Idiopathic inflammatory myopathies (IIM) are a heterogenous group of autoimmune diseases including four main groups: dermatomyositis (DM), anti-synthetase syndrome (ASyS), immune-mediated necrotizing myopathies (IMNM), and inclusion body myositis (IBM) (1, 2). IIM may manifest as a muscle-specific autoimmune disorder (IBM and IMNM) or as a systemic condition primarily affecting the skin, joints, and/or lungs (DM and ASyS).
The complexity of disease activity assessment in IIM arises from its heterogeneous nature. A core set of disease activity measures, aimed at evaluating improvement through a total improvement score calculated from two time points, has been proposed (3). However, reliable biomarkers are still required to assess disease activity at a single time point. While creatine kinase (CK) levels, one of the core set measures, are well correlated with disease activity in IMNM (4), they may lack sensitivity in patients with ASyS or DM (5).
Interferons (IFNs) play a significant role in the pathophysiology of IIM (6–8). There are three main types of IFN. Type I IFN are mainly represented by 13 subtypes of IFN-α and IFN-β. Type II IFN is only represented by IFN-γ and signal through a distinct receptor. Due to the low circulating levels of these cytokines, the IFN signature—an overexpression of IFN-stimulated genes —is typically used as an indirect measure, rather than direct IFN quantification. This signature has been identified in blood (9), muscle (7) and skin (10) of DM patients, and is associated with disease activity (8, 11). Type II IFN is related to CD8+ T cells and has been involved in ASyS and IBM (12, 13). There appears to be a type II IFN signature in IMNM muscle biopsies (14), but the data are still uncertain.
Despite the identification of IFN signatures in various tissues, results have been inconsistent, particularly with respect to the different subtypes of myositis (15, 16). No studies have evaluated the precise quantification of various cytokines along the IFN pathway in the different myositis subgroups. Recent advances in ultrasensitive technology have enabled the detection of very low concentrations of proteins, such as IFN-α and IFN-γ, at femtomolar levels (17), making it possible to measure these cytokines with greater accuracy.
The aim of this study was to assess the potential of type I and II IFN, using an ultrasensitive digital ELISA technology, as a blood biomarker of activity for IIM.
2 Patients and methods2.1 Patients and seraPatients were prospectively enrolled between 2011 and 2018 for the first cohort, in a tertiary center of IIM (Pitié-Salpêtrière Hospital, Paris, France).
A validation cohort was established for the dermatomyositis and anti-synthetase syndrome subgroups in an independent cohort of patients sampled between 2018 and 2023. They fulfilled the American college of rheumatology/European league against rheumatism (ACR/EULAR) classification criteria for myositis (18). Patients were classified into four categories: IBM [Lloyd’s criteria (19)], IMNM [ENMC 2017 (20)], ASyS in presence of anti-synthetase antibody and according to ENMC criteria (21) and DM (1, 22)
Sera were collected at diagnosis and/or during the follow-up and were rapidly (<3h) frozen after one centrifugation. All the sera were thawed only once to avoid potential freeze/thaw effects. Patients who had increased dose of corticosteroids (>0.5mg/kg and/or pulses) the week before the sampling were excluded as it may rapidly abrogate the IFN levels (23). Moreover, patients with active infectious diseases (e.g. flue or viral B hepatitis) were excluded. Thirty-three age-matched healthy donors (HD) from a French blood bank were used as negative controls.
2.2 Disease activity assessmentUsing International Myositis Assessment and Clinical Studies Group core set measures the following assessments were performed: Manual Muscle Testing 8 (MMT8), Creatine Kinase (CK) level for the muscle domain and for extra-muscular domains, we used Myositis Disease Activity Assessment Tool (MDAAT) scoring [0-10] the extramuscular manifestation (3). Finally, to assess the global disease activity we used the Physician Global Activity (PGA) (3). Disease activity was assessed at the time of blood collection and the result was represented in a numeric scale (from 0 to 10; 0 corresponding to the remission without treatment and 10 the maximum disease activity).
2.3 IFN serum measurementIFN-α and IFN-γ serum concentrations were measured using the high sensitivity Single Molecule Array (Simoa®) technology (Digital ELISA technology) (Quanterix SimoaTM IFN-α Reagent Kit, Lexington, MA, USA and Quanterix SimoaTM IFN-γ Reagent Kit, Lexington, MA, USA) according to the manufacturer protocols and as previously reported (9, 11, 17–19, 24, 25). Briefly, we collected 5 ml of blood from each patient and 400µl of serum per well was required for each SIMOA analysis.
The limit of detection (LOD) was 0.0035 pg/ml for IFN-α and 0.026 pg/ml for IFN-γ. The positivity threshold was defined as the mean plus three times the standard deviation of the 33 healthy donors (HD) and was 0.22 pg/ml for IFN-α and 1.97 pg/ml for IFN-γ.
For IFN-β serum quantification, Elisa test was used (PBL Assay Science, Piscataway, NJ, USA). The LOD was calculated by the mean value of the blank plus two times the standard deviation (positivity at 95% confidence) calculated on logarithmic values and was 1.24 pg/ml and the positivity threshold was defined by the mean plus three times the standard deviation of the HD and was 2.50 pg/ml.
2.4 Myositis specific antibody detectionThe screening for Myositis-Specific Antibody (MSA) was performed with different line blot commercial assays as previously reported (1) using Euroimmun® or Dteck® immunoassays including anti-melanoma differentiation-associated protein 5 (anti-MDA5), -transcription intermediary factor-γ (anti-Tif1γ), -complex nucleosome remodeling histone deacetylase (anti-Mi2), -nuclear matrix protein 2 (anti-NXP2) and -SUMO-activating enzyme subunit 1 (anti-SAE1) for DM; -histidyl-ARNt synthetase (anti-Jo1), -threonine-ARNt synthetase (anti-PL7), -alanine-ARNt synthetase and -glycine-ARNt synthetase (anti-EJ) for ASyS; -signal recognition particle (anti-SRP) and -3-hydroxy-3-methylglutaryl-coenzyme A reductase (anti-HMGCR) for IMNM.
2.5 Statistical analysisQuantitative variables were expressed as median with inter-quartile range, and numbers with proportions for categorical variables. Multiple comparisons were performed using Kruskal-Wallis test then Dunn’s post-hoc test for quantitative data. To analyse the correlation between IFN and disease activity assessed by the PGA, we performed Spearman’s rank correlation tests using Graphpad Prism 10®.
Positive threshold to discriminate active from inactive patient was assessed by ROC curve analysis. We used the Youden indice to minimise both the number of false-positive and false-negative results. CK and IFN values were transformed through a base-10 logarithm for analysis.
After verifying the absence of multicollinearity, we included IFN-α, IFN-γ and CK levels in binary multivariate logistic regression to determine the association with disease activity (binary outcome using PGA>5 to define active patients). P<0.05 was considered statistically significant.
2.6 EthicalWritten informed consent from each study patient and approval by local Ethics Committee (CPP Ile De France VI (2013-12-19), CCTIRS (N°14.323) and CNIL (AR158656)) were obtained.
3 Results3.1 Patients’ characteristicsOne hundred fifty-two patients were included in the first cohort (DM, n=50; ASyS, n=46; IMNM, n=32 and IBM, n=24) compared with 33 healthy donors. Main patients’ characteristics are shown in Table 1.

Table 1. Patients characteristics in the first cohort at time of blood sampling.
As expected, IBM patients were older and displayed a lower MMT8 score compared to DM and AsyS. MSA were detected in 70.6% of DM patients in the cohort 1 (anti-Mi2, n=10; -Tif1γ, n=12; -NXP2, n=7; -MDA5, n=5 and -SAE, n=2) AsyS were all positive (anti-Jo1, n=38; -PL7, n=4; -PL12, n=3 and –EJ, n=1), and all IMNM patients were seropositive (anti-SRP, n=13, -HMGCR, n=19). The disease duration was 301 [46-1411) days in DM, 857 [59-1935] in ASyS, 820 [196,8-2275] in IMNM and 1328 [646-2246] in IBM.
No difference was observed in the therapeutic profile, including the use of corticosteroids and immunosuppressors, between IMNM, DM, and AsyS while IBM patients did not receive any treatment.
3.2 Increased levels of type I and II IFNs depend on the myositis subgroupsSerum IFN-α level was significantly higher in DM (0.07 [0.03-0.23] pg/ml) and ASyS (0.07 [0.02-0.16] pg/ml) compared to HD (0.02 [0.01-0.05] pg/ml; p<0.005 and p<0.05 respectively) whereas it was not significantly different in IMNM (0.03 [0.01-0.09] pg/ml) or IBM (0.02 [0.02-0.03] pg/ml) compared to HD (Figure 1A).

Figure 1. (A) Interferon alpha. (B) Interferon Beta. (C) Interferon gamma. IFN levels in IIM. DM, Dermatomyositis; ASyS, Anti-synthetase syndrome; IMNM, Immune-mediated necrotizing myopathies; IBM, Inclusion body myositis; HD, healthy donors - - mean ± 3 standard deviation or positivity threshold, *: p<0.05, ** p<0.005, ***: p<0.0005, **** p<0.00005.
Only DM patients had significantly higher IFN-β level (1.24 [1.24-6.31] pg/ml) compared to HD (1.24 [1.24-1.24] pg/ml, p<0.005) (Figure 1B).
IFN-γ level was significantly increased in all IIM subgroups (ASyS (1.05 [0.47-2.46] pg/ml), DM (0.90 [0.55-2.09] pg/ml), IMNM (0.96 [0.42-1.29] pg/ml) and IBM (0.93 [0.42-2.09] pg/ml)) compared with HD (0.46 [0.29-0.59] pg/ml), p<0.05) (Figure 1C).
3.3 IFN levels and disease activityCorrelation between IFNs levels and disease activity showed that PGA was strongly correlated with type-I IFNs, IFN-α (r=0.76 [0.60-0.86], p<0.001) and IFN-β (r=0.58 [0.35-0.74], p<0.01) in DM. A weak correlation with IFN-γ (r=0.36 [0.05-0.56], p=0.02) was observed. When we look at the correlations of the different IFN types with the core set measures assessing disease activity in DM, IFN-α correlates more with extra muscular domain (r=0.62 [0,47-0,73]) than muscle domain (CK: r= 0.29 [0,09-0,48] p<0.05 and MMT8: r= -0,31 [-0.48- -0.11] p<0.05). IFN-β correlates more with muscle domain (CK: r=0.45 [0.26-0.6] p<0.05 and MMT8: r=-0.34 [-0.51- -0.14] p<0.05) than extra muscular assessment (MDAAT: r=0.32 [0.12-0.50] p<0.05).
ASyS also demonstrated that PGA correlated with IFN-α (r=0.55 [0.34-0.76], p<0.001) and IFN-γ levels (r=0.46 [0.15-0.66], p=0.003). Of note, no ASyS patient presenting an active disease had increased IFN-β level. When we look at the correlations of the different IFN types with the core set measures, IFN-α correlates with extramuscular domain (r=0.34 [0,11-0,54]), CK (r= 0.45 [0,22-0,63] p<0.05 and MMT8: r= -0,27 [-0.49- -0.02] p<0.05). In ASyS patients, IFN-γ correlates more with muscle domain (CK: r=0.39 [0.08-0.63] p<0.05 and MMT8: r=-0.46 [-0.67- -0.17] p<0.05) than extra muscular assessment (r=0.31 [0.02-0.56] p<0.05).
In IMNM, only IFN-γ level was significantly correlated with disease activity (r=0.48 [0.14-0.71], p=0.006) whereas IFN-α (r=0.23 [-0.14-0.55], p=0.2) and IFN-β (r=-0.07 [-0.43-0.31], p=0.7) were not. IFN-γ correlates with CK levels (r=0.39 [0.08-0.63] p<0.05) but not with MMT8: r=-0.03 [-0.38- 0.33] p=0.87). Of note, correlation between CK levels and disease activity was very high (r=0.87 [0.73-0.94], p<0.001) for IMNM patients.
In IBM, IFN-γ correlates with CK (r=0.69 [0.35- 0.87] p<0.05) (Figure 2D) but not with MMT8.
Multivariate analysis including IFN-α, and IFN-γ showed that only IFN-α was associated with active disease in DM patients (OR=9.5 [3.1-45.9], p<0.001). Concerning ASyS patients, only IFN-α was statistically associated with disease activity (OR=5 [1.9-17.9], p=0.004), and there was a trend for IFN-γ (p=0.08). No IFN subtype was associated with disease activity in the IMNM subgroup.
3.4 Validation cohortGiven the correlation between type 1 IFN and disease activity in DM and ASyS, and that only IFN-γ correlate with IMNM disease activity whereas creatine kinase is a reliable biomarker in this condition, we focus on IFN type 1 in DM and ASyS. Thus, we build a second independent cohort to validate our observations in DM and ASyS.
Seventy patients were included (DM n=49 and ASyS n=21) in the validation cohort. Main patients’ characteristics are shown in Table 2. MSA were detected in 84% in the cohort 2 (anti-Mi2, n=6; -Tif1γ, n=12; -NXP2, n=7; -MDA5, n=12 and -SAE, n=2). ASyS were all but one antibody positive (anti-Jo1, n=12; -PL7, n=3; -PL12, n=2 -OJ n=1 and –EJ, n=2) Of note, in both cohort, 54 patients were naïve from treatment, including 28 DM patients, 21 ASyS patients and 6 IMNM patients.
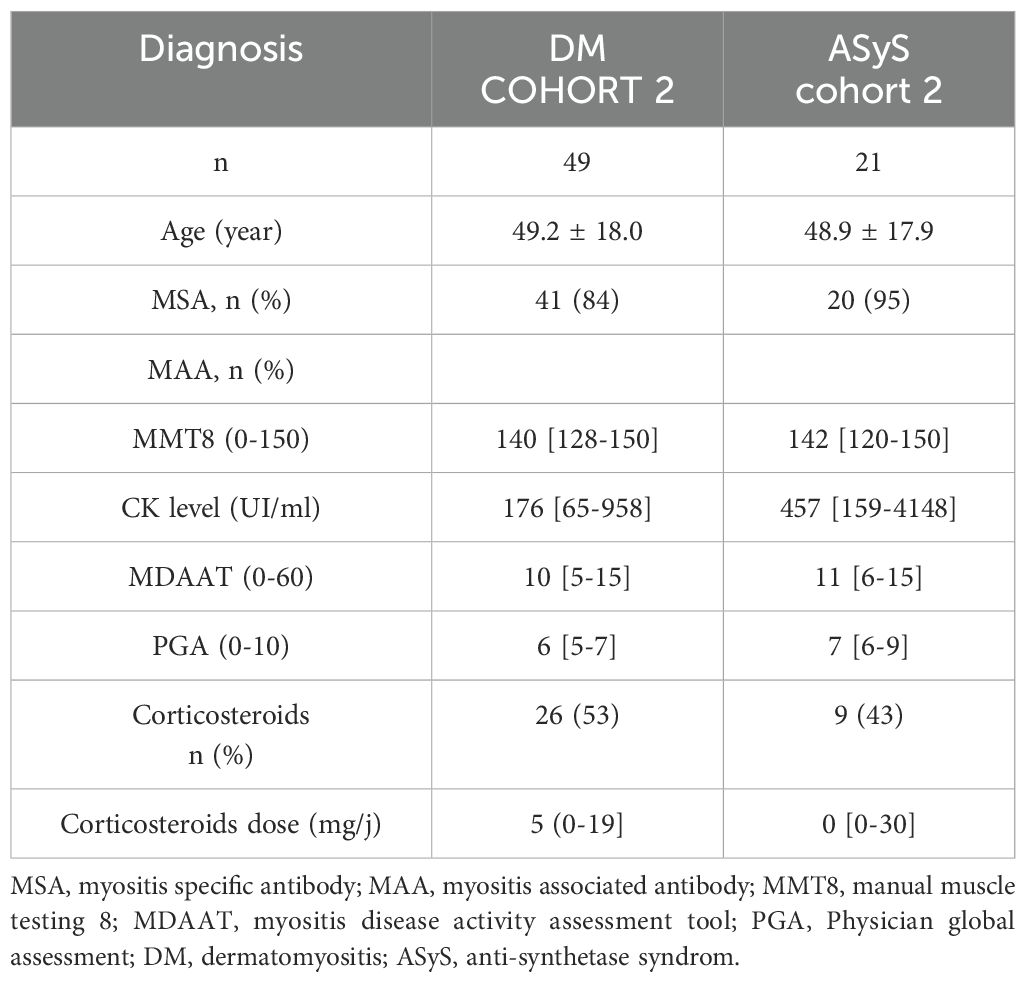
Table 2. Patients characteristics in the second cohort at time of blood sampling.
Correlation between IFN-α levels and disease activity in DM showed a good correlation (r=0.68 [0.49-0.81] p<0.05. Correlation with IFN-β was weak (r= 0.39 [0.11-0.61] p<0.05).
In ASyS, there was only a trend for the correlation between IFN-α and disease activity (r=0.37 [0.12-0.71] p=0.11).
When we pooled the two cohorts, DM patient (n=99) harboured a strong correlation between disease activity and type-I IFN, (IFN-α (r=0.70 [0.58-0.79], p<0.001) (Figure 2A) and IFN-β (r=0.49 [0.31-0.63], p<0.01) (Figure 2B)). ASyS also demonstrated that disease activity correlated with IFN-α in both cohort (n=67) (r=0.46 [0.23-0.63], p<0.001) (Figure 2C).
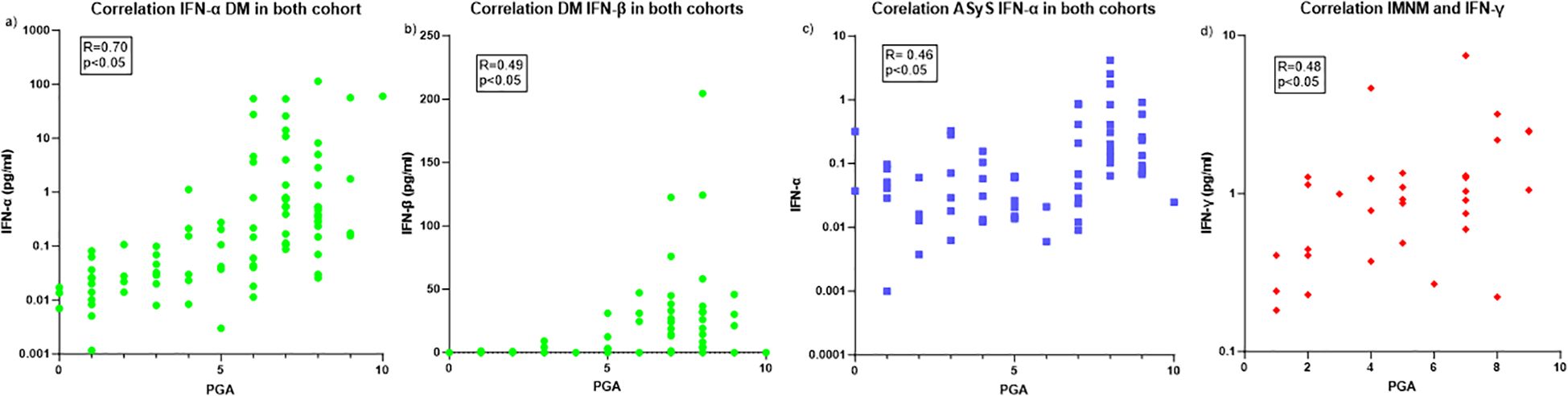
Figure 2. Correlation between IFNs and disease activity. (A) Correlation between IFN-α and DM disease activity. (B) Correlation between IFN-β and DM disease activity. (C) Correlation between IFN-α and ASyS disease activity. (D) Correlation between IFN-γ and IMNM disease activity. DM, Dermatomyositis; ASyS, Anti-synthetase syndrom; PGA, physician global assessment.
3.5 Sensitivity and specificity of IFNs to discriminate active and inactive DM and ASyS patients in both cohortNext, we aimed to define the threshold level of IFN corresponding to active disease if there were a correlation between IFN and disease activity.
Active DM patients had higher level of IFN-α (0.49 [0.15-3.7] pg/ml) compared to non-active DM patients (0.03 [0.01-0.07] pg/ml, p<0.001) (Supplementary Figure 1A). ROC analysis showed an area under the curve (AUC) at 0.90 (IC95 0.84-0.96; p<0.001). For a threshold of 0.11 pg/ml, the sensitivity was 88% and the specificity 80% to discriminate active from non-active DM patients.
Active DM patients had higher level of IFN-β (4.58 [0.0-30.7]) compared to non-active DM patients (0 [0-0] pg/ml, p=0.0001) with an AUC at 0.71 (IC95 0.61-0.81; p<0.05) because of a lot of false negative. Of note, IFN-γ levels were higher in active DM patients (1.417 [0.81-2.74] pg/ml) compared to inactive ones (0.64 [0.38-1.20] pg/ml, p=0.007).
Active ASyS patients had higher IFN-α level (0.08 [0.03-0.38] pg/ml) compared to non-active ASyS patients (0.04 [0.01-0.09] pg/ml, p<0.001) with an AUC=0.69, IC95(0.56-0.82); p<0.05). Active ASyS patients had higher level of IFN-γ (2.02 [0.52-3.24] pg/ml) compared to non-active ASyS patients (0.86 [0.41-1.49] pg/ml, p<0.05) (Supplementary Figure 2D) and AUC=0.69, IC95(0.53-0.95); p<0.05.
Of note, for IMNM, active patients didn’t have higher level of IFN-γ (1.06 [0.75-2.18] pg/ml) than non-active patients (0.44 [0.31-1.19] pg/ml) p=0.06.
3.6 Different IFN secretion profiles depending on IIMs subgroups and myositis specific antibodiesThe levels of IFN-α, IFN-β, and IFN-γ for each patient are presented in Figure 3, where all tested patients are grouped by IIMs subtypes and according to disease activity.
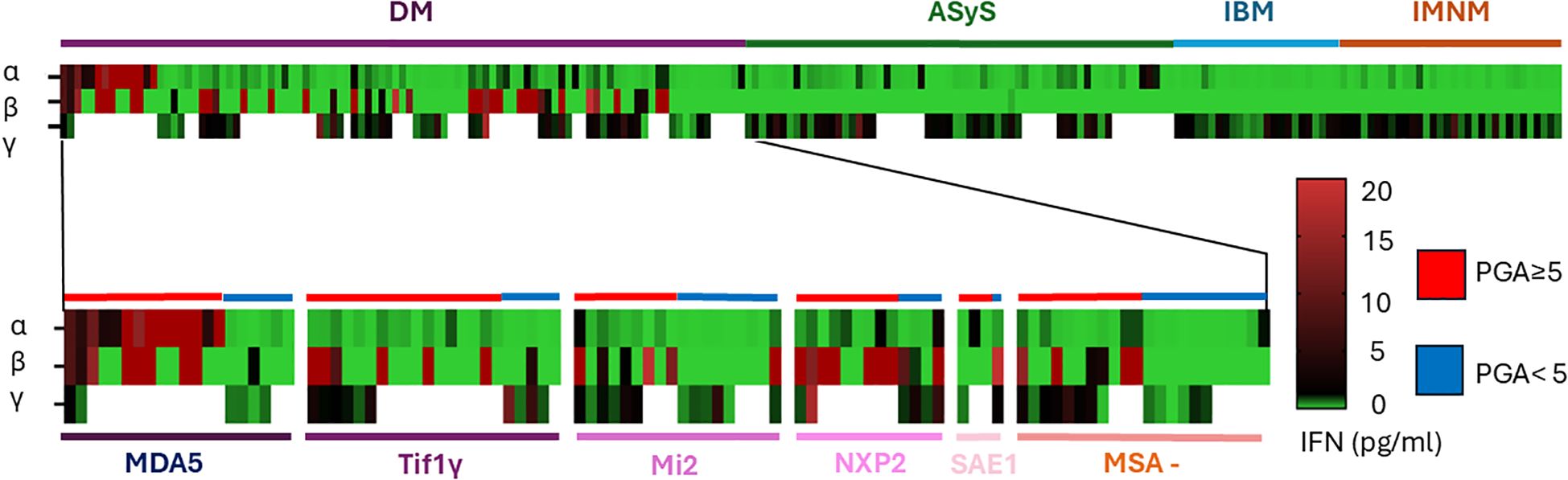
Figure 3. Different cytokine profile according to subgroup of myositis and antibody in DM. Each rows represent a patient, each colomn an IFN subtype and the color define the quantity of IFN secretion in pg/ml.
In DM, patients with anti-MDA5 antibodies had higher levels of IFN-α than the others patients (6.58 [0.12-47.16] pg/ml vs 0.14 [0.02-0.22] pg/ml p<0.005) (Figure 3 and Supplementary Figure 2A). Anti-NXP2 positive antibody patients had higher IFN-β level (25.6 [0.45-67.03] pg/ml) than patients with Tif1γ antibodies (0.0 [0.0-7.3] pg/ml p<0.05) but not again the other ASM (Figure 3 and Supplementary Figure 2). Focusing on naïve patients (n=28/99), 23 patients had an increased level of IFN-α above the 2 SD threshold (0.22 pg/ml), 18 for IFN-β (2.50 pg/ml) and only 2 for IFN-γ.
4 DiscussionIn this study, using two independent cohorts, we demonstrated that type I IFN, particularly IFN-α, are reliable biomarkers of disease activity in patients with DM and ASyS. While IFN-α levels were elevated in both conditions, IFN-β was only significantly increased in DM patients. Furthermore, IFN-α showed a strong association with the MDA5-positive subgroup and exhibited superior performance as a biomarker for disease activity in DM. Type II interferon (IFN-γ) was elevated across all myositis subgroups, with the strongest correlation observed in ASyS, suggesting its relevance as a disease activity biomarker in this cohort.
The concept of an interferon signature has been previously documented in DM, with studies identifying the expression of IFN-stimulated genes in muscle, skin, and blood samples (26–28). The IFN score, which combines the expression of multiple IFN-stimulated genes, has been used to assess disease activity, particularly in DM. While it serves as a valuable tool, it is not yet standardized for routine clinical use and requires RNA extraction (7).
Moreover, although prior research has shown that the IFN score correlates with cutaneous disease activity in DM (11), our study extends these findings by linking serum IFN levels with overall disease activity, rather than focusing solely on skin involvement. Only one previous study employed Digital ELISA to measure IFN-α blood levels in adult DM (11), noting the strong correlation between IFN-α and the IFN gene signature (11, 24). This earlier study demonstrated a significant correlation between IFN-β levels and cutaneous disease activity, but did not evaluate overall disease activity. Additionally, this technology has been successfully applied to other autoimmune diseases, such as lupus, where it showed a correlation with disease activity (25).
Historically, IFN-β has been considered the most reliable biomarker for DM activity (29). However, our study reveals that both IFN-α and IFN-β are reliable biomarkers in DM, with IFN-β being more specific to DM, whereas IFN-α appears to have broader relevance across various myositis subgroups.
Notably, we observed that some DM patients, particularly those with anti-MDA5 or anti-SAE antibodies, had elevated levels of IFN-α but not IFN-β. This finding suggests that the IFN pathway may vary depending on the DM subgroup, with significant therapeutic implications. This variability is particularly important, as therapeutic monoclonal antibodies may block only the IFN-α or IFN-β pathways.It is important to note that the detection limit for IFN-α was 300 times lower than that for IFN-β. This suggests that the utility of monitoring IFN-β levels could increase as the detection limit improves. Currently, no commercially available assays for IFN-β have been developed by the manufacturer.
We also observed that IFN-γ, a type II interferon, was elevated in all myositis subgroups, with the strongest correlation with disease activity observed in ASyS. This finding is consistent with previous studies indicating that patients with anti-Jo1 antibodies exhibit an IFN signature and supports the notion that ASyS may be considered a type II interferonopathy (30). This distinction underscores the different underlying immune pathways in these conditions: DM is characterized by a type I interferon signature (IFN-α and IFN-β), whereas ASyS is predominantly associated with a type II interferon signature. Muscle tissue analysis has shown that DM patients express IFN-related proteins (31, 32), while ASyS patients predominantly overexpress MHC-II, a protein induced by type II IFN (33). These findings highlight the need for tailored therapeutic strategies, as the IFN pathways involved in these diseases differ.
In the case of IMNM, only IFN-γ levels correlated with disease activity, with CK levels being a better biomarker, as previously shown (4). IFN-γ levels may reflect a involvement of Th1 immune responses such as CD8+ T cells, which play a key role in the pathophysiology of IBM and ASyS (12, 13) but these cells are sparse or absent in IMNM. On the other hand, macrophages, which are the predominant immune cells infiltrating IMNM muscle tissue, may contribute to the increased levels of IFN-γ (4). These findings further emphasize the distinct immune mechanisms driving disease activity in the different subtypes of myositis.
A key limitation of this study is the absence of a universally accepted gold standard for assessing disease activity in myositis. Both DM and ASyS are multisystemic diseases, making the measurement of disease activity challenging. In the absence of specific biomarkers, disease activity is typically assessed using a combination of clinical, radiological, and functional criteria. However, many of these methods, particularly in non-muscular disease domains (e.g., skin, lungs, joints), rely on subjective clinical evaluation or imaging techniques that may not directly measure inflammatory disease activity or could be influenced by sequelae of previous diseases. The lack of objective biomarkers for these disease domains complicates the overall assessment of disease activity. To address this, the ACR/EULAR has developed composite improvement scores to assess disease activity based on changes over time (3).
While IFN levels may not yet fully replace clinical tools, our study suggests that monitoring IFN levels could be more sensitive in assessing disease activity than traditional clinical measures. Further validation in larger, independent cohorts, as well as longitudinal studies, is needed to establish IFN-α and IFN-γ as routine biomarkers for disease monitoring and to refine therapeutic strategies, particularly in DM.
5 ConclusionIn conclusion, this study supports the role of type I IFN (IFN-α and IFN-β) and type II IFN (IFN-γ) as reliable biomarkers for disease activity in DM and ASyS. Serum IFN-α levels, measured using SIMOA technology, correlate closely with clinical disease activity in DM, while IFN-γ could be a useful biomarker in ASyS. The findings also suggest that myositis-specific antibodies are associated with distinct IFN secretion profiles, which may help guide personalized treatment strategies. These results should be confirmed in independent prospective studies to validate the clinical utility of IFN biomarkers and optimize therapeutic approaches, especially in DM.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by CPP Ile De France VI (2013-12-19). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsLB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CA: Investigation, Methodology, Supervision, Writing – review & editing. AL: Data curation, Investigation, Supervision, Writing – review & editing. SoM: Investigation, Methodology, Software, Validation, Writing – review & editing. DA: Conceptualization, Investigation, Software, Validation, Writing – review & editing. KD: Investigation, Methodology, Software, Supervision, Writing – review & editing. VB: Investigation, Methodology, Software, Writing – review & editing. OL-C: Investigation, Methodology, Writing – review & editing. ST: Investigation, Writing – review & editing. KM: Conceptualization, Investigation, Software, Writing – review & editing. SaM: Validation, Writing – review & editing. AM: Investigation, Methodology, Validation, Writing – review & editing. BH: Investigation, Methodology, Supervision, Writing – review & editing. MR: Writing – review & editing. GG: Investigation, Software, Supervision, Writing – review & editing. DD: Conceptualization, Investigation, Software, Validation, Writing – review & editing. OB: Conceptualization, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – review & editing. YA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1529582/full#supplementary-material
References1. Mariampillai K, Granger B, Amelin D, Guiguet M, Hachulla E, Maurier F, et al. Development of a new classification system for idiopathic inflammatory myopathies based on clinical manifestations and myositis-specific autoantibodies. JAMA Neurol. (2018) 75:1528−37. doi: 10.1001/jamaneurol.2018.2598
PubMed Abstract | Crossref Full Text | Google Scholar
2. Selva-O’Callaghan A, Pinal-Fernandez I, Trallero-Araguás E, Milisenda JC, Grau-Junyent JM, Mammen AL. Classification and management of adult inflammatory myopathies. Lancet Neurol. (2018) 17:816−28. doi: 10.1016/S1474-4422(18)30254-0
PubMed Abstract | Crossref Full Text | Google Scholar
3. Aggarwal R, Rider LG, Ruperto N, Bayat N, Erman B, Feldman BM, et al. 2016 American College of Rheumatology/European League Against Rheumatism criteria for minimal, moderate, and major clinical response in adult dermatomyositis and polymyositis: An International Myositis Assessment and Clinical Studies Group/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Ann Rheum Dis mai. (2017) 76:792−801. doi: 10.1002/art.40064
PubMed Abstract | Crossref Full Text | Google Scholar
4. Allenbach Y, Arouche-Delaperche L, Preusse C, Radbruch H, Butler-Browne G, Champtiaux N, et al. Necrosis in anti-SRP+ and anti-HMGCR+myopathies: Role of autoantibodies and complement. Neurology. (2018) 90:e507−17. doi: 10.1212/WNL.0000000000004923
PubMed Abstract | Crossref Full Text | Google Scholar
5. Mathur T, Manadan AM, Thiagarajan S, Hota B, Block JA. The utility of serum aldolase in normal creatine kinase dermatomyositis. J Clin Rheumatol. (2014) 20:47−8. doi: 10.1097/RHU.0000000000000062
PubMed Abstract | Crossref Full Text | Google Scholar
6. Bolko L, Jiang W, Tawara N, Landon-Cardinal O, Anquetil C, Benveniste O, et al. The role of interferons type I, II and III in myositis: A review. Brain Pathol. (2021) 31:e12955. doi: 10.1111/bpa.12955
PubMed Abstract | Crossref Full Text | Google Scholar
7. Greenberg SA, Pinkus JL, Pinkus GS, Burleson T, Sanoudou D, Tawil R, et al. Interferon-alpha/beta-mediated innate immune mechanisms in dermatomyositis. Ann Neurol. (2005) 57:664−78. doi: 10.1002/ana.20464
PubMed Abstract | Crossref Full Text | Google Scholar
8. Greenberg SA, Higgs BW, Morehouse C, Walsh RJ, Kong SW, Brohawn P, et al. Relationship between disease activity and type 1 interferon- and other cytokine-inducible gene expression in blood in dermatomyositis and polymyositis. Genes Immun. (2012) 13:207−13. doi: 10.1038/gene.2011.61
PubMed Abstract | Crossref Full Text | Google Scholar
9. Baechler EC, Bauer JW, Slattery CA, Ortmann WA, Espe KJ, Novitzke J, et al. An interferon signature in the peripheral blood of dermatomyositis patients is associated with disease activity. Mol Med. (2007) 13:59−68. doi: 10.2119/2006-00085.Baechler
PubMed Abstract | Crossref Full Text | Google Scholar
10. Wong D, Kea B, Pesich R, Higgs BW, Zhu W, Brown P, et al. Interferon and biologic signatures in dermatomyositis skin: specificity and heterogeneity across diseases. PLoS One. (2012) 7:e29161. doi: 10.1371/journal.pone.0029161
PubMed Abstract | Crossref Full Text | Google Scholar
11. Huard C, Gullà SV, Bennett DV, Coyle AJ, Vleugels RA, Greenberg SA. Correlation of cutaneous disease activity with type 1 interferon gene signature and interferon β in dermatomyositis. Br J Dermatol. (2017) 176:1224−30. doi: 10.1111/bjd.15006
PubMed Abstract | Crossref Full Text | Google Scholar
12. Allenbach Y, Chaara W, Rosenzwajg M, Six A, Prevel N, Mingozzi F, et al. Th1 response and systemic treg deficiency in inclusion body myositis. PLoS One. (2014) 9:e88788. doi: 10.1371/journal.pone.0088788
PubMed Abstract | Crossref Full Text | Google Scholar
13. Hervier B, Perez M, Allenbach Y, Devilliers H, Cohen F, Uzunhan Y, et al. Involvement of NK cells and NKp30 pathway in antisynthetase syndrome. J Immunol. (2016) 197:1621−30. doi: 10.4049/jimmunol.1501902
PubMed Abstract | Crossref Full Text | Google Scholar
14. Pinal-Fernandez I, Casal-Dominguez M, Derfoul A, Pak K, Plotz P, Miller FW, et al. Identification of distinctive interferon gene signatures in different types of myositis. Neurology. (2019) 93:e1193−204. doi: 10.1212/WNL.0000000000008128
PubMed Abstract | Crossref Full Text | Google Scholar
15. Rigolet M, Hou C, Baba Amer Y, Aouizerate J, Periou B, Gherardi RK, et al. Distinct interferon signatures stratify inflammatory and dysimmune myopathies. RMD Open. (2019) 5:e000811. doi: 10.1136/rmdopen-2018-000811
PubMed Abstract | Crossref Full Text | Google Scholar
16. Tabata MM, Hodgkinson LM, Wu TT, Li S, Huard C, Zhao S, et al. The type I interferon signature reflects multiple phenotypic and activity measures in dermatomyositis. Arthritis Rheumatol. (2023) 75:1842−9. doi: 10.1002/art.v75.10
PubMed Abstract | Crossref Full Text | Google Scholar
17. Wilson DH, Rissin DM, Kan CW, Fournier DR, Piech T, Campbell TG, et al. The simoa HD-1 analyzer: A novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J Lab Autom. (2016) 21:533−47. doi: 10.1177/2211068215589580
PubMed Abstract | Crossref Full Text | Google Scholar
18. Lundberg IE, Tjärnlund A, Bottai M, Werth VP, Pilkington C, de Visser M, et al. 2017 European league against rheumatism/american college of rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol (Hoboken NJ). (2017) 69:2271−82. doi: 10.1002/art.40320
PubMed Abstract | Crossref Full Text | Google Scholar
19. Lloyd TE, Mammen AL, Amato AA, Weiss MD, Needham M, Greenberg SA. Evaluation and construction of diagnostic criteria for inclusion body myositis. Neurology. (2014) 83:426−33. doi: 10.1212/WNL.0000000000000642
PubMed Abstract | Crossref Full Text | Google Scholar
20. Allenbach Y, Mammen AL, Benveniste O, Stenzel W. Immune-Mediated Necrotizing Myopathies Working Group. 224th ENMC International Workshop:: Clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14-16 October 2016. Neuromuscul Disord. (2018) 28:87−99. doi: 10.1016/j.nmd.2017.09.016
PubMed Abstract | Crossref Full Text | Google Scholar
21. Stenzel W, Mammen AL, Gallay L, Holzer MT, Kleefeld F, Benveniste O, et al. 273rd ENMC International workshop: Clinico-Sero-morphological classification of the Antisynthetase syndrome. Amsterdam, The Netherlands, 27-29 October 2023. Neuromuscul Disord. (2024) 45:104453. doi: 10.1016/j.nmd.2024.104453
PubMed Abstract | Crossref Full Text | Google Scholar
22. Mammen AL, Allenbach Y, Stenzel W, Benveniste O. ENMC 239th workshop study group. 239th ENMC international workshop: classification of dermatomyositis, amsterdam, the Netherlands, 14-16 december 2018. Neuromuscul Disord. (2020) 30:70−92. doi: 10.1016/j.nmd.2019.10.005
PubMed Abstract | Crossref Full Text | Google Scholar
23. Flammer JR, Dobrovolna J, Kennedy MA, Chinenov Y, Glass CK, Ivashkiv LB, et al. The type I interferon signaling pathway is a target for glucocorticoid inhibition. Mol Cell Biol. (2010) 30:4564−74. doi: 10.1128/MCB.00146-10
PubMed Abstract | Crossref Full Text | Google Scholar
24. Rodero MP, Decalf J, Bondet V, Hunt D, Rice GI, Werneke S, et al. Detection of interferon alpha protein reveals differential levels and cellular sources in disease. J Exp Med. (2017) 214:1547−55. doi: 10.1084/jem.20161451
PubMed Abstract | Crossref Full Text | Google Scholar
25. Mathian A, Mouries-Martin S, Dorgham K, Devilliers H, Barnabei L, Ben Salah E, et al. Monitoring disease activity in systemic lupus erythematosus with single-molecule array digital ELISA quantification of serum interferon-α. Arthritis Rheumatol (Hoboken NJ). (2019) 71(5):756–65. doi: 10.1002/art.40792
PubMed Abstract | Crossref Full Text | Google Scholar
26. Allenbach Y, Leroux G, Suárez-Calvet X, Preusse C, Gallardo E, Hervier B, et al. Dermatomyositis with or without anti-melanoma differentiation-associated gene 5 antibodies: common interferon signature but distinct NOS2 expression. Am J Pathol. (2016) 186:691−700. doi: 10.1016/j.ajpath.2015.11.010
PubMed Abstract | Crossref Full Text | Google Scholar
27. Salajegheh M, Kong SW, Pinkus JL, Walsh RJ, Liao A, Nazareno R, et al. Interferon-stimulated gene 15 (ISG15) conjugates proteins in dermatomyositis muscle with perifascicular atrophy. Ann Neurol. (2010) 67:53−63. doi: 10.1002/ana.21805
留言 (0)