
Papanicolaou (Pap) smear has been the cornerstone of organized population-based cervical cancer screening programs demonstrating remarkable success in reducing the incidence and mortality from cervical cancer in developed nations.[1] The major strengths of cytology-based screening have been the inherent simplicity, relatively low cost, and large knowledge base of various cytological patterns of precancerous lesions.[2] Although cytology identifies the women who are at higher risk of harboring high-grade cervical premalignant lesions or invasive cancer, a diagnostic test such as colposcopy is crucial for women with abnormal cytology for localization of the abnormality, confirmation of diagnosis, and appropriate management.
PRINCIPLES OF COLPOSCOPYColposcopy is a procedure that allows examination of the cervix and vagina using a powerful variable intensity light source and changeable magnification ranging from ×4 to ×30. It involves observation of the changes on the cervical epithelium after consecutive application of normal saline, 5% dilute acetic acid, and Lugol’s iodine. Disease categorization is based on the integration of all the findings detected by colposcopy. If any abnormality is identified on colposcopy, a guided biopsy needs to be directed from the most severe area to confirm or rule out high-grade premalignant or malignant lesions.
DOCUMENTATION OF COLPOSCOPY FINDINGS USING THE INTERNATIONAL FEDERATION OF CERVICAL PATHOLOGY AND COLPOSCOPY (IFCPC) 2011 NOMENCLATUREThe IFCPC introduced a new terminology in the year 2011 to improve the objectivity of colposcopy and ensure comparability of the results for research purpose.[3] The current IFCPC terminology has replaced the previous terminologies such as “satisfactory” and “unsatisfactory” colposcopies. Using this nomenclature, the colposcopists are required to start examination with a general assessment of the cervix, which involves looking for (i) adequacy of exposure, (ii) visibility of squamocolumnar junction, and (iii) type of transformation zone (TZ). The subsequent examination involves categorization of colposcopy findings to normal, abnormal, and suspicious for invasion. The abnormal colposcopy findings are further classified into (i) Grade 1 changes (Minor) that are generally suggestive of benign or low-grade abnormalities, (ii) Grade 2 changes (Major) that are associated with the high-grade lesions, and (iii) non-specific that includes leukoplakia, erosion, and the changes seen following Lugol’s iodine application. The “Miscellaneous” category in the classification includes findings such as polyp and condyloma.
The colposcopy terminology defined by 2011 IFCPC nomenclature is summarized in [Table 1].
Table 1:: IFCPC 2011 nomenclature.
General assessment • Adequate/inadequate for the reason… (i.e.: cervix obscured by inflammation, bleeding, and scar)To improve the predictive value of colposcopy and make the diagnosis more objective, the Swede scoring has been introduced. Swede score assigns a score of 0–2 for five different parameters listed in Table 2. A total score of less than 5 reasonably excludes high-grade lesions and biopsy may be avoided. A score of 5 to 7 is observed in low-grade or high-grade lesions and a biopsy is indicated to confirm the diagnosis. A Swede score exceeding 7 indicates the presence of high-grade lesion or even invasive cancer and if facilities are available, these lesions may be directly taken up for treatment (See and Treat) in the same sitting.
Table 2:: Swede score.
Swede scores 0 1 2 Aceto uptake Zero or transparent Thin, milky Distinct, stearin-like Margin and surface Zero or diffuse Sharp but irregular, jagged, geographical, satellites Sharp and even, difference in surface levels including “cuffing” Vessels Fine, regular Absent Coarse or atypical vessels Lesion size <5 mm 5–15 mm or spanning 2 quadrants >15 mm or spanning 3–4 quadrants or endocervically undefined Iodine staining Brown Faintly or patchy yellow Distinct yellow Interpretation of colposcopy findingsA provisional colposcopy diagnosis is arrived at after completion of documentation of colposcopy findings using the IFCPC nomenclature and calculation of Swede score. [Table 3] shows colposcopy images of various lesions detected on colposcopy.
Table 3:: Salient features of various lesions detected on colposcopy.
Lesion type Salient features Colposcopy image Leukoplakia • White patch with shiny, waxy surface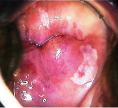 Condyloma
• Single or multiple bright white distinct, irregular lesions
Condyloma
• Single or multiple bright white distinct, irregular lesions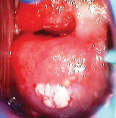 Condyloma/subclinical papillomavirus infection (SPI)
• Thin/milky acetowhite patches
Condyloma/subclinical papillomavirus infection (SPI)
• Thin/milky acetowhite patches Low-grade squamous intraepithelial lesion
• Thin acetowhite epithelium
Low-grade squamous intraepithelial lesion
• Thin acetowhite epithelium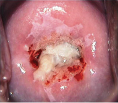 High-grade squamous intraepithelial lesion
• Dense acetowhite epithelium
High-grade squamous intraepithelial lesion
• Dense acetowhite epithelium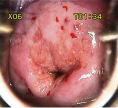 Invasive carcinoma
• Dense acetowhite area with/without erosion
Invasive carcinoma
• Dense acetowhite area with/without erosion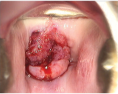 Adenocarcinoma
• Multiple dense acetowhite areas on columnar epithelium (grated coconut appearance)
Adenocarcinoma
• Multiple dense acetowhite areas on columnar epithelium (grated coconut appearance)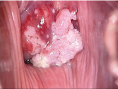 ACCURACY OF COLPOSCOPY
ACCURACY OF COLPOSCOPY
Colposcopy is subjective in nature and its accuracy is mostly dependent on the quality of training and skill of the colposcopist to recognize cervical abnormalities. The reported sensitivities and specificities of colposcopy vary widely across studies. In a meta-analysis on the performance of colposcopy by Mitchell et al., the average sensitivity and specificity of colposcopy for normal diagnosis as threshold in comparison with all other cervical abnormalities (atypia, low-grade SIL, high-grade SIL, and cancer) was reported as 96% and 48% respectively. Improvement in specificity was observed (69%) with marginal reduction in sensitivity (85%) when normal cervix and low-grade SIL as threshold was compared with high-grade SIL and cancer.[4] Colposcopy diagnosis correlates well with high-grade abnormalities with sensitivities reported as high as 85–94% for detection of cervical precancer and cancer. However, owing to low reproducibility and specificity (50%), it is less efficient for women with minor cytological abnormalities.[5]
Women with negative colposcopy may have a risk of subsequent development of high-grade disease, suggesting the possibility of missing lesions.[6] In the atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesion triage study (ALTS) study, the sensitivity of initial colposcopy for the subsequent development of CIN3 over 2 years of follow-up was reported to be only 53%.[7]
Most of the studies on the accuracy of colposcopy come from settings where referrals are based on cytological abnormalities. This is potentially advantageous for the colposcopists because the grade of cytological abnormality provides some indication of the changes to be expected during colposcopy procedure. Colposcopy evaluation is somewhat tricky when referrals are based on VIA or HPV positive screening test results where no such background information provided by cytology is available. In a population-based study conducted in Eastern India to evaluate the performance of colposcopy to assess women with positive VIA and/or (HPV) tests, the sensitivity and specificity of colposcopy to detect HSIL+ lesions were 84.8% and 66.1% respectively.[8] Colposcopy underestimated the severity of disease in 52.6% of women with HSIL diagnosis on biopsy. Other studies have also shown low agreement between colposcopic diagnosis and histologic grade of disease.[9,10]
“See and treat” APPROACHThe traditional standard of care of women presenting with abnormal cytology is performing colposcopy for localization of abnormality, taking guided biopsy from colposcopically detected abnormal area and treatment based on the histopathology results. This requires multiple visits to the clinic by the women and may lead to dropouts. In the “see and treat” approach, the women with colposcopically suspected high-grade lesions are offered treatment in the same sitting without waiting for histopathology confirmation. This approach has been proved to minimize the rates of loss to follow-up and also to utilize the scarce medical resources in remote areas in an efficient way. However, this strategy has the inherent risk of over-treatment, which is outweighed by the risk of the women with high-grade lesions remaining untreated and subsequently developing invasive cancer. The use of Swede scoring to decide on “see and treat” can reduce the over-treatment.
Although the “see and treat” strategy is commonly used when colposcopy is done to evaluate the women with abnormal, the strategy is now often used for the management of women positive on alterative screening tests such as VIA. In a community-based study conducted in India, trained nurses performed colposcopy on the VIA-positive women and if abnormal lesions were suspected on colposcopy, women were offered treatment by cryotherapy in the same sitting (provided the lesion fulfilled the eligibility criteria for treatment by ablation). Nearly 75% of eligible women accepted treatment at the same visit and the cure rates were 81.4% for women with CIN 1; 71.4% for CIN 2 and 68.0% for CIN 3.[11]
MANAGEMENT OF WOMEN WITH ABNORMALITIES ON CYTOLOGY Management of women with ASC-US cytologyASC-US is the most common abnormality and the least reproducible of all the cytologic categories. It carries a very low risk of having high-grade CIN and majority of these abnormalities regress spontaneously.[12] In a study by the Kaiser Permanente Northern California (KPNC) health system, the 5-year cumulative risk of CIN 2+ disease among women aged 30–64 years for baseline cytology of ASCUS was just 6.9%.
A large multicenter, randomized trial of triaging of ASCUS and LSIL (ALTS) evaluated three alternative strategies to manage the women with ASCUS cytology; immediate colposcopy, repeat cytology, or triage by high-risk HPV DNA test.[7] HPV testing identified 96% of women with CIN 3+, while referring 56% of the women to colposcopy; repeat cytology using a triage threshold of ASCUS had a much lower sensitivity (85%) to detect CIN 3+, while referring 58% women to colposcopy. Repeat cytology also resulted in a delay in referral by at least 6 months which may affect compliance to recall. In this study, sensitivity of baseline colposcopy for the subsequent detection of CIN 3+ was only 53%.
Another multicenter study conducted in the United Kingdom, which evaluated the management of borderline or ASCUS cytology concluded that there was no clear benefit of immediate colposcopy referral as it lead to overtreatment with associated after effects in the young women.[13] Based on these studies, the current recommendation to manage the women with ASC-US smear is to perform reflex oncogenic HPV testing (from the same sample in which liquid-based cytology was done originally) or to recall the women to collect samples for HPV test. Women with ASC-US smear but negative on HR-HPV test are returned to normal recall. The HPV-positive women should be immediately referred to colposcopy.
Management of women with LSILThe natural history of LSIL matches that of HPV infection with rapid regression in the majority of cases. Immediate referral for colposcopy following a single LSIL cytology report may lead to unnecessary detection of insignificant cervical abnormalities. A systemic review and meta-analysis exploring the management strategies of ASCUS or LSIL at primary screening showed that performing immediate colposcopy detected a significant number of CIN1 that would anyway regress to normal in majority of women.[14]
Studies have shown that with an LSIL referral, the rates of CIN2+ and CIN3+ are 17% and 12% respectively.[15,16] A multicenter study in the United Kingdom concluded that although immediate colposcopy on women referred for LSIL detected CIN2+ lesions, it lead to large number of referrals with no high-grade abnormalities.[13] In the context of low and middle income countries where colposcopy services are limited, factors like age of women and availability of a triage test are to be considered to understand who should undergo diagnostic colposcopy and who should be followed up or returned to routine screening protocol.
In younger women, as 90% of the LSIL regresses within 3 years,[17] a repeat smear is suggested at 12 months rather than immediate referral for colposcopy.[18] In older women, studies have observed that the regression rates are much lower.[19] Most likely, many of the LSIL detected in adults reflect persistent infections with underlying CIN 2 or 3, helping to explain these differences. In settings where HPV triage is possible, all women with LSIL cytology and with HRHPV positive test reports should be referred for colposcopy. If colposcopy detects any lesion, then guided biopsy should be obtained from the abnormal area. In absence of any detectable lesion, cervical biopsy from the TZ should be considered.[20] Recent studies have shown that p16/Ki-67 dual staining could be useful to identify underlying high-grade CIN in cases reported as LSIL.[21]
Management of women with ASC-HWomen with ASC-H on the Pap smear have higher risk of harboring underlying cervical lesions. In a retrospective study of 517 ASC-H cases, biopsy reported 2.9% of cervical cancer and 1.7% of AIS.[22] Data from the KPNC study showed that the cumulative 5-year risk of developing CIN 3+ lesions for women with ASC-H cytology was 7.8%, which is significantly higher compared to 3.8% for ASCUS/LSIL.
Colposcopy is recommended in women with ASC-H cytology. Reflex HPV testing is not recommended in these cases. Biopsies are indicated from colposcopically detectable lesions. If no lesion is found on colposcopy, recommendations include repeat colposcopy and repeat cytology after 6 months (ASCCP 2012). If these repeat tests are negative, women may return to regular screening. The finding of ASC-H with Type III TZ on colposcopy requires excision procedure for diagnostic purposes, even if no apparent lesion is visible.
Management of women with HSILWomen with HSIL on cytology are at substantial risk of developing CIN 2+ lesions and cervical cancer, which is confirmed from the KPNC cohort.[12] Cervical cancer is found at colposcopy in some 2% of women with HSIL, although risk rises with age. Studies have shown CIN 2 or greater in 53– 66% of cases in which colposcopic biopsies are undertaken, and up to 90% if an immediate loop electrosurgical excision procedure (LEEP) is performed. The 5-year risk of CIN 3+ among women 30 years of age and older with HPV-positive HSIL in the KPNC cohort, was 50% and the 5-year cancer risk was 7%. This mandates for the immediate colposcopy without any triaging method.
Colposcopic-guided biopsy and ECC should always be carried out when the cytology indicates HSIL. In the absence of any detectable lesion on colposcopy and when the TZ is not seen completely, a diagnostic excision procedure should be done.
A case-control study compared cost-effectiveness, patient compliance, and pathology of 100 women with HSIL smear treated with single visit colposcopy and LLETZ intervention. CIN 2 and CIN 3 were confirmed in 94% of patients, CIN 1 in 2%, and 3% had microinvasive cancer to a depth of 0.5–1.5 mm, and 1% did not have any evidence of CIN. Cost analysis revealed savings of $35,000 for the institution. Patient compliance was improved with a kept appointment rate of 82%. This study concluded that “See and treat” intervention in these high-risk women was an effective tool convenient for patients with only one disruption of daily schedules.[23]
Management of women with Glandular abnormalities on cytologyThe glandular abnormalities reported on Pap smear are AGCNOS, AGC-N, or AIS. The prevalence of AGC accounts for 0.08–5.96% of all the cases of Pap abnormalities, and it has been shown that 8% of diagnosed cases are associated with malignant lesions. AGC-associated neoplastic findings may be related to gynecological or extrauterine malignancies. Thus, when AGCs are encountered, in addition to the evaluation of the cervix, it is advised to look for other gynecological and non-gynecological malignancies. Endometrial sampling is recommended along with colposcopy and endocervical sampling in women 35 years of age and older with all subcategories of AGC and AIS. Daniel et al. in their study of 456 cases found AIS in 20%, carcinoma of the cervix in 9%, and endometrial pathology in 29%, including carcinoma of the endometrium in 10%, and the rate of CIN 2 and CIN 3 was 36% on histopathology.[24] In the KPNC cohort, CIN 3+ was found in 9% and malignancy in 3% of women with AGC cytology, aged above 30 years. Considering the high rate of underlying pathology, colposcopy is indicated without any triaging method. All women with an AGC on cytology should have endocervical curettage. Women over 35 years of age or with a history of abnormal bleeding should have endometrial sampling.
In glandular neoplasia of “endocervical type,” the prevalence of invasive adenocarcinoma, CGIN, and CIN is high. AGC-N is associated with higher rates of abnormalities and thus, in the absence of an abnormality found on colposcopy, a diagnostic excision procedure is recommended either by cold knife cone biopsy, laser cone biopsy, or LEEP. Hysterectomy should not be performed as a diagnostic excision procedure.
If the cytology report is AGC-NOS and there is no lesion identified on colposcopy, follow-up every 6 months with repeat cytology, colposcopy, and endocervical curettage till 2 years is recommended. If all the tests are negative, she can enter the routine screening schedule.
CIN is found in approximately half of women with AIS.[25] Since glandular and squamous lesions often coexist, identification of CIN does not exclude AIS or adenocarcinoma.
MANAGEMENT OF WOMEN WITH CYTOLOGICAL ABNORMALITIES IN PREGNANCYPrecancerous lesions do not pose any risk to the pregnancy and poses no immediate risk to the mother. The safety of delaying treatment of pregnant women has been demonstrated in a number of cohort and retrospective uncontrolled studies.[26,27] The primary aim of colposcopy in pregnancy is to rule out invasive cancer.
Treatment during pregnancy carries substantial risk for hemorrhage and pregnancy loss. Treatment of premalignant lesions and endocervical curettage is contraindicated in pregnancy.
Women with an ASC-US or LSIL reports during pregnancy should have repeat cytology testing at 3 months post-pregnancy. Pregnant women with HSIL, ASC-H, or AGC should be referred for colposcopy within 4 weeks. If CIN3 or invasion is suspected on colposcopy, biopsy should be taken as it is found that biopsy is a safe procedure.[28] Invasive cancers detected on histopathology should be immediate treatment based on stage. If CIN 2 or CIN 3 is detected during pregnancy the colposcopy should be repeated 2 months after delivery.
留言 (0)