Vascular calcification (VC) is a pathological process involving hydroxyapatite mineral deposition in the vascular system (1). VC correlates strongly with a range of cardiovascular diseases, including those affecting the thoracic and abdominal aorta. Abdominal aortic calcification (AAC) is an early indicator of aortic wall atherosclerosis and is typically driven by inflammatory processes (2). Thus, AAC may be a marker of subclinical atherosclerosis and a predictor of vascular-related morbidity and mortality (3). Nevertheless, the complete pathogenesis of VC remains unclear, and no specific preferred treatment approach has yet been established (4). Therefore, a deeper understanding of the factors that influence VC and implement effective preventive measures is urgently needed. Given the pivotal role of inflammatory mediators in the pathogenesis of aortic calcification, increasing interest has been paid to utilizing this process as a peripheral biomarker for early mortality prediction in patients with aortic calcification (5).
Neutrophils and lymphocytes are primary inflammatory cells in peripheral blood. Their ratio, that is, the neutrophil-to-lymphocyte ratio (NLR), is a reliable biomarker of systemic inflammatory status. Accumulating evidence (6) has focused on the important effects of NLR on individuals at high cardiovascular risk. A study found (7) that the higher the NLR, the higher the cardiovascular risk, which in turn is closely related to the progression of target organ damage and arteriosclerosis (8). Neutrophils can aggravate endothelial cell injury by releasing inflammatory mediators and oxidative stress factors, leading to the deterioration of vascular function and the formation of atherosclerotic plaques (9). Lymphocytes are important in the formation of inflammation and immune regulation. Therefore, elevated NLR may indicate an increased inflammatory state, increased vascular damage, and an increased risk of cardiovascular death. In this context, the NLR may be a novel inflammatory biomarker for the prognosis of numerous cardiovascular diseases.
Although AAC is an inflammatory process, the precise relationship between the NLR and AAC remains unclear, meriting further investigation. To address this research gap, our primary objective was to deepen our understanding of how NLR influences AAC and the implications of this association. Established evidence among adults in the U.S. is scarce. Thus, our study aimed to provide additional insights into the interplay between the NLR and AAC. Therefore, this retrospective cross-sectional study analyzed data from 3,047 participants from the National Health and Nutrition Examination Survey (NHANES) 2013–2014 cohort.
2 Materials and methods 2.1 Study populationThe NHANES is a nationally representative survey conducted by the National Center for Health Statistics (NCHS) that employs stratified, multistage probability cluster sampling to assess the health or nutritional status of the non-institutionalized US population (10). The sample for the survey was selected as a representative of the U.S. population across ages. Information on AAC was provided only in the NHANES 2013–2014 cycles for adults aged 40–80 years.
From the initial pool of 10,175 individuals in the 2013–2014 NHANES cycle, we excluded those <40 years of age (n = 6,360), with missing AAC scores (n = 675), and with incomplete neutrophil and lymphocyte data (n = 93), resulting in a final sample of 3,047 participants (Figure 1).
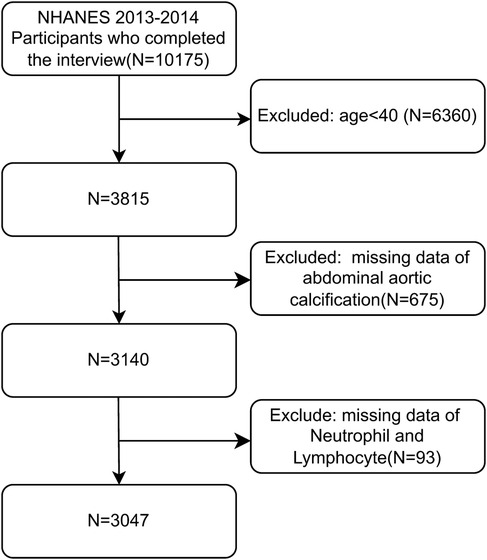
Figure 1. Flowchart of this study.
The NHANES protocol was approved by the NCHS Research Ethics Review Board (11), and written informed consent was obtained from all participants at the time of enrollment. The Ethics Committee of Hiser Hospital, affiliated with Qingdao University, determined that the present study was exempt from review because it used publicly available de-identified data and waived the requirement for informed consent. This study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Reporting Guidelines.
2.2 Study variables 2.2.1 NLRThe NHANES and complete blood count (CBC) profiles were used to acquire hematologic parameters. The CBC parameters were derived based on the Beckman–Coulter method of counting and sizing in conjunction with an automated diluting and mixing apparatus for sample processing. The NLR was calculated for each participant by dividing the total absolute neutrophil count by the total absolute lymphocyte count. Details of the laboratory methodology are available at: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/CBC_H.htm
2.2.2 AACAAC was evaluated in this study using the Kauppila AAC score, which was obtained from dual-energy x-ray absorptiometry (DXA) scans of the lateral lumbar spine (12). The calcification severity was indicated by the AAC score, with a total AAC score ranging from 0 to 24. Higher AAC scores indicated more severe calcification and an AAC score of >6 is usually used as the cutoff for severe AAC (13). We considered the NLR as a continuous variable and used a three-category variable in our analysis to determine the relationship between the NLR and AAC.
2.3 CovariablesThe covariates included sex, age (<65 or ≥65 years), race, income-to-poverty ratio (PIR) (<1.3 or ≥1.3), education level (high school and below or above high school), body mass index (BMI) (<25, 25–30, or ≥30 kg/m2), drinking, smoking, hypertension, diabetes mellitus (DM), coronary heart disease (CHD), angina pectoris, heart failure (HF), chronic kidney disease (CKD), stroke, triglyceride, total cholesterol, platelet count (PLT), blood urea nitrogen (BUN), serum creatinine (SCr), serum uric acid (SUA), serum total calcium (SCa), and serum phosphorus (SPh) levels.
2.4 Statistical analysisAll normally distributed continuous variables are reported as means ± SD, whereas skewed continuous variables are presented as medians [interquartile range (IQR)]. Categorical variables are expressed as frequencies and percentages. Statistical analyses included the use of the chi-square or Fisher's exact tests for categorical variables, one-way analysis of variance (ANOVA) for variables with a normal distribution, and the Kruskal–Wallis H test for variables with a skewed distribution to assess differences among the various NLR groups. Multiple imputations were used to fill in missing baseline data.
Multinomial logistic regression was conducted to investigate the association between the NLR and AAC. The NLR was considered both a continuous and a categorical variable, comprising the following categories: Q1 (<1.64), Q2 (1.64–2.34), and Q3 (>2.34). These categories were selected based on clinical interest and literature (14–16). Three models were constructed in this study. Model 1 was unadjusted. Model 2 was adjusted for sex, age, race, PIR, and education level. Model 3 was additionally adjusted for BMI, alcohol consumption, smoking, hypertension, DM, CHD, angina pectoris, HF, CKD, stroke, triglycerides, total cholesterol, PLT, BUN, SCr, SUA, SCa, and SPh levels.
A multivariate regression model was employed for trend analysis, with the NLR utilized as both continuous and three-category variables. A restricted cubic spline model was used to generate smooth curves and investigate the potential non-linear dose-response relationships between the NLR and SAAC. Subgroup analysis was conducted to investigate the association between the NLR and AAC based on the identified subgroup variables. All statistical analyses were conducted using R Statistical Software, version 4.2.2 (http://www.R-project.org, The R Foundation) and the Free Statistics Analysis Platform (17), version 2.0 (Beijing, China; http://www.clinicalscientists.cn/freestatistics). Statistical significance was defined as a two-sided p < 0.05.
3 ResultsAfter rigorous screening based on the predefined inclusion and exclusion criteria, this study included a total of 3,047 patients (1,469 males and 1,578 females) (Figure 1). The overall prevalence of severe calcification disease in the study population was 10.8%. The participants were classified into three quantiles based on their NLR: quantile 1 (<1.64%), quantile 2 (1.64%–2.34%), and quantile 3 (>2.34%). The baseline patient characteristics are shown in Table 1. Sex, age, race, BMI, alcohol consumption, smoking, hypertension, DM, CHD, angina pectoris, HF, CKD, stroke, triglyceride levels, total cholesterol, BUN, SPh, and SCr differed significantly between the three NLR groups, while PIR, PLT, SUA, and SCa did not (all p > 0.05).

Table 1. Characteristics of the study population based on NLR tertiles.
3.1 Association between the NLR and SAACMultivariable logistic regression analyses adjusted for potential confounders (Table 2, Model 3), NLR expressed as a continuous variable was positively associated with the probability of SAAC [odds ratio (OR) = 1.08, 95% confidence interval (CI) = 1.00–1.16, p = 0.049]. The multivariable-adjusted regression ORs (95% CI) of NLR associated with groups 2 and 3 were 1.04 (95% CI = 0.73–1.48) and 1.42 (95% CI = 1.02–1.96), respectively, compared with NLR group 1 (Table 2, Model 3). The NLR was considered a continuous variable in the restricted cubic spline analysis. The results of multivariable-adjusted restricted cubic spline analysis revealed a linear relationship between the NLR and SAAC (Figure 2, p for non-linearity = 0.244, excluding the highest 1% for each NLR measure). The observed trend indicated that as the NLR increased, the risk of SAAC also increased.
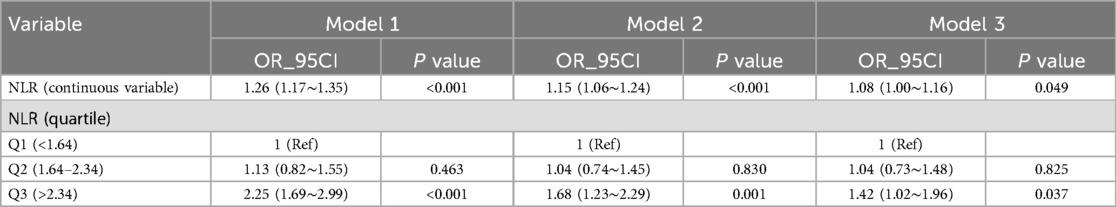
Table 2. Multivariable logistic regression to assess the association of NLR with severe AAC.
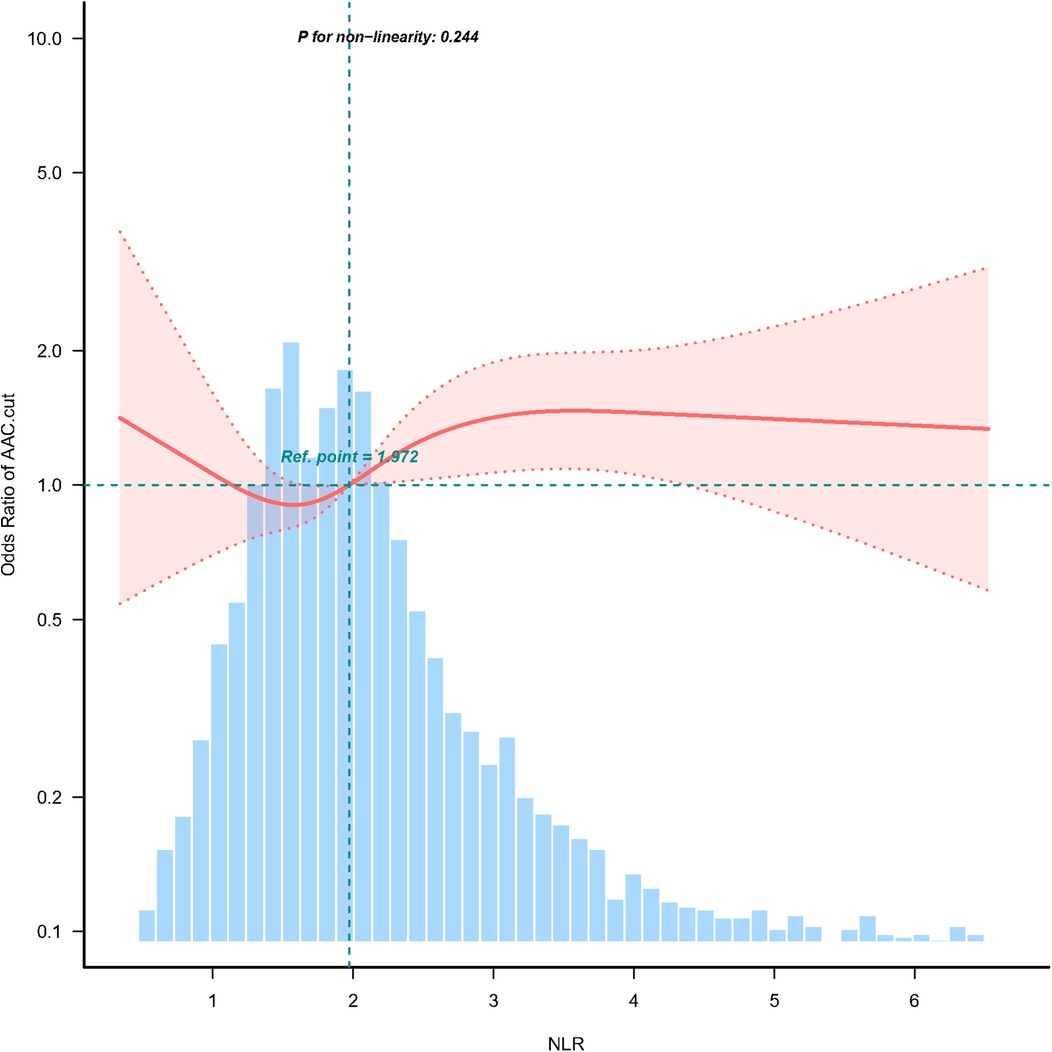
Figure 2. Association between NLR and SAAC odds ratio. Solid and dashed lines represent the predicted values and 95% confidence intervals. They were adjusted for sex, age, race, PIR, education level, BMI, alcohol consumption, Smoking, Hypertension, DM, CHD, Angina pectoris, HF, CKD, Stroke, triglycerides, total cholesterol, PLT, BUN, SCr, SUA, SCa, and SPh levels. Only 99% of the data is shown.
3.2 Subgroup analysisSubgroup analyses were conducted to examine the association between the NLR, incident AAC, and SAAC. These analyses were stratified by age, sex, hypertension, DM, and BMI. The results demonstrated consistent associations between NLR and SAAC across all subgroups and sensitivity analyses, with no statistically significant differences observed (all p-values for interaction >0.05) (Figure 3).
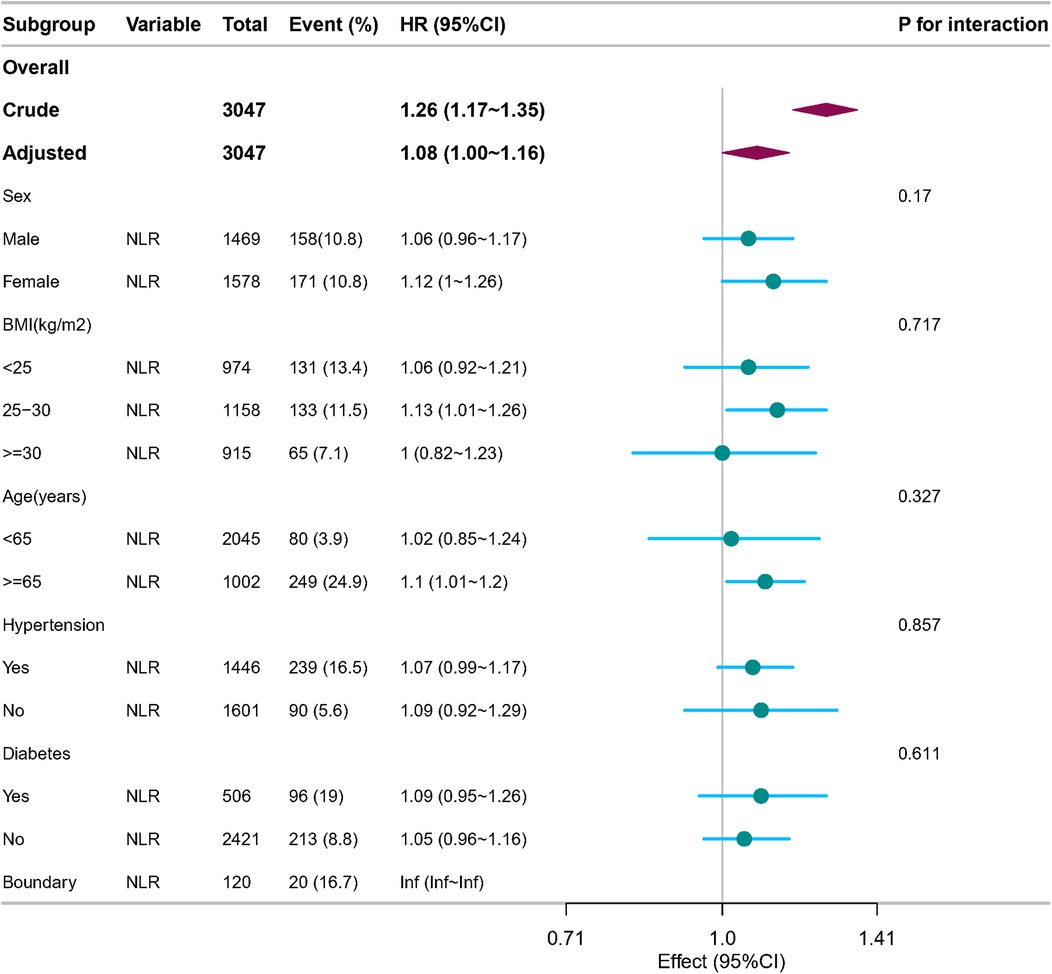
Figure 3. Forest plot of multivariable logistics analysis between NLR and SAAC. Except for the stratification component itself, each stratification factor was adjusted for sex, age, race, PIR, education level, BMI, alcohol consumption, Smoking, Hypertension, DM, CHD, Angina pectoris, HF, CKD, Stroke, triglycerides, total cholesterol, PLT, BUN, SCr, SUA, SCa, and SPh levels.
4 DiscussionThe results of this comprehensive retrospective cross-sectional study, which analyzed data from the 2013–2014 NHANES, demonstrated that the NLR is an independent predictor of SAAC. This association was consistent across various clinical subgroups. Our findings have several important clinical implications.
Inflammation is strongly associated with the risk of cardiovascular mortality (18). The CANTOS trial (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) showed that targeted anti-inflammatory therapy—at least with interleukin-1ß inhibition—could significantly lower the rates of heart attack and cardiovascular death. Among patients at “residual inflammatory risk,” anti-inflammatory therapy reduces the risk of cardiovascular events (19, 20). Inflammatory markers, which are simple, measurable biological indicators, are important in guiding the long-term follow-up treatment of patients at high cardiovascular risk.
In the inflammatory expression stage, neutrophils suggest non-specific inflammation, while lymphocytes have defense and regulatory roles in the inflammatory response. NLR, as a combination of the two, can more comprehensively reflect the inflammatory state and immune status of the organism. This study investigated the non-linear relationship between the NLR and SAAC using data from the NHANES 2013–2014. Our findings are consistent with those of previous observational studies. For instance, Zhou et al. (14) reported a significant correlation between the NLR and elevated SAAC in a specific population (β = 2.37; 95% CI = 1.79–3.42, p = 0.025). Similarly, a recent prospective longitudinal cohort study involving patients with end-stage renal disease (ESRD) also demonstrated a significant association between the NLR and SAAC (21). Moreover, NLR is an independent predictor of thoracic peri-aortic calcification (TAC) in patients with ESRD. A recent study employing Mendelian randomization further confirmed these associations (22). These findings illustrate the potential of the NLR in risk stratification of patients with SAAC.
Further research is required to validate these results, investigate the detailed relationship between the NLR and SAAC, and elucidate the potential underlying mechanisms of this relationship. Recent studies have begun to unravel the relationship between the NLR and SAAC. For example, a cross-sectional analysis of baseline data from a multicenter cohort revealed the independent association between the highest tertile of NLR with AAC (OR = 2.876, 95% CI = 1.250–6.619, p = 0.013) (15). Our study extends these findings by demonstrating that a high NLR is associated with an increased risk of developing SAAC. Furthermore, our results indicated that with increased NLR, the risk of SAAC increased by 8% in the NHANES 2013–2014 dataset.
The correlations between the NLR and incident SAAC, although slight, were consistent across diverse event subtypes. These correlations persisted across the various subgroups defined by age, race, BMI, DM, and hypertension. These significant associations with SAAC are consistent with findings in the existing literature. Therefore, the results of the subgroup analyses demonstrate the overall stability of the findings.
A comprehensive understanding of the potential mechanisms and clinical implications is essential for elucidating the relationship between NLR and SAAC. Previous studies have suggested a metabolic link between the NLR and SAAC (23). Chronic inflammatory infiltration is often accompanied by osteochondral metaplasia and neovascularization (24). Moreover, the rate of hemodynamic progress is related to leukocyte chemotaxis and the expression of tumor necrosis factor-alpha (TNF-α) (25). Additionally, metalloproteinase (MMP) expression induces an inflammatory reaction (26). Finally, a variety of cytokines produced by inflammatory cells may accelerate and induce VC (27).
In summary, our study contributes to the growing body of evidence on the association between the NLR and SAAC. Further studies are required to gain a deeper understanding of the mechanisms and clinical implications of this relationship. This study represents an inaugural comprehensive investigation of the association between the NLR and the risk of developing AAC and cardiovascular events based on data obtained from the NHANES 2013–2014. The key strengths of this study are its large sample size, national population coverage, and representativeness, which provide robust statistical power to explore the relationship between the NLR and SAAC. The use of a territory-wide validated electronic healthcare database containing comprehensive records of diagnoses, hospitalizations, and medication dispensing details helped reduce the impact of common biases observed in observational studies, such as selection and recall biases (28).
The methodology in the present study was sound, demonstrating both novel and potential clinical applications. As a recently identified inflammatory marker, the NLR is easily calculated and readily available in clinical settings. An independent association was also identified between the increase in NLR and the incidence of severe AAC, thereby providing substantial evidence for identifying SAAC.
This study has some limitations. For instance, the cross-sectional nature of this study precludes the establishment of direct causality, and the possibility of residual confounding by unmeasured variables cannot be discounted. Despite adjusting for relevant confounders in the multivariate model, unknown residual confounders such as dietary factors or family income may have influenced the observed associations. Nevertheless, the homogeneity of the study population and adjustment for significant confounders suggests that any potential bias is likely minimal. Furthermore, due to the constraints of the NHANES database and the lack of data on AAC progression, a more comprehensive assessment of the relationship between NLR and AAC was not possible. Assessment of aortic calcification using DXA is less accurate than CT, but lateral spine images obtained with DXA to detect the prevalence of VFA can detect AAC with reasonably good sensitivity and specificity (29, 30). A high level of quality control was maintained throughout the DXA data collection and scan analysis (31) to ensure accuracy and consistency. In the future, further studies using more precise techniques are necessitated. Finally, only including the U.S. population aged >40 years limits the generalizability of our results. Further investigations across diverse populations are required to validate these findings.
In conclusion, the results of this study indicate a positive correlation between the NLR and SAAC in US adults aged >40 years. Thus, the NLR may be a novel inflammatory marker with implications for AAC.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe requirement of ethical approval was waived by the Ethics Committee of Hiser Hospital, affiliated with Qingdao University for the studies involving human participants because the present study was exempt from review as it used publicly available deidentified data. As such the ethics committee/institutional review board also waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsHD: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft. MS: Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Software, Supervision, Writing – review & editing. ZhZ: Formal Analysis, Methodology, Project administration, Resources, Visualization, Writing – original draft. KF: Methodology, Resources, Software, Validation, Visualization, Writing – review & editing. ZiZ: Data curation, Software, Visualization, Writing – review & editing. YC: Funding acquisition, Project administration, Supervision, Writing – review & editing. WS: Formal Analysis, Funding acquisition, Resources, Software, Supervision, Validation, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Traditional Chinese Medicine Science and Technology Project of Shangdong Province of China (Grant number: M-2023033). The funder did not contribute to the study's design, collection, analysis, and interpretation of data.
AcknowledgmentsWe are grateful to thank all of the participants, the staff, and the other study investigators for their valuable contributions. We appreciatively thank Dr. Jie Liu (Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital) for his consultation on the statistical support, and comments regarding the manuscript. We would like to thank Editage (http://www.editage.cn) for English language editing.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Adatorwovor R, Roggenkamp K, Anderson JJ. Intakes of calcium and phosphorus and calculated calcium-to-phosphorus ratios of older adults: NHANES 2005–2006 data. Nutrients. (2015) 7(11):9633–9. doi: 10.3390/nu7115492
PubMed Abstract | Crossref Full Text | Google Scholar
2. Narula N, Dannenberg AJ, Olin JW, Bhatt DL, Johnson KW, Nadkarni G, et al. Pathology of peripheral artery disease in patients with critical limb ischemia. J Am Coll Cardiol. (2018) 72(18):2152–63. doi: 10.1016/j.jacc.2018.08.002
PubMed Abstract | Crossref Full Text | Google Scholar
3. Gonçalves FB, Voûte MT, Hoeks SE, Chonchol MB, Boersma EE, Stolker RJ, et al. Calcification of the abdominal aorta as an independent predictor of cardiovascular events: a meta-analysis. Heart. (2012) 98(13):988–94. doi: 10.1136/heartjnl-2011-301464
Crossref Full Text | Google Scholar
4. Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res. (2011) 109(6):697–711. doi: 10.1161/CIRCRESAHA.110.234914
PubMed Abstract | Crossref Full Text | Google Scholar
5. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010
PubMed Abstract | Crossref Full Text | Google Scholar
6. Chen TT, Lee KY, Chang JH, Chung CL, Tran HM, Manullang A, et al. Prediction value of neutrophil and eosinophil count at risk of COPD exacerbation. Ann Med. (2023) 55(2):2285924. doi: 10.1080/07853890.2023.2285924
PubMed Abstract | Crossref Full Text | Google Scholar
7. Xu JP, Zeng RX, Zhang YZ, Lin SS, Tan JW, Zhu HY, et al. Systemic inflammation markers and the prevalence of hypertension: a NHANES cross-sectional study. Hypertens Res. (2023) 46(4):1009–19. doi: 10.1038/s41440-023-01195-0
PubMed Abstract | Crossref Full Text | Google Scholar
8. Buonacera A, Stancanelli B, Colaci M, Malatino L. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Mol Sci. (2022) 23(7):3636. doi: 10.3390/ijms23073636
PubMed Abstract | Crossref Full Text | Google Scholar
10. Borrud L, Chiappa MM, Burt VL, Gahche J, Zipf G, Johnson CL, et al. National health and nutrition examination survey: national youth fitness survey plan, operations, and analysis, 2012. Vital Health Stat 2. (2014) 2(163):1–24.
11. Xie R, Xiao M, Li L, Ma N, Liu M, Huang X, et al. Association between SII and hepatic steatosis and liver fibrosis: a population-based study. Front Immunol. (2022) 13:925690. doi: 10.3389/fimmu.2022.925690
PubMed Abstract | Crossref Full Text | Google Scholar
12. Mazziotti G, Tupputi U, Ferrante G, Guglielmi G. Abdominal aortic calcification as a marker of relationship between atherosclerosis and skeletal fragility. J Clin Densitom. (2020) 23(4):539–42. doi: 10.1016/j.jocd.2020.05.001
PubMed Abstract | Crossref Full Text | Google Scholar
13. Górriz JL, Molina P, Cerverón MJ, Vila R, Bover J, Nieto J, et al. Vascular calcification in patients with nondialysis CKD over 3 years. Clin J Am Soc Nephrol. (2015) 10(4):654–66. doi: 10.2215/CJN.07450714
PubMed Abstract | Crossref Full Text | Google Scholar
14. Zhou S, Cai B, Zhang Y, Wang L, Liu X, Xu G. The relationship between neutrophil-to-lymphocyte ratio and aortic arch calcification in ischemic stroke patients. J Stroke Cerebrovasc Dis. (2017) 26(6):1228–32. doi: 10.1016/j.jstrokecerebrovasdis.2017.01.012
PubMed Abstract | Crossref Full Text | Google Scholar
15. Ban TH, Choi BS, Yoon SA, Kim Y, Jin K, Kim GH, et al. Clinical significance of neutrophil-to-lymphocyte ratio on the risk of abdominal aortic calcification and decreased bone mineral density in patients with end-stage kidney disease. PLoS One. (2023) 18(10):e0286612. doi: 10.1371/journal.pone.0286612
PubMed Abstract | Crossref Full Text | Google Scholar
16. Dong K, Zheng Y, Wang Y, Guo Q. Predictive role of neutrophil percentage-to-albumin ratio, neutrophil-to-lymphocyte ratio, and systemic immune-inflammation index for mortality in patients with MASLD. Sci Rep. (2024) 14(1):30403. doi: 10.1038/s41598-024-80801-8
PubMed Abstract | Crossref Full Text | Google Scholar
17. Chen H, Tang H, Zhang X, Huang J, Luo N, Guo Q, et al. Adherence to life’s essential 8 is associated with delayed biological aging: a population-based cross-sectional study. Rev Esp Cardiol (Engl Ed). (2025) 78(1):37–46. doi: 10.1016/j.rec.2024.04.004.38663840
PubMed Abstract | Google Scholar
19. Crea F, Liuzzo G. Addressing acute coronary syndromes: new challenges and opportunities after the CANTOS trial (canakinumab anti-inflammatory thrombosis outcomes study). Circulation. (2018) 137(11):1100–2. doi: 10.1161/CIRCULATIONAHA.117.032178
PubMed Abstract | Crossref Full Text | Google Scholar
20. Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. (2018) 391(10118):319–28. doi: 10.1016/S0140-6736(17)32814-3
PubMed Abstract | Crossref Full Text | Google Scholar
21. Turkmen K, Ozcicek F, Ozcicek A, Akbas EM, Erdur FM, Tonbul HZ. The relationship between neutrophil-to-lymphocyte ratio and vascular calcification in end-stage renal disease patients. Hemodial Int. (2014) 18(1):47–53. doi: 10.1111/hdi.12065
PubMed Abstract | Crossref Full Text | Google Scholar
22. Cho KI, Cho SH, Her AY, Singh GB, Shin ES. Prognostic utility of neutrophil-to-lymphocyte ratio on adverse clinical outcomes in patients with severe calcific aortic stenosis. PLoS One. (2016) 11(8):e0161530. doi: 10.1371/journal.pone.0161530
PubMed Abstract | Crossref Full Text | Google Scholar
23. Song J, Zheng Q, Ma X, Zhang Q, Xu Z, Zou C, et al. Predictive roles of neutrophil-to-lymphocyte ratio and C-reactive protein in patients with calcific aortic valve disease. Int Heart J. (2019) 60(2):345–51. doi: 10.1536/ihj.18-196
PubMed Abstract | Crossref Full Text | Google Scholar
25. Weber A, Pfaff M, Schottler F, Schmidt V, Lichtenberg A, Akhyari P. Reproducible in vitro tissue culture model to study basic mechanisms of calcific aortic valve disease: comparative analysis to valvular interstitials cells. Biomedicines. (2021) 9(5):474. doi: 10.3390/biomedicines9050474
PubMed Abstract | Crossref Full Text | Google Scholar
26. Jenke A, Kistner J, Saradar S, Chekhoeva A, Yazdanyar M, Bergmann AK, et al. Transforming growth factor-beta1 promotes fibrosis but attenuates calcification of valvular tissue applied as a three-dimensional calcific aortic valve disease model. Am J Physiol Heart Circ Physiol. (2020) 319(5):H1123–41. doi: 10.1152/ajpheart.00651.2019
PubMed Abstract | Crossref Full Text | Google Scholar
27. Bogdanova M, Zabirnyk A, Malashicheva A, Semenova D, Kvitting JP, Kaljusto ML, et al. Models and techniques to study aortic valve calcification in vitro, ex vivo and in vivo. An overview. Front Pharmacol. (2022) 13:835825. doi: 10.3389/fphar.2022.835825
PubMed Abstract | Crossref Full Text | Google Scholar
28. Cheng TD, Ferderber C, Kinder B, Wei YJ. Trends in dietary vitamin A intake among US adults by race and ethnicity, 2003–2018. JAMA. (2023) 329(12):1026–9. doi: 10.1001/jama.2023.0636
PubMed Abstract | Crossref Full Text | Google Scholar
29. Schousboe JT, Debold CR. Reliability and accuracy of vertebral fracture assessment with densitometry compared to radiography in clinical practice. Osteoporos Int. (2006) 17(2):281–9. doi: 10.1007/s00198-005-2010-5
PubMed Abstract | Crossref Full Text | Google Scholar
30. Schousboe JT, Lewis JR, Kiel DP. Abdominal aortic calcification on dual-energy x-ray absorptiometry: methods of assessment and clinical significance. Bone. (2017) 104:91–100. doi: 10.1016/j.bone.2017.01.025
留言 (0)