This study aimed to investigate the rates, types, and risk factors of surgical site infection (SSI) following intracranial neurosurgical procedures evaluated by a Korean SSI surveillance system.
MethodsThis was a prospective observational study of patients who underwent neurosurgical procedures at 29 hospitals in South Korea from January 2017 to June 2017. The procedures included craniectomy, craniotomy, cranioplasty, burr hole, and ventriculoperitoneal shunt. Univariate and multivariate logistic regression analyses were performed.
ResultsOf the 1576 cases included, 30 showed infection, for an overall SSI rate of 1.9%. Organ/space infection was the most common, found in 21 out of the 30 cases (70%). Staphylococcus aureus was the most common (41%) of all bacteria, and Serratia marcescens (12%) was the most common among gram-negative bacteria. In univariate analyses, the p-values for age, preoperative hospital stay duration, and over T-hour were <0.2. In a multivariate analysis of these variables, only preoperative hospital stay was significantly associated with the incidence of SSI (p<0.001), whereas age and over T-hour showed a tendency to increase the risk of SSI (p=0.09 and 0.06).
ConclusionSurveillance systems play important roles in the accurate analysis of SSI. The incidence of SSI after neurosurgical procedures assessed by a national surveillance system was 1.9%. Future studies will provide clinically useful results for SSI when data are accumulated.
Key Words: Surgical site infection · Neurosurgery · Risk factors · Korea · Incidence.
INTRODUCTION Surgical site infection (SSI) is the third most frequently reported nosocomial infection, accounting for 14-16% of all infections among hospitalized patients and 38% among surgical patients [9,19]. SSI is associated with substantial morbidity and mortality, prolonged hospital stay, and increased expenses [24]. Although the SSI rate after neurosurgery is generally known to be lower than that after other surgery, SSI can cause catastrophic consequences such as altered consciousness and the need for reoperation. Reoperation is a burden to both the patient and physician. Reported rates of SSI following intracranial neurosurgical procedures have been variable, ranging from 8% in a published series [20]. After neurosurgical procedures, SSI most commonly presents as meningitis, epidural abscess, subdural empyema, or brain abscess [14].Many efforts have been made to prevent SSI, one of which is the use of infection surveillance systems. The Korean National Healthcare-Associated Infection Surveillance System (KONIS) is operated by the Korean Centers for Disease Control and Prevention (CDC). It monitors the current status of SSI in Korea as a whole and helps develop means to reduce SSI rate. Hospitals of all sizes are enrolled in the KONIS system. This study aimed to investigate the rates, types, and risk factors for SSI following intracranial neurosurgical procedures.
MATERIALS AND METHODS This was a prospective observational study of patients who underwent neurosurgical procedures at a total of 29 hospitals in South Korea from January 2017 to June 2017. The neurosurgical procedures included craniectomy, craniotomy, cranioplasty, burr hole, and ventriculoperitoneal (VP) shunt. Patients who survived at least 7 days after surgery were included in the study. Prophylactic antibiotics were given to all patients in accordance with the Ministry of Health guidelines. The first-generation cephalosporin was administered within 1 hour before skin incision, and the duration of administration did not exceed 48 hours after surgery. Patients who under 18 years of age, be transferred, with a fever greater than 38 degrees within 24 hours before surgery, with American Society of Anesthesiologists (ASA) class 4 or more, underwent more than two operations during the hospital stay and underwent emergency operations were excluded from prophylactic antibiotics guidelines. For surveillance, surgical procedures were classified according to the KONIS-SSI code, referencing the 2017 electronic data interchange (EDI) insurance code. The KONIS-SSI codes for the surgical procedures in this study were craniotomy (CRAN) and ventricular shunt (VSHN). Table 1 shows the Health Insurance EDI codes for CRAN and VSHN. Patients were monitored for 90 days after surgery; those discharged or transferred were monitored at outpatient clinics. Patients who underwent reoperation, passed away within 48 hours after surgery, or did not have the incision site closed were excluded. Data were collected by the surgeon performing the surgery and nurse practitioners who had completed the KONIS-SSI steering committee course at least once a year. Data included were age, sex, duration of preoperative hospital stay, elective or emergency surgery, endoscopic procedure, combined surgery, reoperation, surgery by trauma, operation time, wound classification (clean, clean-contaminated, contaminated, and dirty) [22], ASA classification [1], National Nosocomial Infection Surveillance (NNIS)-derived risk index [11], infection, and infecting microorganisms. These values were recorded on standardized worksheets specially designed according to the guidelines in the KONIS-SSI manual. SSI criteria and wound classification The criteria used to define SSI were established by the CDC in 2017 [4,5]. In accordance with these criteria, SSI was defined as any infection occurring within 1 month of the operation when no prosthetic material was left in the wound, or within 1 year when prosthetic material remained within the operation site. However, since the monitoring period was 90 days, SSI was evaluated as an infection within 90 days even if the prosthetic material was inserted. Metal plates and screws used to fix the bone flap were considered prosthetic material. SSIs were classified depending on the degree of infection as follows : superficial incisional, deep incisional, and organ/space. These categories involved skin or subcutaneous tissue at the incision, deep soft tissue (fascia and muscle layers) at the incision, or any part of the anatomy (organs or organ spaces), respectively. Because no clear distinction exists between superficial and deep incisional infection in brain surgery, we included skin or subcutaneous tissue in the definition of superficial incisional infection, and fascia/muscle layers and aponeurosis/skull bone in that of deep incisional infection. Organ/space infection was diagnosed as infection of the central nervous system, such as intracranial infection (brain abscess, subdural or epidural infection, encephalitis), and meningitis or ventriculitis [5]. A method suggested by Narotam et al. [22] was applied to wound classification in neurosurgical procedures as follows : “dirty” procedures included brain abscess, subdural empyema, and osteitis surgical treatments when sepsis was already present; “contaminated” procedures included mainly trauma patients with open compound cranial fractures or scalp lacerations older than 4 hours; “clean-contaminated” procedures included paranasal sinuses or mastoid entry, repair of cranial base fractures, and aseptic surgical technique breaches; “clean” procedures included most of the scheduled surgeries. Statistical analysisTo compare differences in variables between the SSI and non-SSI groups, Fisher’s exact test or the Wilcoxon rank sum test were used. Spearman correlation analysis was used to examine the correlation between hospital scale and SSI rate. p-values of <0.05 were considered significant. Univariate and multivariate logistic regression analyses were conducted with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). The objective of the univariate analysis was to determine the risk factors linked to SSI, followed by multivariate analysis for variables with p-values <0.2.
RESULTS Population A total of 1576 cases (CRAN, 1495; VSHN, 81) fulfilled the inclusion criteria and were enrolled in the study from September 2016 to June 2017. Comparisons between the SSI and non-SSI groups were carried out with VP shunt cases (VSHN) excluded because of the low number of SSIs (two cases). For the CRAN code, the mean age of the non-SSI group was significantly higher than that of the SSI group (57 vs. 52 years; p=0.045), and the mean preoperative hospital stay was significantly longer in the SSI group than in the non-SSI group (13 vs. 28 days; p =0.014). No difference was found between the groups in the ASA class (Table 2). Surgical procedures For the variables of endoscopic, emergency, combined, reoperation, and trauma, no significant differences were found between the two groups. In addition, no difference was observed in wound classification. Although the differences in mean operation time and over T-hour (the exact 75th percentile of procedure duration in minutes) were not significant, over T-hour in the SSI group tended to be higher than in the non-SSI group (24.7 vs. 39.3%; p =0.077) (Table 2). SSI incidence We investigated the SSI rate by NNIS risk index category. For CRAN, the SSI rate was higher as the risk index increased from 1.39% for risk index 0 to 2.59% for risk index 2. Risk index 3 was not significantly different because it was only present in two cases. The overall SSI rate for CRAN was 1.9%. For VSHN, risk index 0 showed an SSI rate of 9.5%; no SSI occurred with risk index 1 or 2. No surgical cases had risk index 3. The overall SSI rate for VSHN was 2.5% (Table 3). In CRAN cases, organ/space infection was the most common, present in 19 out of 28 cases. The 2 VSHN cases were organ/space infection (Table 4). The overall SSI rates for CRAN and VSHN varied depending on hospital size; however, the correlation was not significant (p =0.702, Table 5). Staphylococcus aureus was the most common (41%) causative organism among all bacteria, and Serratia marcescens (12%) among gram-negative bacteria (Fig. 1). SSI risk factors Univariate and multivariate analyzes were performed to identify risk factors for SSI. In univariate analyses, the p-values for age, preoperative hospital stay duration, and over T-hour were pTable 6). DISCUSSION The importance of infection surveillance was acknowledged more than 40 years ago by the CDC and the Joint Commission on Accreditation of Hospitals, and has proven effective in preventing SSIs [6,8]. The Study on the Efficacy of Nosocomial Infection Control, which investigated whether a surveillance program could reduce the infection rate, concluded based on U.S. data that SSIs can be reduced by up to 32% if the following four conditions are met : 1) surveillance is continuous, 2) management efforts are aggressive, 3) infection control personnel are qualified, and 4) feedback on the infection rate is provided to the surgeon, especially on SSI [13]. Therefore, continuous SSI surveillance and feedback on the surveillance results are important for the prevention of SSI.In Korea, a nationwide surveillance system for SSI was first applied to orthopedic artificial joint surgery in 2006, and neurosurgical procedures including craniotomy and VP shunt have been included in the survey since 2009. However, because the surgeon’s participation in the infection surveillance system has not been mandatory, the accuracy of SSI diagnosis and surveillance is evaluated to have been low. Therefore, the 2017 KONIS manual mandated the participation of surgeons. This study is the first in a national survey of SSI in the neurosurgical field using the 2017 KONIS manual. We analyzed the SSI incidence in the neurosurgical field to find possible means to prevent SSI.
In this study, the overall SSI rate for 1576 cases was 2.1% (CRAN, 1.9%; VSHN, 2.5%). The results were similar to those of previously published studies. The incidence of postoperative wound infection documented in the literature range from as low as 1.25% to as high as 17% without, and from 0.3% to 3.0% with prophylactic antibiotics [15,16,20,22,23]. In the present study, the SSI incidence with prophylactic antibiotics was within the previously reported range. Among the total of 30 infection cases, organ/space infections accounted for 70%, followed by superficial (17%) and deep (13%) infections. Patir et al. [23] reported 5% wound infection and 6% meningitis rates among 413 cases. Korinek et al. [16] reported a 5.3% incision infection rate combined with scalp infection and bone flap osteitis, and a 1.52% meningitis incidence among 331 cases. Buang and Haspani [3] reported scalp incision infection in 4.9%, bone flap osteitis in 2.3%, and organ/space infection in 0.5% of the cases. Although exact comparison among studies is difficult because of different infection classifications and overall infection rates, we found the organ/space infection rate to be higher than that of incisional infection, in contrast to previous studies. No studies have found any association between SSI and hospital size. However, nosocomial infection rates have been reported to increase with increasing hospital size [10,21], possibly because of infection control personnel qualification, facility hygiene and maintenance, and pathogen variety depending on hospital size. However, the present study did not find any correlation between SSI and hospital size. Because the number of infections was low, an accurate analysis may not be possible, requiring further study. In this study, gram-positive bacteria caused 70% of the infections, among which S. aureus was the most common (41%) followed by S. epidermidis (11%). Our finding of gram-positive cocci as the main causative organisms of SSI is similar to the results of other series [7,15,16]. S. aureus is a member of the normal flora of the body, frequently found in the nose, respiratory tract, and on the skin. Although S. aureus is not always pathogenic, it is a common cause of skin and soft tissue infections [26]. Nasal carriers of S. aureus are at increased risk for S. aureus SSIs [27]. A recent meta-analysis suggested that implementing a standard procedure including screening for nasal S. aureus, decolonizing carriers with mupirocin ointment, and chlorhexidine bathing before surgery reduced S. aureus SSIs [25]. Although decolonizing is not known to be effective in reducing SSI after neurosurgery, it may still be helpful in reducing SSI. Among gram-negative bacteria, Serratia marcescens was the most common (12%). Serratia marcescens is an opportunistic pathogenic gram-negative bacillus that has emerged as a serious cause of nosocomial infections [18]. Serratia marcescens is capable of thriving in diverse environments including water and soil, but most commonly in healthcare settings. In the last few decades, it has become more common in intensive care units (ICUs) as a cause of bacteremia, pneumonia, and urinary tract infections [12,28,29]. Many patients who undergo neurosurgical procedures require ICU therapy, which may lead to infection by Serratia marcescens. To prevent it, thorough management of infections such as pneumonia and urinary tract infection is necessary. Previously reported risk factors for SSI after neurosurgical procedures include cerebrospinal fluid (CSF) leak, multiple operations, operation time longer than 4 hours, higher ASA class, clean-contaminated or dirty wound, and surgeon grade [2,3,15,17,22]. In our study, only preoperative hospital stay duration was found to be a risk factor for CRAN. In addition, an increase in over T-hour increased the risk of SSI, albeit the effect was not statistically significant. CSF leak, although identified as a risk factor in many studies, was not investigated because of the lack of data. The duration of preoperative hospital stay is lengthened by poor general condition, histories such as diabetes mellitus and hypertension, infection status such as pneumonia, urinary tract infection, and other complications in the case of trauma. These conditions can increase the risk of SSI. However, the poor general condition is not enough to explain the duration of preoperative hospital stay because other variables, such as ASA class and age, are also related to poor general condition. Future research will need additional analysis of the causes of longer preoperative periods. Long surgery is thought to increase the time of potential exposure to infection, often because of excessive bleeding.This study has several limitations. First, although the total number of patients was high, the infection group size was small. Second, data of the presence of a CSF leak, ventricular drain, or foreign body, previously identified as risk factors, were limited and not analyzed. Additional data collection will be needed in future studies to analyze SSI in the neurosurgical field. Third, the diagnostic criteria for incisional infection included pus discharge, tenderness, local edema, redness, and fever at the time of bacterium isolation from the skin incision site or subcutaneous tissue culture. However, it is possible that the surgeon did not always diagnose infection when observing some of the above symptoms, reserving the diagnosis for cases with definitive evidence of infection such as pus at the skin incision site. Fourth, in accordance with the SSI criteria used, postoperative infections were defined as those occurring within 1 year of the operation when prosthetic material remained within the operation site. The SSI rate could be undervalued because our study analyzed preliminary data that was only 90 days in duration. In future studies, a 1-year monitoring period will be necessary.
In the meantime, a nationwide survey on SSI in Korea was conducted by internal medicine staff. However, there may be differences in the diagnosis of SSI judged by surgeons and physicians. This study was the first national survey of SSI under surgeon’s diagnosis. Although there is a lack of finding risk factors for SSI due to the lack of analysis of various variables, we believe that it is also very meaningful to show reliable results on the SSI rate in the neurosurgical field. In future studies, when data is accumulated, and appropriate factors are analyzed, more valuable results can be obtained to help reduce SSI incidence.
CONCLUSIONSSIs can cause many serious problems in neurosurgery. Identifying SSI risk factors can help reduce mortality, morbidity, and improve patient care. An SSI surveillance system is important for the accurate analysis of SSI. The incidence of SSI after neurosurgical procedures assessed by a national surveillance system was 1.9%. Preoperative hospital stay duration was an independent risk factor for SSI. Age and over T-hour tended to increase the risk of SSI.
Fig. 1.Microorganisms identified in surgical site infection.
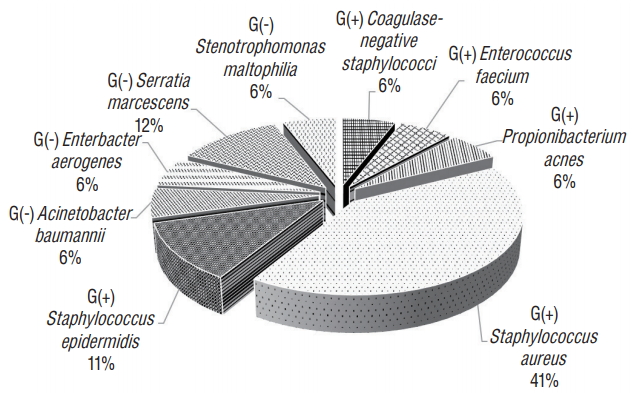 Table 1.
Table 1.
KONIS-SSI and EDI codes for neurosurgical procedures included in the study
KONIS-SSI code Type of surgery Definition and surveillance scope 2017 Health Insurance EDI standard code CRAN Craniotomy The skull is opened to expose the brain or the meninges, except for a simple puncture or a burr hole. N0331, N0333, N0334, N0335, S0471, S0472, S0475, S0476, S4621, S4622, S4633, S4634, S4635, S4636, S4637, S4641, S4642, S4653, S4654, S4655, S4656, S4657, S4658, S4661, S4662, S4681, S4683, S4733, S4734, S4735, S4736, S4737, S4760, S4771, S4772, S4780, S4792, S4793, S4794, S4796, S4797, S4798, S4799, S4801, S4802, S4803 VSHN Ventricular shunt A short circuit is inserted from the ventricle to another site; includes correction or removal of a shunt. S4712 Table 2.General characteristics of non-SSI and SSI groups of CRAN cases
Non-SSI group (n=1467) SSI group (n=28) p-value Sex (female) 766 (52.2) 14 (50.0) 0.816 Age (years) 57 (4-90) 52 (25-84) 0.045* Endoscopic operation 78 (5.3) 0 0.396 Emergency operation 556 (37.9) 13 (46.4) 0.357 Trauma 122 (8.3) 0 0.162 Combined operation 31 (2.1) 0 1.000 Reoperation 61 (4.2) 2 (7.1) 0.332 Duration of preoperative hospital stay 13 (0-214) 28 (4-300) 0.014* Wound classification Clean 1412 (96.3) 28 (100.0) 1.000 Clean-contaminated 24 (1.6) 0 Contaminated 24 (1.6) 0 Dirty 7 (0.5) 0 ASA classification 1 178 (12.1) 2 (7.1) 0.869 2 738 (50.3) 15 (53.6) 3 412 (28.1) 10 (35.7) 4 104 (7.1) 1 (3.6) 5 35 (2.4) 0 Operation time (minutes) 206 (20-1750) 264 (80-534) 0.389 Over T-hour† 362 (24.7) 11 (39.3) 0.077 Table 3.Pooled mean and key percentiles of the distribution of SSI rates by risk index
KONIS-SSI code Risk index Number of participating hospitals Number of operations Number of infections Infection rate (%) CRAN 0 23 649 9 1.39 1 25 728 16 2.20 2 20 116 3 2.59 3 2 2 0 0.00 Total 25 1495 28 1.87 VSHN 0 7 21 2 9.52 1 7 46 0 0.00 2 5 14 0 0.00 3 0 0 0 0.00 Total 8 81 2 2.47 Table 4.Type of SSIs according to operative procedure
KONIS-SSI code Type of surgery Number of operations Number of infections Superficial incisional infections Deep incisional infections Organ/space infections CRAN Craniotomy 1495 28 5 4 19 VSHN Ventricular shunt 81 2 0 0 2 Total 1576 30 5 4 21 Table 5.SSI rate by hospital size
Number of beds Number of operations Number of infections Infection rate (%) ≤299 36 0 0.00 300-499 85 4 4.71 500-749 287 3 1.05 750-899 361 7 1.94 900-999 142 5 3.52 1000-1499 232 2 0.86 ≥1500 433 9 2.08 Total 1576 30 1.90 Table 6.Univariate and multivariate analysis of factors influencing SSI in craniotomy (CRAN)
Univariate Multivariate OR 95% CI p-value Adjusted OR 95% CI p-value Sex (female vs. male) 0.92 0.43, 1.93 0.816 Age 0.98 0.96, 1.01 0.167* 0.98 0.95, 1.00 0.090 Endoscopic OP (yes vs. no) <0.001 <0.001, >999.999 0.971 Emergency OP (yes vs. no) 1.42 0.67, 3.01 0.359 Trauma (yes vs. no) <0.001 <0.001, >999.999 0.964 Combined OP (yes vs. no) <0.001 <0.001, >999.999 0.982 Reoperation (yes vs. no) 1.77 0.41, 7.64 0.442 Duration of preoperative hospital stay 1.02 1.02, 1.03 <0.001* 1.02 1.01, 1.03 <0.001† Wound classification (contaminated and dirty vs. clean and clean-contaminated) <0.001 <0.001, >999.999 0.982
留言 (0)