Infections caused by carbapenemase-producing Enterobacterales are on the rise and represent a significant threat to global healthcare services (Zhang et al., 2017). Among the various types of carbapenemases, KPC and NDM are the two most prominent classes found predominantly in Enterobacterales (Yang et al., 2023). Patients with CPE infections often face limited treatment options due to the scarcity of effective antibiotics (Grundmann et al., 2017). Historically, the primary clinical burden of CPE has been the increasing incidence of hospital-acquired infections caused by Klebsiella pneumoniae and Escherichia coli. However, other carbapenemase-producing Enterobacterales species, including Enterobacter cloacae complex, Serratia marcescens, and Citrobacter youngae, have also been identified in hospital settings (Nordmann and Poirel, 2014). Recent studies have highlighted the emergence of carbapenem-resistant Citrobacter species in various sites, such as the human gut, respiratory tract, urinary tract, and bloodstream, associated with severe diseases such as neonatal meningitis and brain abscesses (Yang et al., 2018; Fupin et al., 2022; Bitar et al., 2019). C. youngae, a Gram-negative member of the Enterobacterales, was originally isolated from human faces and was long considered a rare nosocomial pathogen. A 12-year study revealed that C. freundii and C. koseri are the two most commonly isolated species in clinical Citrobacter infections, with infections caused by other species, including C. youngae, accounting for only 5% of cases. The overuse and misuse of antibiotics have contributed to an increase in bacterial resistance and made treatment more difficult. In addition, concerns have been raised about food safety and cross-infection in hospital settings (Lee et al., 2019). C. youngae infections have been reported in neonates, young children, and immunocompromised patients, resulting in conditions such as bloodstream, urinary tract, gastrointestinal tract, and abdominal cavity infections (Zhang et al., 2008; Song et al., 2023; Al-Mulla et al., 2014; Chen et al., 2013; McAteer et al., 2023).
In C. youngae, the chromosomally encoded AmpC β-lactamase (cAmpC) plays a key role in mediating resistance to β-lactam antibiotics. This enzyme can hydrolyse a wide range of β-lactams, including cephalothin and extended-spectrum cephalosporins, and is remarkably unaffected by traditional β-lactamase inhibitors (Ranjan and Ranjan, 2013). Overexpression of chromosomal AmpC β-lactamase, coupled with reduced outer membrane permeability, often results in decreased susceptibility to carbapenems, particularly imipenem. As a result, clinical microbiology laboratories may have difficulty in accurately diagnosing carbapenemase production in these species (Woodford et al., 2007). Epidemiological studies have shown that blaCMY genes encoding AmpC β-lactamases are widely distributed in Enterobacterales. These genes are often associated with multidrug resistance and have been detected in both plasmid-encoded and chromosomally encoded forms (Lee et al., 2019). The blaCMY-2 is one of the most commonly reported AmpC β-lactamase genes in Enterobacterales, including Escherichia coli, Salmonella spp., and Klebsiella pneumoniae (Zhang et al., 2008; Song et al., 2023; Al-Mulla et al., 2014; Chen et al., 2013; McAteer et al., 2023). Their global spread poses a significant challenge to antimicrobial therapy, particularly in hospital-acquired infections (Ranjan and Ranjan, 2013).
According to previous epidemiological studies, OXA-48-like carbapenemases are the most commonly found in Citrobacter species, followed by NDM, VIM, and others (Biez et al., 2023). While KPC-type carbapenemases are highly prevalent in carbapenem-resistant Enterobacterales (CRE) overall, they are less commonly detected in Citrobacter species. Research has shown that Citrobacter spp. harbor a wide variety of plasmids, including a relatively high proportion of carbapenemase-encoding plasmids, suggesting that plasmid-mediated transfer of carbapenemase genes can occur between Citrobacter spp. and other bacteria (Arana et al., 2017). The emergence of the blaKPC gene in C. youngae poses a serious public health threat. Our study identifies the presence of blaKPC and the novel blaCMY variant blaCMY-190 (accession number OR896917) in Citrobacter spp. and highlights the importance of continuous monitoring and vigilance for this emerging resistance mechanism.
Materials and methods Strain and antimicrobial susceptibility testA carbapenem-resistant C. youngae strain was isolated from the peritoneal effusion at a tertiary hospital in Shaanxi Province, Northwest China. Strain identification was conducted using Illumina MiSeq technology, which is capable of generating millions of short (100–300 bp), low-error (0.1%) paired-end reads. Minimum inhibitory concentrations (MICs) were determined using the Clinical and Laboratory Standards Institute (CLSI) broth microdilution method. With the exception of tigecycline, colistin, and cefoperazone–sulbactam, all drug breakpoints were interpreted according to CLSI M100-33 guidelines (Clinical and Laboratory Standards Institute, 2023). Tigecycline MICs were interpreted using the US Food and Drug Administration (FDA) Enterobacterales breakpoint (FDA, 2021), and the colistin MICs were interpreted using the European Committee on Antimicrobial Susceptibility Testing (EUCAST) standards. Quality control strains, E. coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853, were used for antimicrobial susceptibility testing. Carbapenemase phenotypes were detected using the imipenem-EDTA double-disk synergy test and the NG-Test Carba-5, while carbapenemase genes (KPC, NDM, OXA, IMP, and VIM) were confirmed using the PCR.
Conjugation, transformation, and plasmid sequencingConjugation and transformation experiments were carried out to investigate plasmid transfer. Briefly, C. youngae, a blaKPC-2-positive isolate, was used as a donor strain, while E. coli J53 (azide-resistant) and E. coli DH5α were used as recipient strains. The conjugation test was carried out on Mueller–Hinton (MH) agar containing azide (100 μg/mL) and ampicillin (50 μg/mL), while the transformation test was performed on MH agar with ampicillin (50 μg/mL). The presence of the blaKPC-2 gene and other resistance genes essential for conjugation was confirmed using antimicrobial susceptibility testing, PCR, and DNA sequencing. Identification of each conjugate colony was performed using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) with MALDI Biotyper software (bioMerieux, located in Marcy-l’Etoile, France) and further confirmed by next-generation sequencing (NGS). Plasmids carrying the blaKPC-2 gene from the conjugants were extracted using the Qiagen Midi Kit (Qiagen, Hilden, Germany) and sequenced on the Illumina NovaSeq (Illumina, San Diego, CA, United States) short-read sequencer (150-bp paired-end reads). Sequencing reads were trimmed using a sickle (GitHub) and assembled de novo using SPAdes 3.12.0. Baseline calibration and assembly evaluation were done using Pilon 1.18. Open reading frame prediction and annotation were performed using RAST version 2.02 and BLAST at NCBI. Plasmid replicon types were determined using a PCR-based plasmid replicon typing method (Carattoli et al., 2005). Plasmid comparisons were performed using BRIG (Alikhan et al., 2011).
Cloning experiments and induced experimentsThe blaCMY-190 gene and its upstream promoter region were amplified from C. youngae-YS01. The pHSG398 vector was digested with EcoRI and KpnI using the ClonExpress RII One-Step Cloning Kit (Vazyme Biotech Co., Ltd., Nanjing, China). The linearised vector, purified PCR product, buffer, and enhanced recombinase were mixed and incubated for 20 min to obtain the recombinant vector. This recombinant vector was then introduced into E. coli DH5α by chemical transformation, followed by the determination of minimum inhibitory concentrations (MICs). In the presence of β-lactamase inducer (cefoxitin 10 μg/mL or cefotaxime 8 μg/mL), E. coli DH5α-PHSG398 and the positive control S. marcescens were incubated at 37°C for 2 h. The β-lactamase activity was then determined by a spectrophotometric method using the Amplite Beta-Lactamase Activity Assay Kit (AAT Bioquest Inc., America). A chromogenic β-lactam substrate (cephalosporin) was used, which changes color from yellow to red upon hydrolysis by β-lactamase. The assay was performed using an absorbance microplate reader, measuring the OD ratio at wavelengths of 490 nm to 380 nm.
Whole-genome sequencing and bioinformatic analysisGenomic DNA was extracted from overnight cultures of single bacterial colonies using a genome extraction kit according to the manufacturer’s instructions (Vazyme, China). DNA was sequenced using Illumina short-read sequencing (150 bp paired-end reads) (Illumina, San Diego, CA, United States). De novo sequence assembly was performed using SPAdes 3.12.0. For isolates suspected of carrying plasmid-encoded carbapenemases, long-read sequencing was conducted using the MinION instrument (Oxford Nanopore Technologies, Oxford, United Kingdom). Long-read sequencing libraries were prepared and multiplexed using a rapid multiplex barcoding kit (Oxford Nanopore Technologies catalog number SQK-RBK004) and sequenced on R9.4.1 flow cells. Base calling of raw reads was performed using Guppy v2.3.1 (Oxford Nanopore Technologies, Oxford, United Kingdom), and hybrid assembly, incorporating both Illumina short reads and Oxford Nanopore long reads, was performed using Unicycler v0.4.8-beta (Wick et al., 2017). Antimicrobial resistance gene analysis and draft genome annotation were performed using BacWGSTdb. Multilocus sequence typing (MLST) was performed using the mlst tool. Genomic comparison of closely related isolates was performed using Proksee. Antimicrobial resistance genes and plasmids were identified using the BLASTn analysis of assembled contigs against the ResFinder and PlasmidFinder databases, using a cutoff of 80% sequence identity and 80% sequence coverage. A maximum likelihood phylogenetic tree was reconstructed using MEGA11.
Results Overview of the Citrobacter youngae isolateCitrobacter youngae was recovered from the peritoneal effusion of a 62-year-old female patient hospitalized for acute liver failure. During her hospital stay, the patient developed a fever, marked abdominal distension, weakness, malaise, and chest tightness on exertion. Initial treatment included intravenous administration of meropenem (1 g Q8H) for 4 days. The patient had several comorbidities, including hepatic malignancy, decompensated cirrhosis secondary to hepatitis B, ascites, acute renal failure, uremia, and portal vein thrombosis. Subsequently, a KPC-2 enzyme-producing C. youngae was isolated from the ascitic fluid, and the intravenous therapy was changed to ceftazidime–avibactam (2.5 g Q8H) for 7 days. Following this treatment, the patient’s temperature normalized, and the infection was effectively controlled. She was discharged 21 days after admission. The antimicrobial susceptibility profile of the C. youngae isolate is detailed in Table 1. The isolate was susceptible to ceftazidime–avibactam (MIC = 1 μg/mL), amikacin (MIC = 2 μg/mL), tigecycline (MIC = 0.125 μg/mL), and colistin (MIC≤0.25 μg/mL). In contrast, it was resistant to imipenem (MIC = 8 μg/mL), meropenem (MIC = 16 μg/mL), cefepime (MIC≥32 μg/mL), cefoperazone–sulbactam (MIC = 64 μg/mL), aztreonam (MIC≥128 μg/mL), and ciprofloxacin (MIC = 8 μg/mL). The results of ATCC strains in this study were within the quality control range.
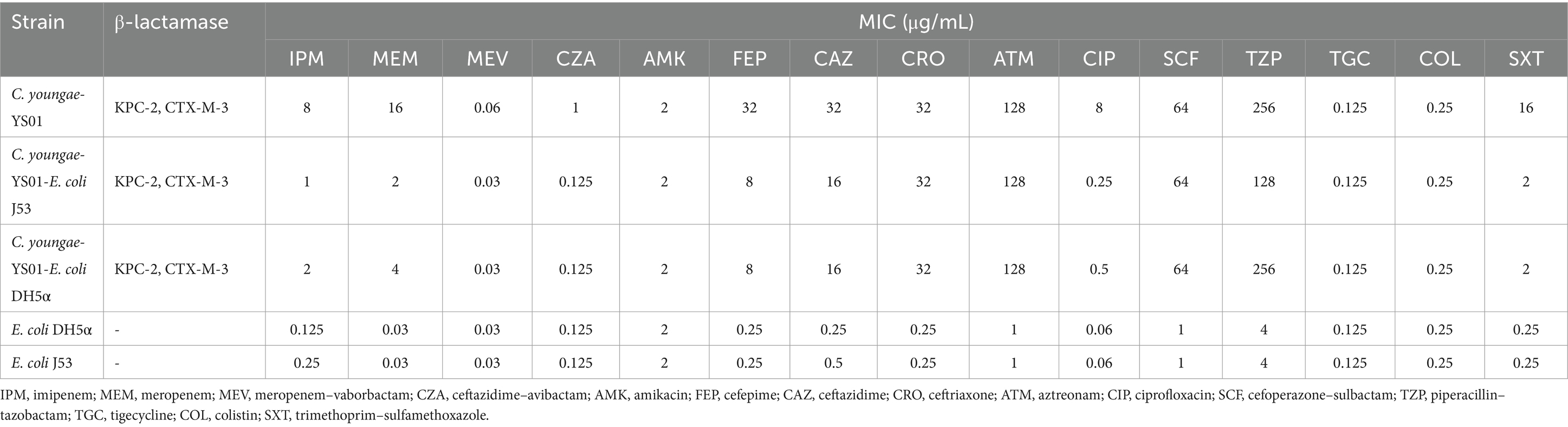
Table 1. Susceptibility of C. youngae clinical isolate, conjugant, and recipient to antimicrobial agents.
Conjugation and transformation experimentThe study also identified the presence of the plasmid-encoded blaKPC-2 gene in C. youngae, marking the first detection of this gene within this bacterial species. This finding raises concerns about the potential for horizontal gene transfer of resistance genes. PCR-based sequencing confirmed the presence of blaKPC-2 in C. youngae. In addition, the plasmid carrying blaKPC-2 was successfully transferred from the C. youngae strain to E. coli J53 and E. coli DH5α. Both conjugants, C. youngae-YS01-E. coli J53 and C. youngae-YS01-E. coli DH5α, exhibited resistance to piperacillin–tazobactam, cefoperazone–sulbactam, and aztreonam, with at least a 4-fold increase in MICs for imipenem and a 60-fold increase for meropenem. Notably, C. youngae-YS01-E. coli DH5α showed higher MIC values for carbapenem antibiotics than C. youngae-YS01-E. coli J53 (Table 1). The conjugate C. youngae-YS01-E. coli J53 was resistant to piperacillin–tazobactam and aztreonam but sensitive or with intermediate susceptibility to imipenem and meropenem. In contrast, the conjugate C. youngae-YS01-E. coli DH5α was resistant to piperacillin–tazobactam, cefoperazone–sulbactam, and aztreonam but showed intermediate susceptibility or resistance to imipenem and meropenem. The MICs of meropenem and imipenem in both conjugates and the transformants increased by at least 4- and 60-fold, and 16- and 120-fold, respectively, compared to the recipient strains E. coli J53 and E. coli DH5α (Table 1).
Sequence analysis, clone experiment, and functional study of blaCMY-190We conducted phenotypic and molecular characterization as well as a functional study of a new CFE subtype designated blaCMY-190, which is encoded by the chromosomal ampC gene and produced by clinical isolates of C. youngae. Sequencing of C. youngae-YS01 revealed an open reading frame (ORF) of 1,143 bp in length, encoding a putative protein of 381 amino acids. This protein showed a high identity (88.05%) with the plasmid-encoded enzyme CFE-1 (accession number NG_048757) when compared with the amino acid sequence of AmpC β-lactamase. The sequence of the cloned DNA fragment and the associated β-lactam drug resistance pattern identified a new CFE-1-like gene distinct from the E. coli plasmid genes for the transcriptional regulator AmpR and AmpC β-lactamase. The deduced amino acid sequence confirmed that the new gene encoding the β-lactamase CMY-190 (Sequence Information: OR896917) is a variant of CFE-1, characterized by 26 amino acid substitutions. In particular, blaCMY-190 confers resistance to third-generation cephalosporins, including ceftriaxone, cefotaxime, and ceftazidime. Compared to the recipient E. coli DH5α-PHSG398, the MICs of cefotaxime, ceftriaxone, and ceftazidime increased 4-fold, while the MICs of cefepime and cefoxitin increased 8- and 32-fold, respectively (Table 2). To assess the inducibility of blaCMY-190 expression, we measured β-lactamase activity in E. coli DH5α strains carrying plasmid pHSG398 using the spectrophotometric method with the chromogenic β-lactam (cephalosporin) nitro-cephalosporin as a substrate. After exposure to a β-lactam inducer (cefoxitin 10 μg/mL or cefotaxime 8 μg/mL), the β-lactamase activity in the bacterial cells was evaluated. The results showed limited induction of β-lactamase activity after exposure to cefotaxime, whereas cefoxitin did not induce further β-lactamase activity. In a parallel experiment, S. marcescens was used as a positive control. When exposed to cefotaxime (8 μg/mL), β-lactamase activity increased 4-fold. In contrast, E. coli DH5α transformants lacking the AmpR plasmid exhibited lower β-lactamase activity and MICs than those carrying AmpR, indicating that the expression of the blaCMY-190 gene requires a functional AmpR regulator.
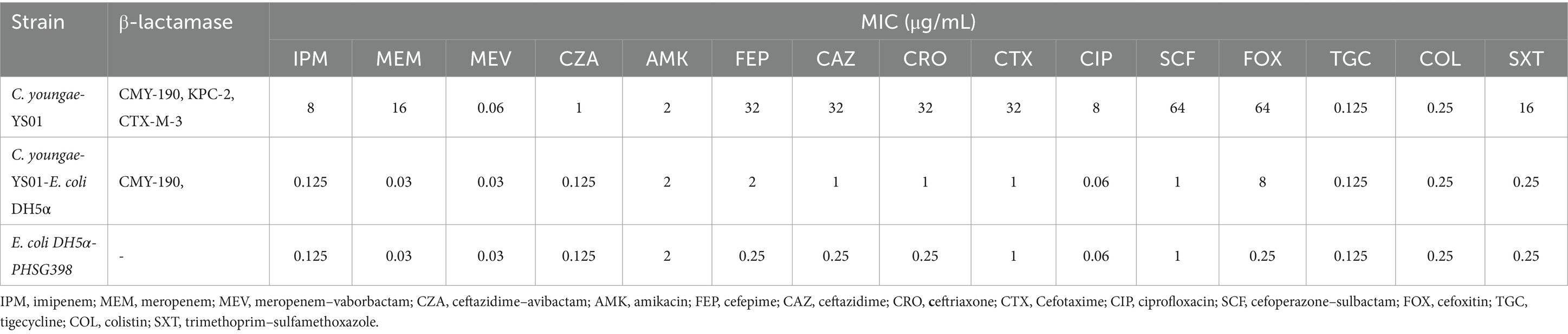
Table 2. In vitro susceptibility of C. youngae-YS01, C. youngae-YS01-E. coli DH5α clone strain, and the E. coli DH5α-PHSG398.
Analysis of the plasmid and genetic environment of the blaKPC-2 and blaCMY-190 genesWhole genome sequencing (WGS) has identified several resistance genes, including the β-lactamase genes blaCMY-190, blaKPC-2, and blaCTX-M-3; the aminoglycoside resistance gene aac (3)-II; and the fluoroquinolone resistance gene qnrS1. Together, these genes confer resistance to carbapenems, aminoglycosides, and quinolones. Restriction maps and nucleotide sequences were generated to further characterize these elements. Analysis of the blaCMY-190 sequence revealed the presence of the genes blc (encoding an outer membrane lipoprotein), RcsA (encoding a transcriptional regulatory protein), and epmB (L-lysine 2, 3-aminomutase) downstream of blaCMY-190. In addition, the ampR gene and the frdABCD operon of C. youngae were identified upstream of the ampC gene. The region surrounding the ampR–ampC genes in C. youngae contained both blc and frdABCD, suggesting a likely common chromosomal location. This genetic element appears to be associated with all known blaCMY-190-carrying structures, whether chromosomal or plasmid-based (Figure 1). The blaKPC-2 gene was found to be flanked by mobile elements related to Tn1721 and IS26, consistent with the predominant genetic structures carrying blaKPC-2 in the domestic environment (Figure 2). This genetic configuration has recently been identified in several Citrobacter species (Zhu et al., 2020; Qiao et al., 2023; Hu et al., 2019). Sequencing results from C. youngae revealed that the CFE gene is located on chromosome 4878723bbp within the ST195 sequence type. Other chromosomal resistance genes include blaCTX-M-3 and QnrB12. Comparative BLAST analysis indicated high similarity between this C. youngae chromosome sequence and a previously isolated strain from Korea (GenBank accession no. CP021963). A phylogenetic tree illustrating the related blaCMY variants is shown in Figure 3. The blaKPC gene was identified on a 64,927 bp IncN plasmid, which, based on BLAST analysis, shows high similarity to the plasmid sequence of an E. coli strain isolated from a hospital in Shanghai, China (79% coverage, 99.92% identity, GenBank accession no. CP028486) (Figure 4).
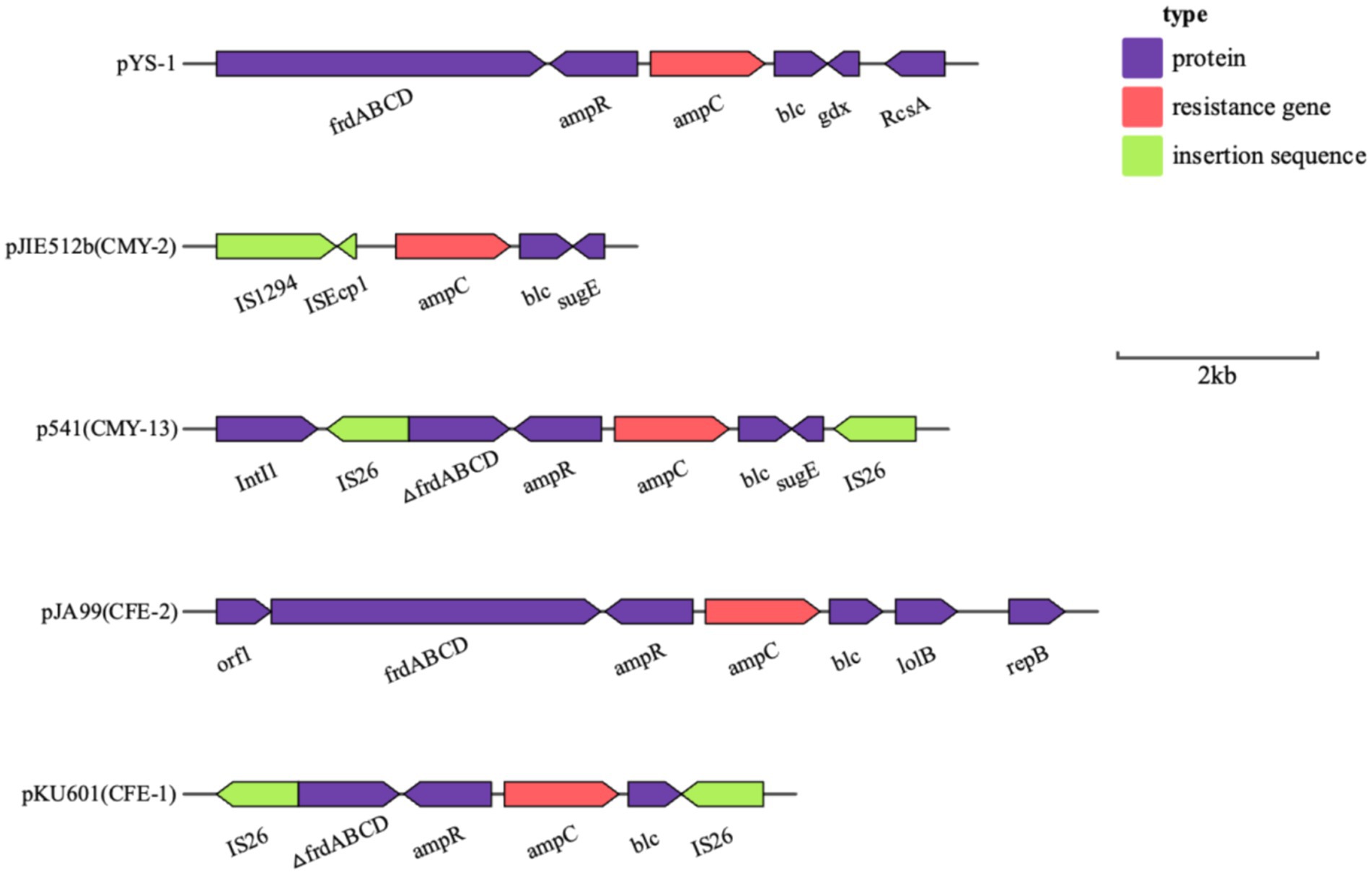
Figure 1. Schematic representation of the genetic background of the blaCMY-190 gene in C. youngae-YS01. Gene and intergenic region sizes are plotted to scale. The open reading frame is shown, with the direction of transcription indicated by thick arrows.
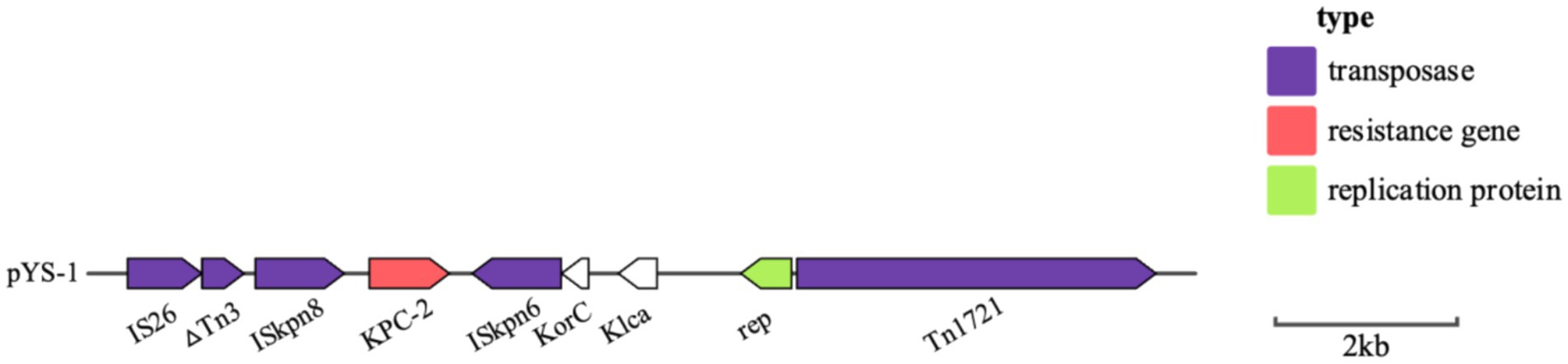
Figure 2. Schematic representation of the genetic background of the blaKPC gene in C. youngae-YS01. Gene and intergenic region sizes are plotted to scale. The open reading frame is shown, with the direction of transcription indicated by thick arrows.
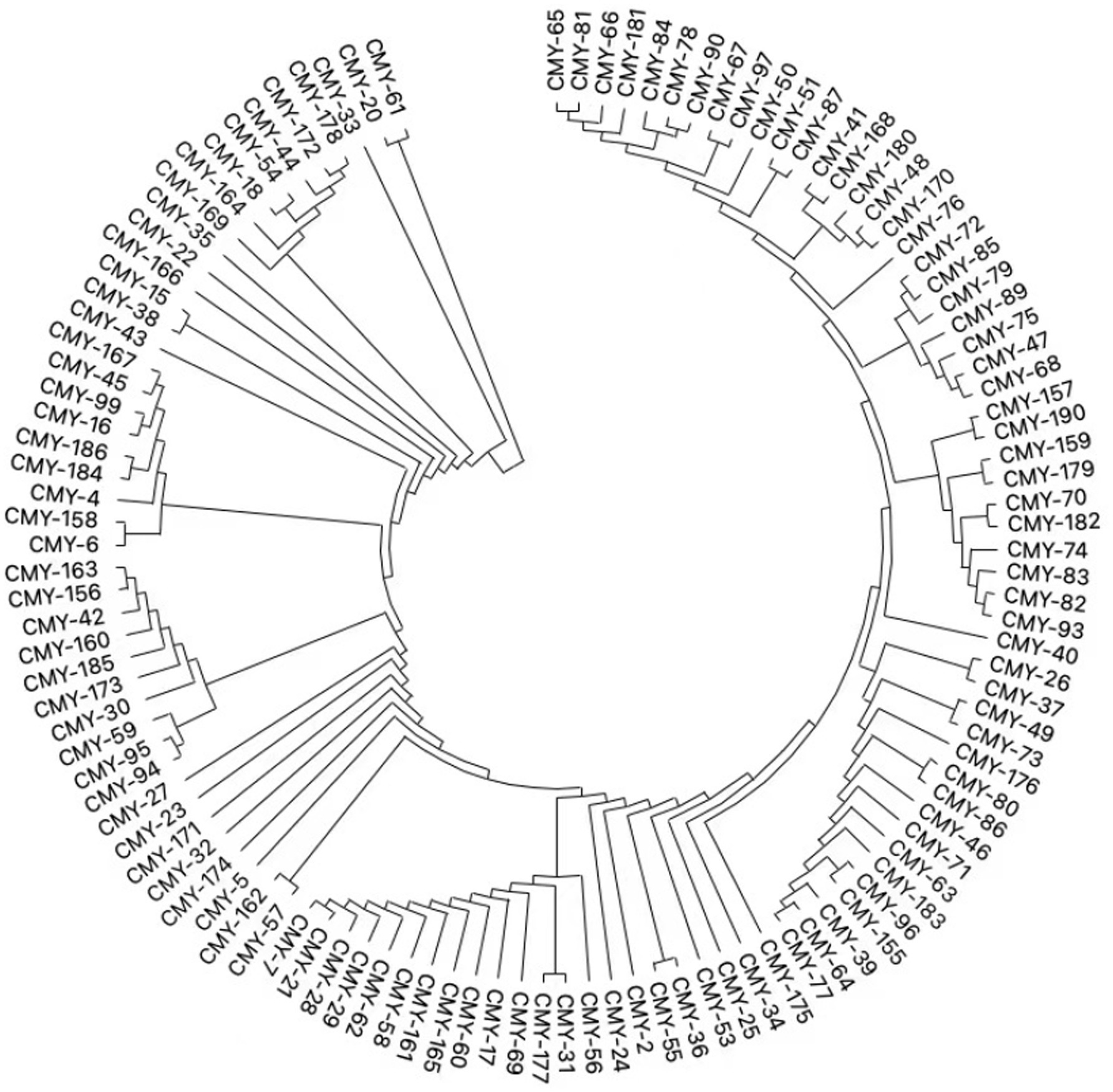
Figure 3. Phylogenetic tree of relative blaCMY variants.
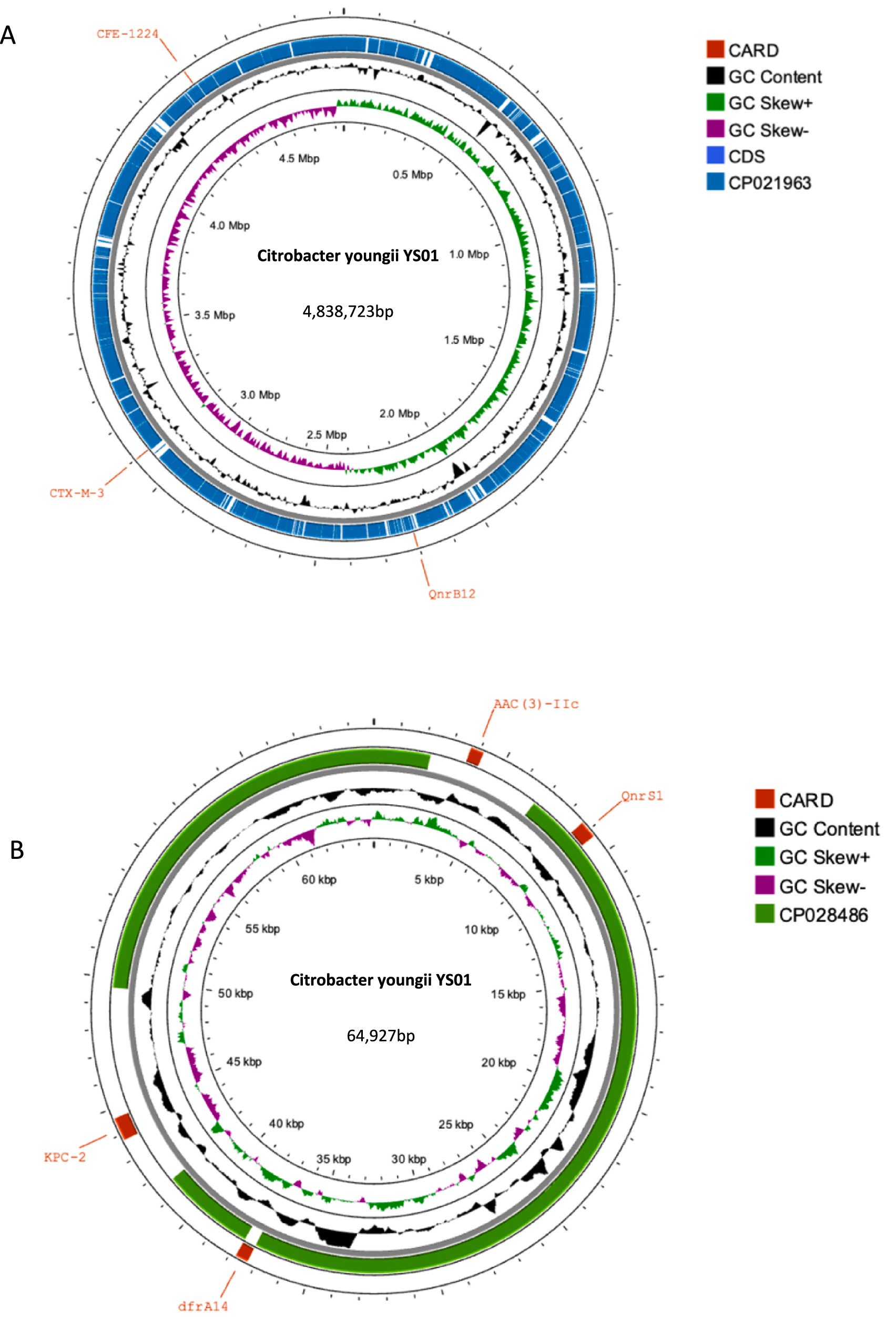
Figure 4. (A) Alignments of chromosomes. Comparison of the chromosomes C. youngae-YS01 and L6 (CP021963) using Proksee. (A) BLAST search for the sequence in GenBank showed that the sequence of C. youngae-YS01 was very similar (99.95% coverage and 100% identity) to that of L6 (4,945,156 bp, GenBank accession no. CP021963), a chromosome of C. youngae isolated from Korea. (B) Alignments of plasmids. Comparison of the plasmids pYS-1 and E41-1 (CP028486) using Proksee. A BLAST search for the sequence in GenBank showed that the sequence of C. youngae-YS01 was very similar (79% coverage and 99.92% identity) to that of E41-1 (52,864 bp, GenBank accession no. CP028486), a plasmid of E. coli isolated from China.
DiscussionAmpC-type β-lactamases are classified as class C enzymes, with different expression patterns depending on their genetic context. These include the inducible expression of chromosomal AmpC β-lactamase, stable de-expression with inducible expression, and plasmid-mediated AmpC β-lactamase. Among these, the blaCMY-2 gene is the most widely reported AmpC gene worldwide. blaCMY-2, initially identified in E. coli and Salmonella isolates, is often associated with transposons, facilitating its transmission across community and healthcare settings. The ability of plasmid-mediated blaCMY-2 to transfer between bacterial species has contributed to its global prevalence, with the incidence in Egypt increasing from 2.91% in 2019 to 4.09% in 2020 and in the United States increasing from 1.3% in 2016 to 3.42% in 2019 (Rodríguez-Guerrero et al., 2022). Typically, the blaCMY gene is associated with the ISEcp1 element, forming the ISEcp1 tnpA-blaCMY-2-blc-sugE arrangement, largely due to a strong promoter within ISEcp1 (Chiu et al., 2021). This genetic configuration, in combination with host factors, significantly influences ceftriaxone susceptibility. Under increasing selective pressure, blaCMY variants with hydrolytic activity against broad-spectrum cephalosporins have emerged, conferring resistance to agents such as cephalosporins, cefoxitin, and aztreonam and even reducing susceptibility to fourth-generation cephalosporins (e.g., cefepime). In addition, the blaCMY gene, in combination with altered membrane permeability, may contribute to imipenem resistance in K. pneumoniae. Of particular concern, blaCMY variants also reduce the susceptibility of Enterobacterales to avibactam. For example, Tyr150Ser and Asn346Ile substitutions in CMY-2 increase resistance to avibactam. In addition, blaCMY-172 and blaCMY-178 are associated with ceftazidime–avibactam resistance in Enterobacterales, with CMY-178 showing a higher level of resistance than CMY-172 (Merida-Vieyra et al., 2020; Zhou et al., 2023).
In this study, we identified a novel AmpC β-lactamase gene, blaCMY-190, in clinical isolates of C. youngae in China. This is the report of a chromosomally encoded AmpC β-lactamase gene, blaCMY-190, carrying the regulatory ampR gene derived from the C. youngae plasmid. Regulation of AmpC β-lactamase expression is closely linked to cell wall recycling and involves the ampR gene, which encodes a transcriptional regulator from the LysR family (Balasubramanian et al., 2014). Research suggests that AmpR binds to a 38-base pair sequence within the intergenic region between ampR and ampC genes. In the absence of β-lactam inducers, AmpR downregulates β-lactamase synthesis by 2.5-fold, whereas in their presence, expression can be increased by 10- to 200-fold (Nakano et al., 2004). Our results describe the sequence structure surrounding the ampR–ampC regions, including blaCMY-190. Unlike most plasmid-encoded AmpC β-lactamase genes, such as blaCMY-2, blaCMY-4, and blaLAT-1, which lack the ampR gene (Biez et al., 2023), Citrobacter spp. and Enterobacter spp. have complete ampR and ampC genes. In addition, these species have a downstream fumarate operon (frdABCD) adjacent to the ampR gene and an outer membrane lipoprotein (blc) located downstream of the ampC gene (Nakano et al., 2004). Analysis of the blaCMY-190 gene reveals a close relationship with the chromosomally encoded AmpC β-lactamase gene in C. freundii. The amino acid sequence of blaCMY-190 shares 88.05% identity with that of CFE-1 from C. freundii JA99, which was isolated from clinical samples in China (Chen et al., 2018). In addition, the amino acid sequence of AmpR shares 99.0% identity with the AmpR sequence of C. freundii JA99. This high degree of similarity strongly suggests that the blaCMY-190 gene is derived from the plasmid ampC gene of C. freundii JA99. This hypothesis is further supported by the presence of the ampR and ampC genes, as well as the frdABCD operon and the blc gene, which surround the ampR and ampC genes in C. freundii species.
Based on previous epidemiological studies, KPC-type carbapenemases are more common in CRE, while their identification in Citrobacter spp. is relatively rare. In Citrobacter spp., OXA-48-like carbapenemases are the predominant type, followed by NDM and VIM variants (Biez et al., 2023). Notably, while KPC-type carbapenemases are highly prevalent in CRE overall, they are less frequently detected in Citrobacter spp. Our study identified a strain of C. youngae carrying the blaKPC-2 gene, making it the first detection of this gene in C. youngae. As a clinically important opportunistic pathogen, C. youngae poses a significant public health challenge due to its carbapenem resistance. This case illustrates how C. youngae can act as a silent reservoir for critical resistance genes. The emergence of the blaKPC-2 gene in C. youngae deserves widespread attention due to its potential impact on clinical infection management. Genetic elements play a crucial role in the spread of resistance genes. In many countries and regions, including Europe (Naas et al., 2008), the United States (Chen et al., 2013), and Brazil (Pereira et al., 2013), the blaKPC gene is mainly located on mobile elements such as Tn4401 and Tn3-Tn4401. These composite transposons belong to the Tn3 family and contain blaKPC-2, transposase, resolvase, and insertion sequences ISKpn6 and ISKpn7, which allow high transposition frequencies for blaKPC-2. In Asia, blaKPC-2 is mainly found on different variants of Tn1721 and IS26 (Fu et al., 2019). Studies indicate that the KPC-2-carrying plasmid in C. youngae is closely related to plasmids isolated from K. pneumoniae in Suzhou. These plasmids share a composite transposition element consisting of Tn1721 and IS26. Homologous recombination mediated by IS26 is likely to lead to the rearrangement and widespread dissemination of these resistance regions, suggesting a potential transfer of blaKPC-2 from K. pneumoniae to C. youngae. The transferability of the plasmid was further confirmed in conjugation experiments. Compared to other Enterobacterales, C. youngae poses a significant risk for the transfer of antimicrobial resistance, which becomes an additional concern factor in clinical settings (Nüesch-Inderbinen et al., 2013; Hawkey et al., 2001). Therefore, early identification of resistance mechanisms and resistance gene transfer pathways is essential to improve clinical anti-infection strategies and to control the potential widespread of Citrobacter spp.
ConclusionThis study reports the first identification of a C. youngae strain carrying both the chromosomally encoded AmpC β-lactamase gene blaCMY-190 and the plasmid-encoded carbapenemase blaKPC-2, detected in a patient’s ascites sample. The patient presented with multiple complications, including hepatic malignancy, decompensated cirrhosis due to hepatitis B, ascites, acute renal failure, uremia, and portal vein thrombosis, and had been treated with a range of antibiotics. The newly identified cAmpC β-lactamase (CMY-190) showed activity against oxyimino-cephalosporins. Our findings highlight the need for increased surveillance and prevention efforts targeting CMY-190 to reduce the spread of multidrug-resistant Gram-negative bacilli in healthcare settings. The detection of blaKPC-2 in C. youngae, possibly by horizontal transfer from K. pneumoniae, is also a first and highlights that C. youngae and other less-reported Enterobacterales may serve as unrecognized reservoirs for carbapenemase genes.
Data availability statementThe datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: Sequence Information: OR896917.
Ethics statementAll experiments were conducted in strict accordance with relevant laws, regulations, and institutional guidelines. No human or animal subjects were involved in this research. The collection and use of bacterial isolates were carried out according to standard microbiological protocols and approved biosafety procedures. Our study was approved by the Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University (2022080).
Author contributionsZL: Data curation, Funding acquisition, Writing – original draft, Writing – review & editing. SS: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. XZ: Investigation, Writing – review & editing. JL: Formal analysis, Writing – review & editing. CT: Methodology, Writing – review & editing. SW: Resources, Writing – review & editing. KL: Conceptualization, Writing – review & editing. JY: Formal analysis, Writing – review & editing. YZ: Funding acquisition, Writing – review & editing. YGu: Investigation, Methodology, Project administration, Writing – review & editing. YGe: Methodology, Project administration, Writing – review & editing. FH: Supervision, Validation, Visualization, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (grant numbers 82172311 and 82302574), the Key Research and Development Plan of Shaanxi Province (2024SF-YBXM-160), the China Antimicrobial Surveillance Network (Independent Medical Grants from Pfizer, 2020QD049), and the Key Research and Development Plan of Shaanxi Province (2020SF-173).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes ReferencesAlikhan, N. F., Petty, N. K., Ben Zakour, N. L., and Beatson, S. A. (2011). BLAST ring image generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402. doi: 10.1186/1471-2164-12-402
PubMed Abstract | Crossref Full Text | Google Scholar
Al-Mulla, N., Elshafie, S., Janahi, M., Al-Nasser, A., Chandra, P., and Taj-Aldeen, S. (2014). Bacterial bloodstream infections and antimicrobial susceptibility pattern in pediatric hematology/oncology patients after anticancer chemotherapy. Infect Drug Resist 7, 289–299. doi: 10.2147/IDR.S70486
PubMed Abstract | Crossref Full Text | Google Scholar
Arana, D. M., Ortega, A., González-Barberá, E., Lara, N., Bautista, V., Gómez-Ruíz, D., et al. (2017). Carbapenem-resistant Citrobacter spp. isolated in Spain from 2013 to 2015 produced a variety of carbapenemases including VIM-1, OXA-48, KPC-2, NDM-1 and VIM-2. J. Antimicrob. Chemother. 72, 3283–3287. doi: 10.1093/jac/dkx325
PubMed Abstract | Crossref Full Text | Google Scholar
Balasubramanian, D., Kumari, H., Jaric, M., Fernandez, M., Turner, K. H., Dove, S. L., et al. (2014). Deep sequencing analyses expands the Pseudomonas aeruginosa AmpR regulon to include small RNA-mediated regulation of iron acquisition, heat shock and oxidative stress response. Nucleic Acids Res. 42, 979–998. doi: 10.1093/nar/gkt942
PubMed Abstract | Crossref Full Text | Google Scholar
Biez, L., Bonnin, R. A., Emeraud, C., Birer, A., Jousset, A., Naas, T., et al. (2023). Nationwide molecular epidemiology of carbapenemase-producing Citrobacter spp. in France in 2019 and 2020. mSphere 8:e0036623. doi: 10.1128/msphere.00366-23
PubMed Abstract | Crossref Full Text | Google Scholar
Bitar, I., Caltagirone, M., Villa, L., Mattioni Marchetti, V., Nucleo, E., Sarti, M., et al. (2019). Interplay among IncA and Bla(KPC)-carrying plasmids in Citrobacter freundii. Antimicrob. Agents Chemother. 63:2609. doi: 10.1128/AAC.02609-18
PubMed Abstract | Crossref Full Text | Google Scholar
Carattoli, A., Bertini, A., Villa, L., Falbo, V., Hopkins, K. L., and Threlfall, E. J. (2005). Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63, 219–228. doi: 10.1016/j.mimet.2005.03.018
PubMed Abstract | Crossref Full Text | Google Scholar
Chen, L., Chavda, K. D., Melano, R. G., Jacobs, M. R., Levi, M. H., Bonomo, R. A., et al. (2013). Complete sequence of a Bla(KPC-2)-harboring IncFII(K1) plasmid from a Klebsiella pneumoniae sequence type 258 strain. Antimicrob. Agents Chemother. 57, 1542–1545. doi: 10.1128/AAC.02332-12
PubMed Abstract | Crossref Full Text | Google Scholar
Chen, K. J., Chen, T. H., and Sue, Y. M. (2013). Citrobacter youngae and Pantoea agglomerans peritonitis in a peritoneal dialysis patient. Perit. Dial. Int. 33, 336–337. doi: 10.3747/pdi.2012.00151
PubMed Abstract | Crossref Full Text | Google Scholar
Chen, C. M., Huang, M., Wu, H. J., Guo, M. K., and Wu, L. T. (2018). Identification of CFE-2, a new plasmid-encoded AmpC β-lactamase from a clinical isolate of Citrobacter freundii. Int. J. Antimicrob. Agents 52, 421–424. doi: 10.1016/j.ijantimicag.2018.06.013
PubMed Abstract | Crossref Full Text | Google Scholar
Chiu, C. H., Lee, J. J., Wang, M. H., and Chu, C. (2021). Genetic analysis and plasmid-mediated blaCMY-2 in salmonella and Shigella and the ceftriaxone susceptibility regulated by the ISEcp-1tnpA-blaCMY-2-blc-sugE. J. Microbiol. Immunol. Infect. 54, 649–657. doi: 10.1016/j.jmii.2020.01.008
PubMed Abstract | Crossref Full Text | Google Scholar
Clinical and Laboratory Standards Institute (2023). M100: Clinical and laboratory standards institute performance standards for antimicrobial susceptibility testing. 33th Edn. Wayne, PA: Clinical and Laboratory Standards Institute.
FDA (2021). Tigecycline-injection products. Silver Spring, MA: FDA.
Fu, P., Tang, Y., Li, G., Yu, L., Wang, Y., and Jiang, X. (2019). Pandemic spread of Bla((KPC-2)) among Klebsiella pneumoniae ST11 in China is associated with horizontal transfer mediated by IncFII-like plasmids. Int. J. Antimicrob. Agents 54, 117–124. doi: 10.1016/j.ijantimicag.2019.03.014
PubMed Abstract | Crossref Full Text | Google Scholar
Fupin, H. U., Yan, G., Demei, Z., Fu, Z., Xiaofei, J., and Yingchun, X.. (2022). CHINET surveillance of antimicrobial resistance among the bacterial isolates in 2021. Chin. J. Infect. Chemother. 22, 521–530. doi: 10.16718/j.1009-7708.2022.05.001
Crossref Full Text | Google Scholar
Grundmann, H., Glasner, C., Albiger, B., Aanensen, D. M., Tomlinson, C. T., Andrasević, A. T., et al. (2017). Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect. Dis. 17, 153–163. doi: 10.1016/S1473-3099(16)30257-2
PubMed Abstract | Crossref Full Text | Google Scholar
Hawkey, P. M., Xiong, J., Ye, H., Li, H., and M’Zali, F. H. (2001). Occurrence of a new metallo-beta-lactamase IMP-4 carried on a conjugative plasmid in Citrobacter youngae from the People’s Republic of China. FEMS Microbiol. Lett. 194, 53–57 doi: 10.1111/j.1574-6968.2001.tb09445.x
Crossref Full Text | Google Scholar
Hu, X., Yu, X., Shang, Y., Xu, H., Guo, L., Liang, Y., et al. (2019). Emergence and characterization of a novel IncP-6 plasmid Harboring Bla (KPC-2) and qnrS2 genes in Aeromonas taiwanensis isolates. Front. Microbiol. 10:2132. doi: 10.3389/fmicb.2019.02132
PubMed Abstract | Crossref Full Text | Google Scholar
Lee, R., Choi, S. M., Jo, S. J., Lee, J., Cho, S. Y., Kim, S. H., et al. (2019). Clinical characteristics and antimicrobial susceptibility trends in Citrobacter Bacteremia: an 11-year single-center experience. Infect Chemother 51, 1–9. doi: 10.3947/ic.2019.51.1.1
PubMed Abstract | Crossref Full Text | Google Scholar
McAteer, J., Lee, J. H., Cosgrove, S. E., Dzintars, K., Fiawoo, S., Heil, E. L., et al. (2023). Defining the optimal duration of therapy for hospitalized patients with complicated urinary tract infections and associated Bacteremia. Clin. Infect. Dis. 76, 1604–1612. doi: 10.1093/cid/ciad009
PubMed Abstract | Crossref Full Text | Google Scholar
Merida-Vieyra, J., de, A., Calderón-Castañeda, Y., and Aquino-Andrade, A. (2020). Detection of CMY-type beta-lactamases in Escherichia coli isolates from paediatric patients in a tertiary care hospital in Mexico. Antimicrob. Resist. Infect. Control 9:168. doi: 10.1186/s13756-020-00840-4
PubMed Abstract | Crossref Full Text | Google Scholar
Naas, T., Cuzon, G., Villegas, M. V., Lartigue, M. F́́., Quinn, J. P., and Nordmann, P. (2008). Genetic structures at the origin of acquisition of the beta-lactamase Bla KPC gene. Antimicrob. Agents Chemother. 52, 1257–1263. doi: 10.1128/AAC.01451-07
PubMed Abstract | Crossref Full Text | Google Scholar
Nakano, R., Okamoto, R., Nakano, Y., Kaneko, K., Okitsu, N., Hosaka, Y., et al. (2004). CFE-1, a novel plasmid-encoded AmpC beta-lact
留言 (0)