Usefulness of Rigid Bronchoscopic Intervention Using Argon Plasma Coagulation for Central Airway Tumors
Abstract ObjectivesArgon plasma coagulation (APC) is a noncontact form of electrocautery that utilizes ionized argon as the electrical current. A rigid bronchoscopic use of APC for the management of central airway obstruction could be safe and rapidly effective. This study evaluated the usefulness of rigid bronchoscopy with APC for the management of central airway obstructions due to benign or malignant tumors.
MethodsTwenty patients with obstructing central airway tumors were retrospectively reviewed from February 2008 to February 2013 at Chonnam National University Hospital. All patients received rigid bronchoscopic tumor removal under general anesthesia. APC was applied before and after tumor removal.
ResultsThe median age of patients was 59 years (interquartile range [IQR], 51 to 67 years) and 70% were female. The causes of airway obstruction included malignancy (n=8) and benign tumor (n=12). Airway tumors comprised intraluminal lesions (n=11, 55%) and mixed intraluminal/extraluminal lesions (n=9, 45%). The median tumor size was 15 mm (IQR, 10 to 18 mm). The median degree of airway obstruction was significantly reduced after intervention (90% [IQR, 88% to 96%] vs. 10% [IQR, 0% to 20%], P<0.001). The median American Thoracic Society dyspnea grade (3 [IQR, 1 to 4] vs. 1 [IQR, 0 to 1], P<0.001) and forced expiratory volume in one second (1.03 L [IQR, 0.52 to 1.36 L] vs. 1.98 L [IQR, 1.57 to 2.64 L], P=0.004) were significantly improved after intervention. There were no procedure-related acute complications and deaths.
ConclusionRigid bronchoscopy with APC is an effective and safe procedure to alleviate central airway obstruction caused by tumors.
Keywords: Argon Plasma Coagulation; Bronchoscopy; Airway Obstruction
INTRODUCTION Central airway obstruction can be caused by a variety of disease processes and exacts significant morbidity and mortality [1]. Patients with central airway obstruction often present with exertional dyspnea, positional wheezing which is not responsive to bronchodilators, and postobstructive pneumonia and may even present within hours of dying due to refractory hypoxia [12]. Patients with malignant central airway obstruction are often poor surgical candidates and have little therapeutic options to relieve dyspnea [2]. Bronchoscopy is widely used to treat obstructive lesions in the airway [345678]. However, in treatment of central airway obstruction, rigid bronchoscopy is preferred over flexible bronchoscopy due to easier securing of the airway and more available therapeutic modalities during intervention [18]. The variety of therapeutic modalities include mechanical debridement, silicone stent placement, laser, cryotherapy, electrosurgery, argon plasma coagulation (APC), photodynamic therapy, and brachytherapy can achieve optimal results in the treatment of central airway obstruction [34567]. APC is a method of noncontact electrocoagulation, using high frequency current by means of ionized argon gas (plasma) [9]. Although APC is frequently used for palliation of malignant obstruction as part of multimodality treatment and also in benign conditions [51011121314], there are very few reports about its use with rigid bronchoscopic intervention for central airway tumors, especially for benign tumors.This study was done to assess the efficacy and possible side effects of APC use in rigid bronchoscopy for the treatment of malignant as well as benign central airway tumors.
MATERIALS AND METHODSBetween February 2008 and February 2013, all patients who received rigid bronchoscopic procedure with APC for benign or malignant central airway obstructive lesions at Chonnam National University Hospital were retrospectively reviewed. Patients with central airway obstruction with symptoms related primarily to airway obstruction and not to systemic disease and/or with an obstruction associated with more than 50% luminal narrowing of trachea or mainstem bronchus received a rigid bronchoscopic tumor removal. Permission was obtained from the Institutional Review Board of Chonnam National University Hospital to review and publish patient records retrospectively (IRB No. CNUH-2014-211). Informed consent was waived because of the retrospective nature of the study.
Rigid bronchoscopic procedure was performed under general anesthesia with the appropriate degree of sedation and muscle relaxation monitored closely by an expert anesthesiologist. After the induction of anesthesia, the patients were intubated with a rigid bronchoscope (Karl-Storz, Tuttlingen, Germany). We used APC to coagulate tissue before and after tumor removal and to vaporize residual tumor after mechanical debulking using the tip of rigid bronchoscopy. Endobronchial APC was performed with an argon plasma coagulator unit (APC 300 and ERBOTOM ICC 200, ERBE USA, Marietta, GA, USA) via a rigid bronchoscope. Energy at 40 to 60 W and argon flow at 1.6 L/minute was applied through a 2.3-mm diameter, 220-cm length APC monopolar probe. Flexible bronchoscopy (Evis BF-1T260, Olympus, Tokyo, Japan) through the rigid tube was employed as necessary to help clear the airways of secretions and blood, and to assess the patency of distal bronchial segments. Immediately following intervention, patients were monitored for less than 2 hours in the postanesthesia care unit adjacent to the operating room, after which they were transferred to an appropriate level of care area (intensive care unit or medical ward) based on their ventilatory status and need for further observation or therapy. If no complications emerged and the treatment was effective, the patients were discharged.
The outcome measures for this study included the degree of airway obstruction, resting American Thoracic Society (ATS) dyspnea grade, and pulmonary functions from baseline. The result was defined as success when the lumen of trachea and mainstem bronchus was reopened to 70% or more of the original diameter and the symptoms were improved. All results are presented as the median and IQR (because the majority of the data did not follow a normal distribution) or number and percent. Categorical variables were analyzed with the Pearson chi-square test or Fisher exact test as appropriate; continuous variables were analyzed with the Mann-Whitney U-test. All data were analyzed with the use of the IBM SPSS ver. 21.0 (IBM Co., Armonk, NY, USA) and statistical significance was considered at P<0.05.
RESULTS Patient characteristics There were 6 males and 14 females with a median age of 59 years (IQR, 51 to 67 years). The most common symptom was dyspnea (16 of 20, 80%). Of all tumors, 8 (40%) were malignant and 12 (60%) were benign. The most common site of tumor and types of obstruction were trachea (12 of 20, 60%) and intraluminal lesions (11 of 20, 55%). Patient demographics and description of airway lesion are detailed in Table 1. Outcomes Treatment outcomes after rigid bronchoscopic intervention using APC are detailed in Table 1. After the rigid bronchoscopic intervention with APC, all patients had successful reestablishment of airway patency. Airway obstruction significantly decreased from preintervention (median, 90%; IQR, 83% to 99%) to postintervention (median, 10%; IQR, 0% to 20%; PFig. 1A). Symptoms were immediately improved after tumor removal in all patients. The ATS dyspnea grade which was measured at admission and discharge (median 4 days from admission; IQR, 2 to 7 days) was significantly improved after tumor removal ([median, 3; IQR, 1 to 4] vs. [median, 1; IQR, 0 to 1], PFig. 1B). Ten of 11 patients who performed spirometry before and after procedure (median 20 days after intervention; IQR, 15 to 28 days) showed significant improvements in forced expiratory volume in one second/forced vital capacity (FEV1/FVC) ratio ([median, 44.4%; IQR, 37.6% to 64.0%] vs. [median, 74.8%; IQR, 67.6% to 85.2%], P=0.003) and FEV1 ([median, 1.03 L; IQR, 0.52 to 1.36 L] vs. [median, 1.98 L; IQR, 1.57 to 2.64 L], P=0.004) (Fig. 1C).Serial follow-up was done with using chest X-ray or chest computed tomography (CT) about 1-3 months after intervention as clinically indicated. Flexible bronchoscopy was added when there was any suspicion of recurrence. The median follow-up duration was 315 days (IQR, 61 to 1,231 days).
After stabilizing the airway with the rigid bronchoscopic treatment, all patients with malignant tumors received additional definitive therapies, except one patient with thyroid cancer who refused additional surgery and only received supportive care. Two patients underwent a second rigid bronchoscopic intervention with APC for recurrent central airway obstructions at 263 days in one patient with an adenoid cystic carcinoma (case 16) and 1,100 days in one patient with a thyroid carcinoma (case 20) after the first intervention. In 12 patients with benign tumors, 10 showed complete removal in bronchoscopic findings after intervention, and there were no tumor recurrences for median 12 months (IQR, 4 to 25 months) follow-up after intervention. Two patients with benign, wide-based tumors that were removed incompletely by rigid bronchoscopic intervention with APC received additional treatments: radiotherapy in one patient with plasmacytoma (case 10) and surgery in one patient with schwannoma (case 5). Representative bronchoscopic images, CT images and histological features of complete removal and incomplete removal of benign central airway tumors are presented in Figs. 2, 3.There were no procedure related complications and deaths.
DISCUSSIONIn this study, APC was an effective and safe tool for alleviating central airway obstruction due to both malignant and benign tumors when combined with rigid bronchoscopic intervention.
The laser is a main therapeutic option for the management of endobronchial tumors, and the most widely used type is the neodymium:yttriumaluminium garnet laser [45]. Many studies have reported effectiveness of this laser therapy for the management of malignant or benign endobronchial obstructions [151617181920]. However, APC could replace laser as a therapeutic modality of thermal ablation, coagulation, and vaporization in airway intervention due to its lower cost, easier use, and flexible flow, which can allow interventions in bronchial segment, and more favorable safety profile than laser [46921]. Endobronchial APC is principally used for the treatment of hemoptysis caused by lesions within the central airways, debulking of exophytic endobronchial tumors, and debulking of granulation tissue arising as a complication of tracheobronchial stent insertion [7]. APC has been used mainly to treat malignant airway obstruction in previous studies [101214], whereas in our study, it was used to treat malignant as well as benign central airway tumors. The treatment for benign tumors using APC was effective and safe, and there was no recurrence after treatment in our study. Despite the safety features of APC, potential complications include airway perforation, airway fire, and gas embolism [9142223]. These complications develop during both rigid and flexible bronchoscopy, but when APC is combined with rigid bronchoscopy, complications might be decreased due to minimized gas flow by mechanical removal of tumors with rigid bronchoscopic tip or rigid bronchoscopic forcep. In the present study, we did not observe these complications and other procedure related acute complications including gas exchange impairment. The positive effects of rigid bronchoscopy are not only probable benefits to reducing complications of APC but also the ability to ventilate the patient through a side port while intervening, efficient suctioning of blood and secretions, and utility of the barrel of the rigid bronchoscope in coring out tumor tissue and dilating stenosis, and the use of accessory instruments [368]. There have been several reports that APC with a flexible bronchoscope is effective and safe in the treatment of tracheobronchial tumors [101224]. Whereas those studies included tumors in the lobar or segmental bronchi, all tumors in our study were located at the level of the trachea and the main bronchi. Because the flexible bronchoscope can occlude the airway and exacerbate respiratory distress, experts recommended a rigid bronchoscopy for providing a secure of airway, supporting proper oxygenation and ventilation, and allowing a use of various instruments [1]. Therefore we used a rigid bronchoscope in all cases. In central malignant airway tumors, bronchoscopic intervention could alleviate life threatening airway obstruction and provide time for additional definitive treatments for longer survival [25]. In our study, 7 of 8 patients with malignant tumors received additional definitive treatments including surgery or radiotherapy and/or chemotherapy after bronchoscopic intervention. In benign tumors, bronchoscopic interventions aim to alleviate airway obstructions and to completely remove tumors. However in wide-based lesions, the tumors may not be removed completely and have risk of relapse after bronchoscopic intervention [26]. Therefore, additional definite treatments, such as surgery, should be considered in these lesions. In our study, 2 of 12 patients with benign tumors had wide-based lesions; the tumors in both patients were incompletely removed by bronchoscopic interventions. The patients received additional definitive treatments such as radiotherapy and surgery.This single-institution retrospective analysis of this study has several limitations. First, the findings of our analysis were limited by small sample size. More cases are needed to confirm our results. Second, the study had relatively short term follow-up period in benign tumors. Slow growing nature of these tumors need longer follow-up period for relapse after bronchoscopic intervention.
In summary, our study demonstrates that rigid bronchoscopic procedure with APC was safe and effective management of benign or malignant central airway obstructions.
ACKNOWLEDGMENTSThis study was supported by a grant (CRI 12057-22) of the Chonnam National University Hospital Research Institute of Clinical Medicine. The funders had no role in the study design, data collection and analysis, the decision to publish or preparation of the manuscript.
CONFLICT OF INTERESTCONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References 1. Ernst A, Feller-Kopman D, Becker HD, Mehta AC. Central airway obstruction. Am J Respir Crit Care Med. 2004 6;169(12):1278–1297. PMID: 15187010.Treatment outcomes before and after rigid bronchoscopy. (A) Changes of ATS dyspnea grade. (B) Changes of FEV1 (L). (C) Changes of degree of stenosis (%). ATS, American Thoracic Society; FEV1, forced expiratory volume in one second.
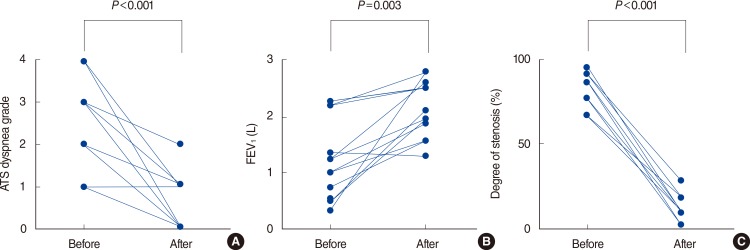 Fig. 2
Fig. 2
Chest computed tomography (CT) and bronchoscopy images of a 67-year-old female patient with primary endobronchial schwannoma completely removed by rigid bronchoscopic intervention with argon plasma coagulation. (A) Bronchoscopic finding showed a polypoid tumor with a smooth surface obstructing the lumen of the trachea. (B) Bronchoscopic finding about 2 months after intervention showed an opened trachea and a small residual nodule confirmed as a granulation tissue by bronchoscopic biopsy. (C) Chest CT before intervention showed a 1.6-cm-sized well defined oval tumor occupying trachea (arrow). (D) Chest CT about 2 months after intervention showed no recurrence in trachea.
 Fig. 3
Fig. 3
Chest computed tomography (CT) and bronchoscopy of a 52-year-old female patient with primary endobronchial schwannoma that was incompletely removed by rigid bronchoscopic intervention with argon plasma coagulation. (A) Chest CT showed a 3×2.7-cm lobulating mass in carina. (B) Bronchoscopic finding showed a large mass with a smooth surface near totally obstructing the lumen of the carina. (C) Bronchoscopic finding immediately after intervention showed opened main bronchi and a wide base of removed tumor.
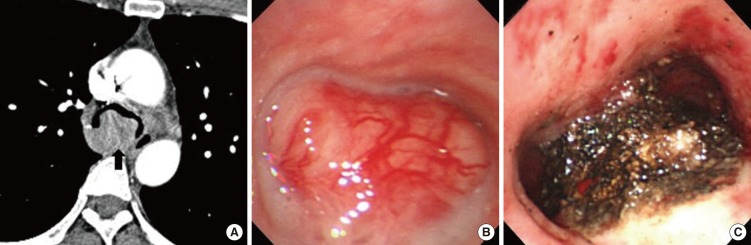 Table 1.
Table 1.
Clinical and outcome data for 20 patients with central airway obstruction
Case Age (year) Sex Pathologic diagnosis Clinical manifestations Location of tumor Types of obstruction Size (mm) Degree of obstruction (%) Role of APC Relapse Additional treatments 1 64 M Hamartoma Dyspnea Trachea Intraluminal 32 90 Complete removal No No 2 56 F Hamartoma Cough, recurrent pneumonia LMB Intraluminal 10 100 Complete removal No No 3 34 F Hamartoma Cough, recurrent pneumonia RMB Intraluminal 5 100 Complete removal No No 4 60 F Hamartoma Dyspnea RMB Intraluminal 10 100 Complete removal No No 5 52 F Schwannoma Dyspnea Trachea Mixed 25 95 Partial removal No Surgery 6 67 F Schwannoma Dyspnea Trachea Intraluminal 15 90 Complete removal No No 7 43 F Carcinoid tumor Hemoptysis LMB Intraluminal 12 80 Complete removal No No 8 57 M Fibroepithelial polyp Dyspnea Trachea Intraluminal 15 80 Complete removal No No 9 73 F Tuberculous granuloma Dyspnea Trachea Mixed 15 90 Complete removal No No 10 47 F Plasmacytoma Dyspnea RMB Mixed 17 70 Partial removal No Radiotherapy 11 60 F Castleman disease Dyspnea Trachea Intraluminal 15 90 Complete removal No No 12 31 F Pleomorphic adenoma Dyspnea Trachea Intraluminal 18 90 Complete removal No No 13 53 M Lung cancer (ADC) Dyspnea LMB Mixed 10 100 Partial removal No Chemotherapy and radiotherapy 14 62 M Lung cancer (SCC) Dyspnea LMB Mixed 10 100 Partial removal No Chemotherapy and radiotherapy 15 69 F Tracheal cancer (SCC) Dyspnea Trachea Mixed NA 90 Partial removal No Chemotherapy and radiotherapy 16 49 F ACC No symptoms Trachea Intraluminal 7 90 Par
留言 (0)