymphoma of T-follicular helper (TFH) origin where the tumor cells typically express PD-1,37 so direct blockade of the tumor suppressive function of PD-1 on the tumor cells might be a direct contributor.38
Lastly, looking at the future of ICI use in PTCL, the EBV-driven T-cell lymphomas such as NK/T cell lymphomas are associated with increased expression of PD-L139 and theoretically likely to respond to checkpoint blockade. There is some limited evidence of clinical activity based on small series.40 41 More data are however needed.
It is critical while designing future studies to think of rational combinations with compounds that would act synergistically with checkpoint blockers and could help prevent HPD. There is a theoretical rational to combining PD-1 blockers with epigenetic modifiers such as romidepsin or azacitidine with possible synergy as these therapies are known to affect the TME and tumor immunogenicity. Few phase I/II studies are doing just that combining pembrolizumab with romidepsin (NCT03278782), or pembrolizumab with decitabine (NCT03240211). There is preclinical evidence based on lymphoma mouse models suggesting that PI3K signaling pathway inhibition leads to a less immunosuppressive TME with downregulation of the tumor-infiltrating Tregs,42 as well as an increase in CD8 +cytotoxic T cell, suggestive of potential synergy when used in combination with checkpoint blockers. Various PI3K inhibitors are currently under study in combination with ICI: Pembrolizumab +copanlisib (NCT02535247), and nivolumab +duvelisib in mycosis fungoides/sezary syndrome (NCT04652960).
In summary, nivolumab had modest clinical activity in patients with R/R PTCL and the study met the criteria at interim analysis to continue accrual. However, due to the occurrence of multiple cases of hyperprogression, the moderate activity of the drug, and the short DOR, a decision was made to halt the study. These findings likely reflect the distinct biology of PTCL and should be considered when designing future studies using checkpoint inhibitors in these diseases. Further studies may be indicated using nivolumab or other ICI in combination with other therapies (rather than a single agent) and will need to be combined with biomarkers to better understand and define HPD in PTCL.
留言 (0)