Membranous nephropathy (MN) is an immune-mediated glomerular disease characterized by the deposition of immune complexes in the glomerular basement membrane and diffuse thickening. Approximately 20% of MN is secondary to autoimmune diseases, infectious diseases, tumors, drugs, or harmful substances and is therefore known as secondary MN. However, approximately 80% of MN still lacks a clear cause and is called idiopathic membranous nephropathy (IMN) (1). IMN is one of the common causes of adult nephrotic syndrome in China, accounting for approximately 20% of cases of primary nephrotic syndrome (2). Although spontaneous remission is a common feature of IMN, IMN has a one-third rule, that is, spontaneous remission in one-third of patients, persistent proteinuria in one-third, and progression to end-stage renal disease in one-third (3).
At present, IMN treatment is mainly based on immunosuppressive agents, such as calcineurin inhibitors, alkylating agents, or rituximab (RTX). However, serious adverse reactions often occur with these treatments, and the recurrence rate of IMN is high after discontinuation of calcineurin inhibitors. Therefore, the optimal management regimen for IMN remains controversial, and the current IMN treatment regimen should be optimized (4).
Traditional Chinese medicine has long been used to treat patients with kidney disease as an alternative therapeutic strategy (5). Preparations of Tripterygium wilfordii are well known for their immunosuppressive and anti-inflammatory properties and are frequently used to treat a variety of autoimmune diseases, including rheumatoid arthritis and Sjogren’s syndrome. Recently, they have also been used to treat glomerular diseases (6, 7). A new Tripterygium wilfordii preparation called Kunxian capsule (KX) has been approved in China. The traditional preparation of Tripterygium wilfordii has some limitations in clinical application, such as reproductive system damage, but KX can significantly lower reproductive toxicity through compatibility with traditional Chinese medicine (8). Studies showed that KX can inhibit kidney damage and T-cell infiltration, and had the potential to protect podocytes (9, 10). However, it is still unknown whether KX is safe and effective for IMN and its therapeutic mechanism of action is unclear. Therefore, we retrospectively described the response rate and side effects that occurred during treatment in 39 patients with IMN who received KX therapy, and explored the molecular targets and possible mechanisms of action of KX during IMN treatment based on network pharmacology.
2 Materials and methods 2.1 Clinical research 2.1.1 PatientsPatients with IMN who visited the Second Hospital of Jilin University between March 2017 and March 2022 were enrolled in this study. The inclusion criteria were as follows: (1) age between 18 and 75 years; (2) pathological diagnosis of IMN (stages I–IV) confirmed by biopsy and treated with KX; and (3) patients who had complete information and were followed up for more than 6 months. The exclusion criteria were as follows: (1) concomitant treatment with immunosuppressants during KX therapy; (2) secondary nephrotic syndrome; (3) fertility needs or pregnancy; (4) life-threatening illnesses, other organ malfunctions, severe infections, or other serious conditions; and (5) incomplete follow-up.
2.1.2 Treatment regimenKX (Guangzhou Baiyunshan Chen Liji Pharmaceutical Factory Co., Ltd., 0.3 g/capsule) was administered orally three times daily at an initial dose of 0.6 g. After remission, the dose was gradually decreased to between 50% and 70% of the oral dose until it was stopped.
2.1.3 Efficacy evaluationBaseline and follow-up data were collected at 1, 3, and 6 months. The primary outcome was the disease response rate, including complete and partial responses. Complete response (CR) was defined as 24 h urinary protein quantification < 0.3 g; partial response (PR) was defined as 24 h urinary protein quantification > 50% decrease from baseline; all other cases were classified as non- response (NR). Any patient who achieved a CR or PR was considered successful.
2.1.4 Statistical analysisStatistical analyses were performed using IBM SPSS Statistics version 25.0. Continuous variables with a normal distribution are shown by mean and standard deviation as, X¯±S whereas continuous variables with a non-normal distribution are shown by median and interquartile ranges as M(P25, P75). Non-normally distributed data was log-transformed to normally distributed data before the statistical analysis. Indicators before and after treatment were compared using the paired sample t-test. To decrease the risk of Type I errors caused by multiple comparisons, Bonferroni correction was applied to adjust level of test. The figures were obtained using GraphPad Prism 9. The metrics that followed a normal distribution were represented using error bar, while the metrics that did not follow a normal distribution were represented using box plot. Statistical significance was defined as a P < 0.05.
2.2 Network pharmacology 2.2.1 Acquisition of potential therapeutic targets of KX for IMNKX is mainly composed of traditional Chinese medicines including Lycii Frucyus, Cuscutae Semen, Epimordii herba, and Tripterygium hypoglaucum. The bioactive ingredients of Lycii Frucyus, Cuscutae Semen, and Epimridii Herba were identified using TCMSP, and those of Tripterygium hypoglaucum were identified using HERB. The bioactive ingredients were screened by setting oral bioavailability (OB) ≥ 30% and drug-like properties (DL) ≥ 0.18 (11, 12). According to these bioactive ingredients, the corresponding targets were obtained from Uniprot (13). With “idiopathic membranous neopathy” as the keywords, the pathogenic targets of IMN were searched in GeneCards (14) and Online Mendelian Inheritance in Man (OMIM) (15). Finally, the above KX targets were intersected with IMN targets to obtain potential therapeutic targets of KX for IMN using the online Venn diagram tool.
2.2.2 Construction of the ingredient-target-disease networkThe potential therapeutic targets and the bioactive ingredients were input into Cytoscape 3.9.1 and free nodes were removed to establish an ingredient-target-disease network and screen out the main bioactive ingredients of KX for IMN.
2.2.3 Construction of protein–protein interaction networkPotential therapeutic targets were submitted to the STRING platform with a confidence score of > 0.40. The PPI network was visualized using Cytoscape 3.9.1, and the core targets were identified.
2.2.4 Enrichment analysisCommon targets of KX and IMN were uploaded to the DAVID database, and gene ontology (GO) functional enrichment and Kyoto Encyclopedia of Genes Genomics (KEGG) pathway analyses were performed. The results were visualized using an online bioinformatics tool.
2.2.5 Molecular docking verificationThe structures of the ingredients with the top three degree values in the ingredient-target-disease network were downloaded from the TCMSP. The protein structures of the top three core targets in the PPI network were downloaded from the PDB database. AutoDockTools1.5.6 and AutoDock4.2.6 were used to perform molecular docking to verify the binding affinity between the targets and the bioactive component of KX. Binding conformations were visualized using PyMOL 2.4.0.
3 Results 3.1 Baseline characteristics of patientsA total of 39 patients were included in the study. Of these, 13 patients had hypertension (33.33%) and 6 had diabetes (15.38%) (Table 1).
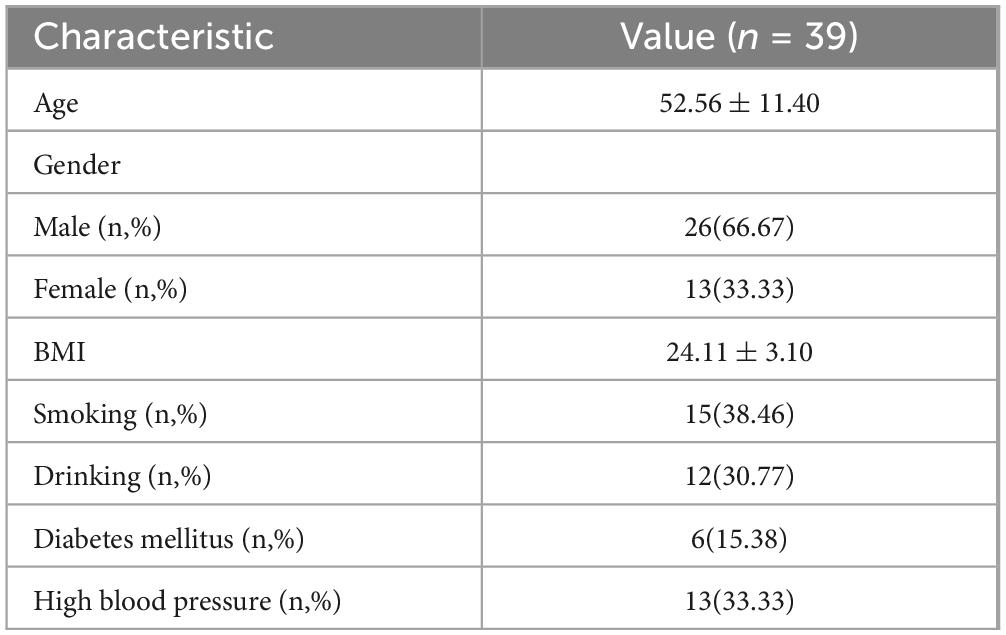
Table 1. Baseline characteristics of patients.
3.2 Changes in plasma albumin and 24 h urine total proteinBaseline 24 h urine protein quantification was 3.34 (1.90, 4.77) g/d; at 1, 3, and 6 months of treatment, it fell to 2.40 (0.91, 3.17) g/d (P < 0.01); 1.50 (0.80, 2.50) g/d (P < 0.01); and 0.70 (0.28, 1.61) g/d (P < 0.01). After 6 months of treatment, the serum albumin increased from (33.63 ± 5.17) g/L at baseline to (36.99 ± 6.07) g/L (P < 0.05) (Figure 1).
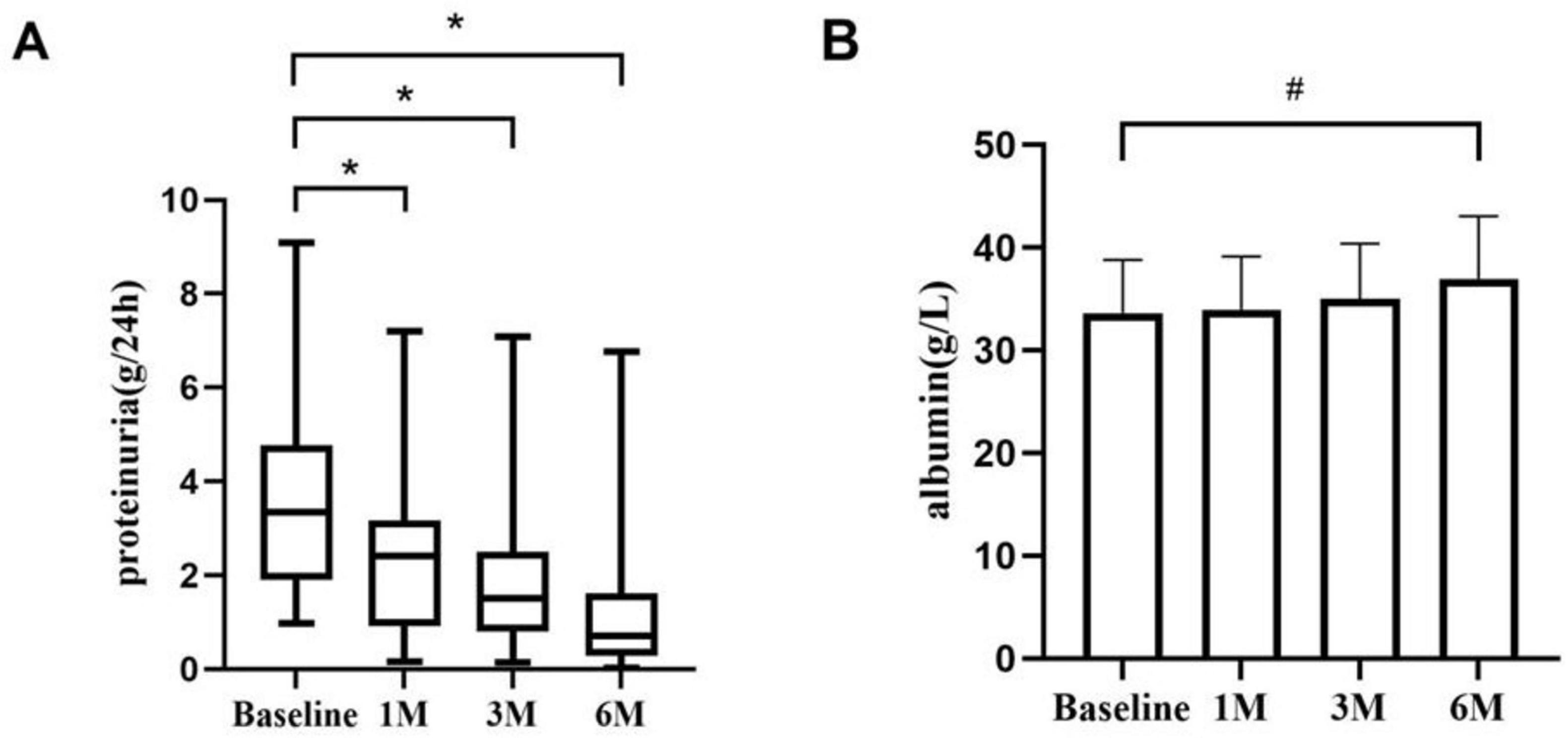
Figure 1. Proteinuria and serum albumin during KX therapy. (A) The proteinuria changes from baseline to 1, 3, and 6 months. There were significant differences in proteinuria at each time point. (B) The changes in serum albumin from baseline to 1, 3, and 6 months.*P < 0.01. #P < 0.05.
3.3 Changes in other biochemical parametersChanges in triglycerides and blood cholesterol levels during KX therapy are shown in Figure 2. Triglyceride levels in patients demonstrated a statistically significant difference at 3 months compared to baseline (P < 0.05). The serum creatinine level and eGFR of patients with IMN remained stable, and there was no significant difference at 1, 3, and 6 months (P > 0.05).
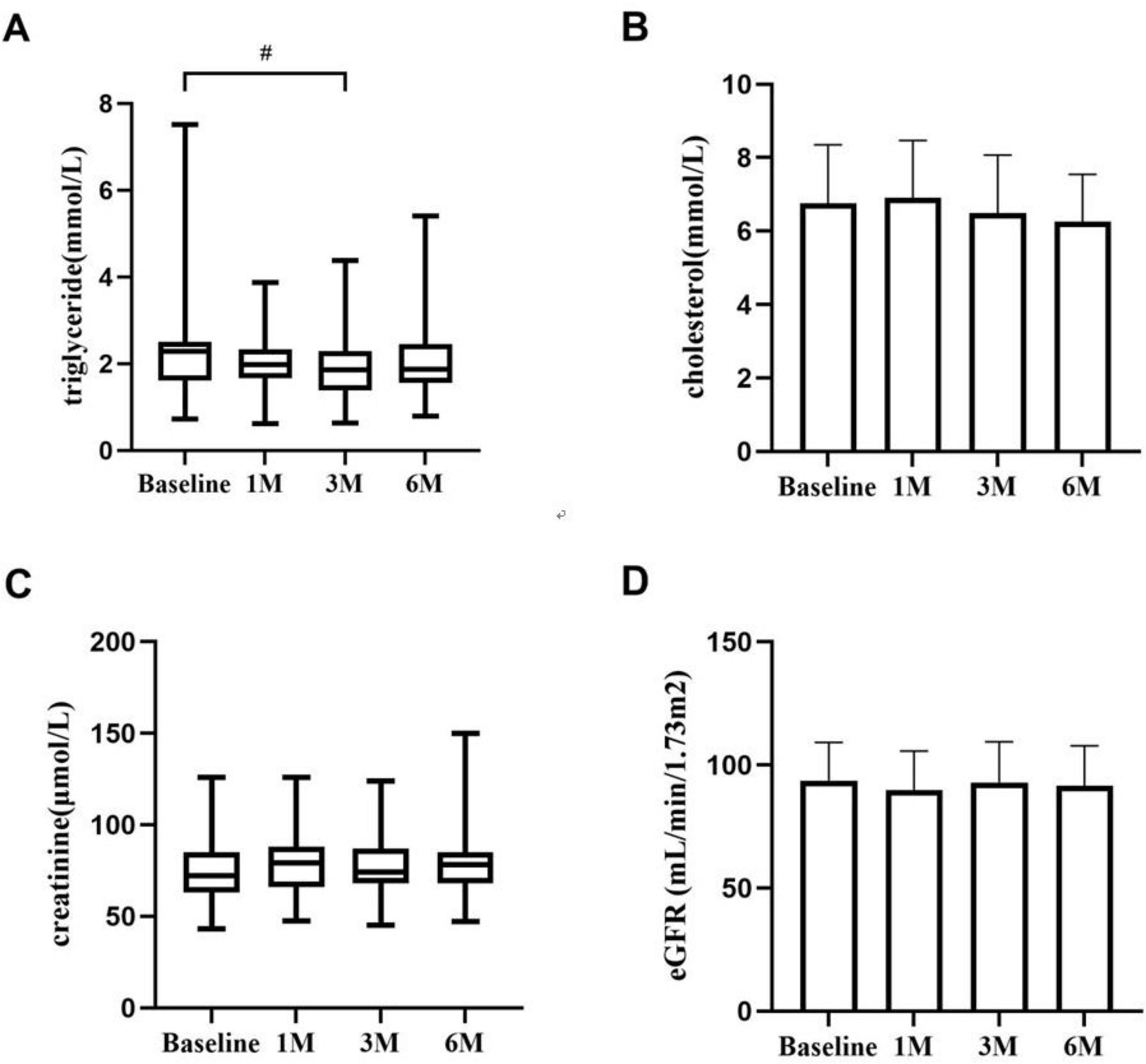
Figure 2. The changes in triglycerides (A), blood cholesterol (B), creatinine (C), and eGFR (D) during KX therapy. #P < 0.05.
3.4 Response after KX therapyAfter receiving KX therapy, 10 patients (25.64%) experienced remission at 1 month, and 17 patients (43.59%) experienced remission at 3 months. At 6 months, 31 patients responded, with an overall clinical response rate of 79.49%, including 10 patients with CR (25.64%) and 21 patients with PR (53.85%) (Figure 3).
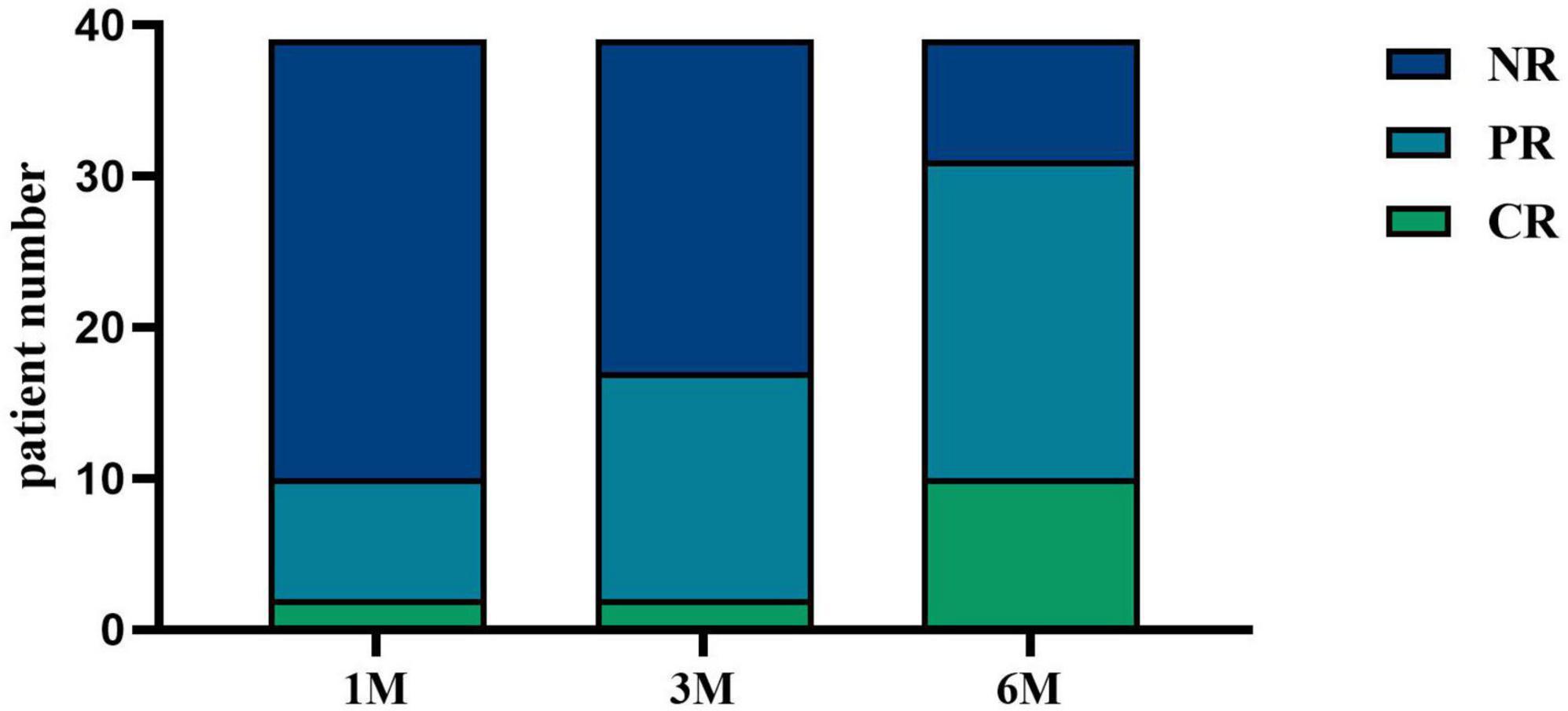
Figure 3. IMN response after KX therapy. CR was defined as 24-h urinary protein quantification < 0.3 g; PR was defined as 24-h urinary protein quantification > 50% decrease from baseline; all other cases were classified as NR. At 1 month, 5.13% (n = 2) of patients achieved CR, and 20.51% (n = 8) achieved PR. At 3 months, 5.13% (n = 2) of patients achieved CR, and 38.46% (n = 15) achieved PR. At 6 months, 25.64% (n = 10) of patients achieved CR, and 53.85% (n = 21) achieved PR.
3.5 Adverse events during KX therapyCommon side effects of KX included liver damage (17.95%), infections (including lung and urinary tract infections) (20.51%), increased creatinine levels (7.69%), and irregular menstruation (2.56%). Most adverse responses improved after symptomatic therapy. Although liver and renal function in some patients did not entirely return to normal at the end of the observation period, they did so during the follow-up period, and no significant fatal adverse reactions occurred.
3.6 Potential therapeutic targets of KX for IMNA total of 86 bioactive ingredients of KX were identified, including 45 from Lycii Frucyus, 11 from Cuscutae Semen, 23 from Epimridii herba, and 7 from Tripterygium hypoglaucum. After eliminating the duplicates, 81 bioactive ingredients of KX and 341 corresponding targets were identified. A total of 5581 targets of IMN were obtained from GeneCards, and 503 IMN targets were obtained from OMIN. A total of 3055 targets of IMN targets were obtained by integrating and removing the duplicates. A total of 341 targets of KX and 3055 targets of IMN were imported into the online Venn diagram tool to obtain 190 potential therapeutic targets (Figure 4).
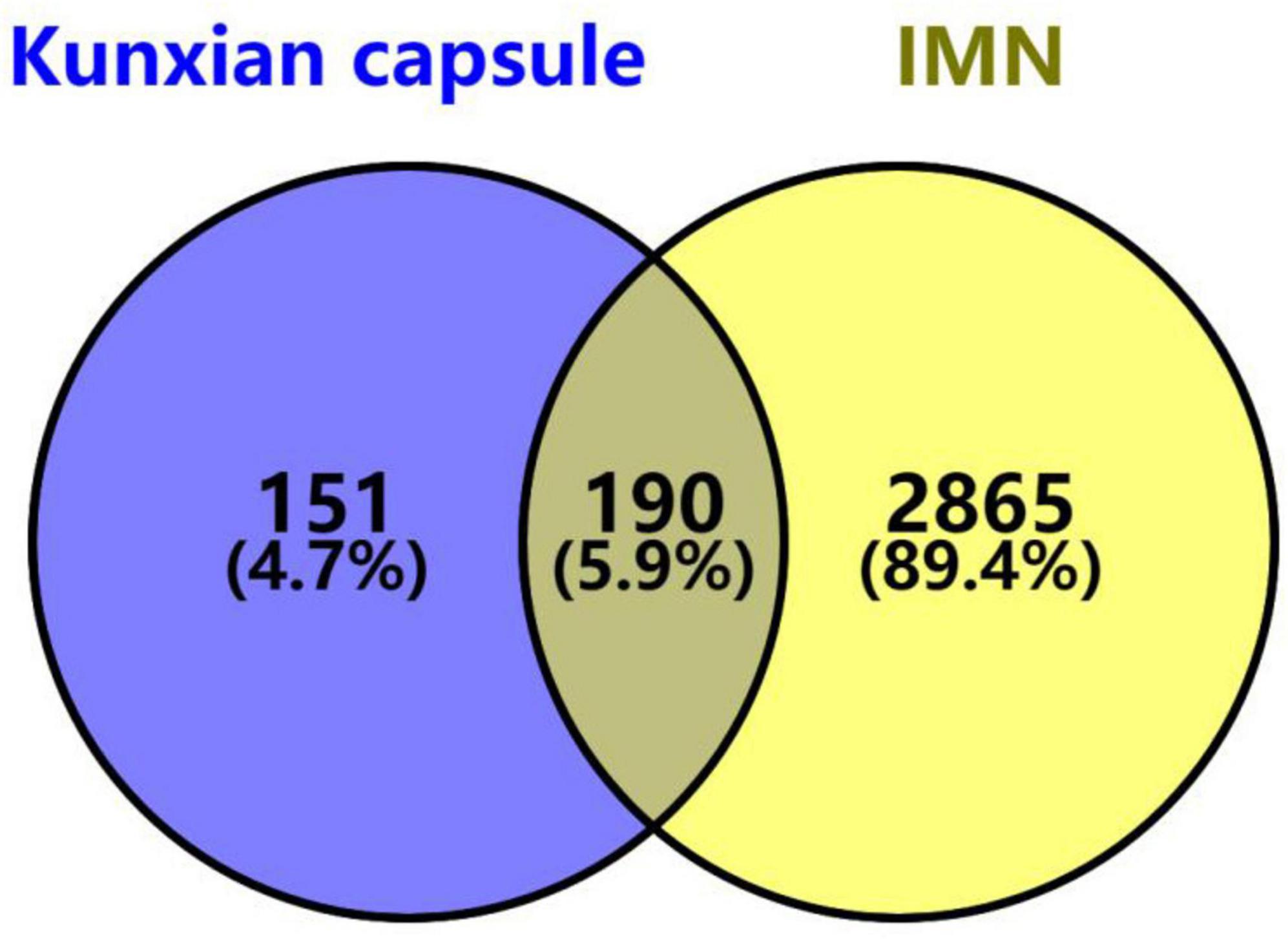
Figure 4. Venn diagram of KX targets and IMN targets. The purple section stands for KX targets, the yellow section stands for IMN targets, and 190 targets in the middle overlapping section are common targets of KX and IMN.
3.7 Ingredients-targets-disease networkThe network comprised 67 bioactive ingredient nodes, 190 potential therapeutic targets, and 848 edges (Figure 5). According to the topological properties of the network, the larger the degree value of the node, the more targets it acts on and the more important the ingredient. The three main bioactive ingredients of KX were quercetin, luteolin, and kaempferol.
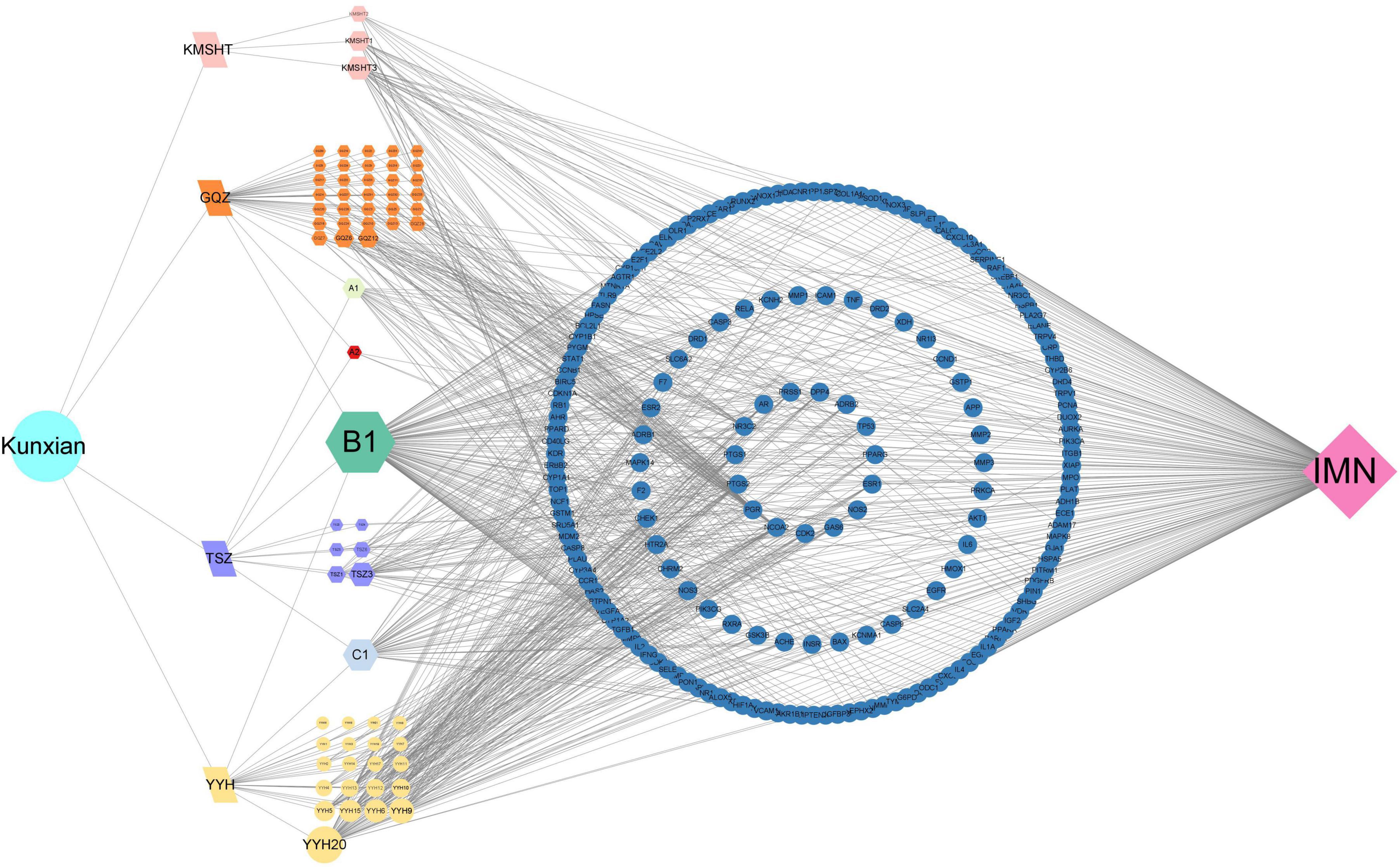
Figure 5. Ingredients of KX-targets-IMN network. The traditional Chinese medicine was represented by letters, “GQZ” for Lycii Frucyus, “TSZ” for Cuscutae Semen, “YYH” for Epimrdii Herba and “KMSHT” for Tripterygium hypoglaucum. The unique ingredients among them were represented by letters and numbers, such as “GQZ1,” and common ingredients were represented with A, B, C and numbers, such as “A1.”
3.8 PPI network constructionThe 190 potential therapeutic targets were uploaded to the STRING platform, and the results were inputted into Cytoscape 3.9.1. A total of 48 core therapeutic targets were screened using a betweenness centrality > 150, closeness centrality > 0.003, and degree centrality > 48. The top three core targets were AKT1, IL6, and TNF (Figure 6).

Figure 6. PPI network of KX targets against IMN. The color and the size of the nodes reflects the degree of connectivity (darker color and bigger circle indicates a higher degree).
3.9 Possible regulatory signaling pathways of KX in treating IMNWe further conducted GO functional analysis and KEGG pathway enrichment analysis to elucidate the biological effects on gene functions and signaling pathways of the related targets of KX in the treatment of IMN (Figure 7). In terms of biological processes, it was mainly related to positive regulation of gene expression and response to xenobiotic stimulus. The cellular components were mainly concentrated in extracellular space. For molecular functions, it can be seen that the targets were mainly enriched in enzyme binding. According to the results of the KEGG pathway enrichment analysis, it was most involved in cancer pathways, the AGE-RAGE signaling pathway in diabetic complications, lipid and atherosclerosis.
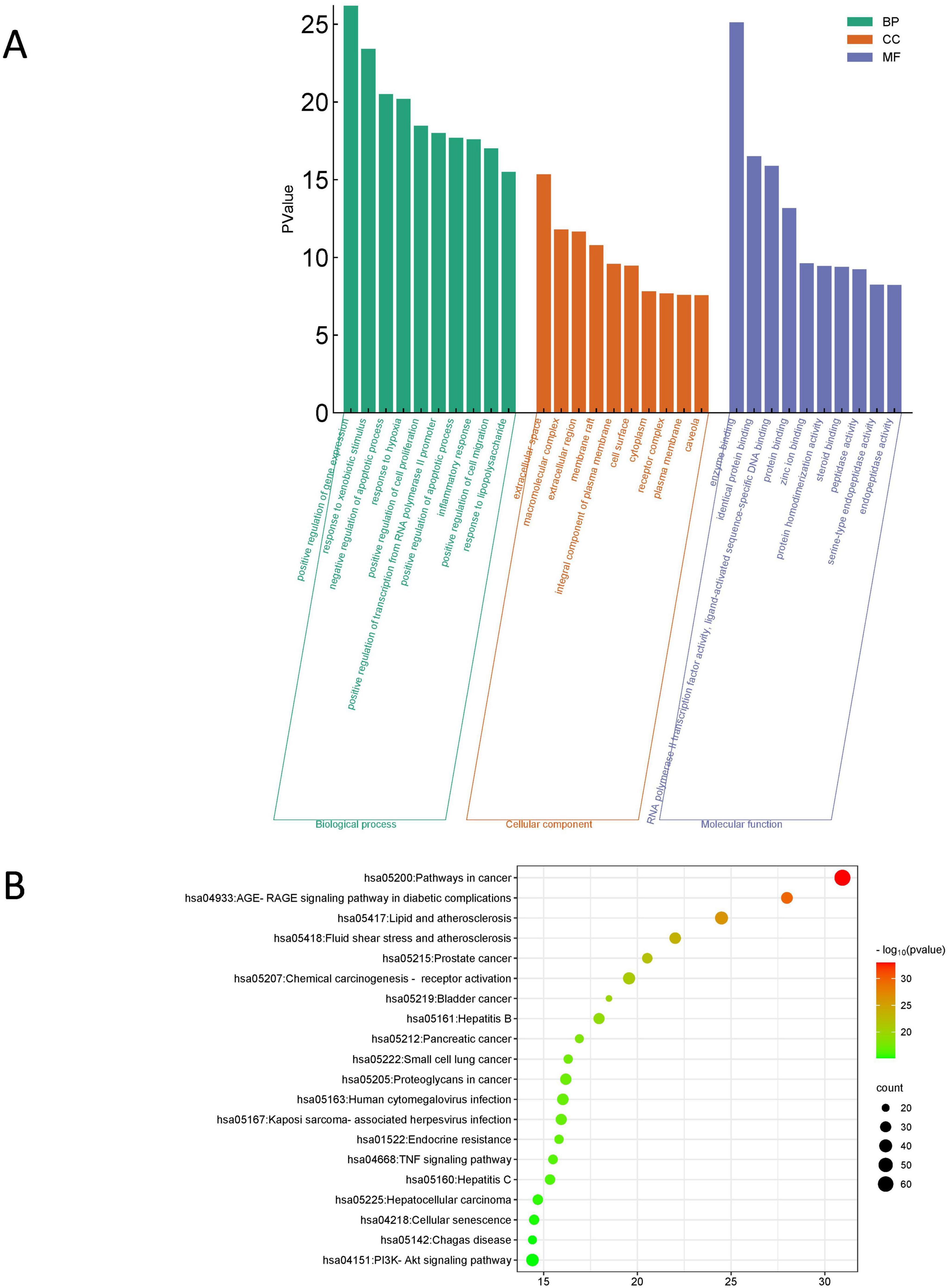
Figure 7. Enrichment analysis of the targets of KX in treating IMN. (A) GO functional analysis. The top 10 items of each part are shown. (B) KEGG pathway enrichment analysis. The sizes of the bubbles are illustrated from big to small in descending order of the number of potential targets involved in the pathways.
3.10 Molecular docking resultsIn the present study, the interactions between the core targets and KX were verified by molecular docking (Figure 8). It is generally believed that the lower the energy when the conformation of the ligand binding to the receptor is stable, the greater the possibility of interaction. Nine pairs were delivered to the docking simulation, and all binding complexes showed good binding affinity (Table 2).
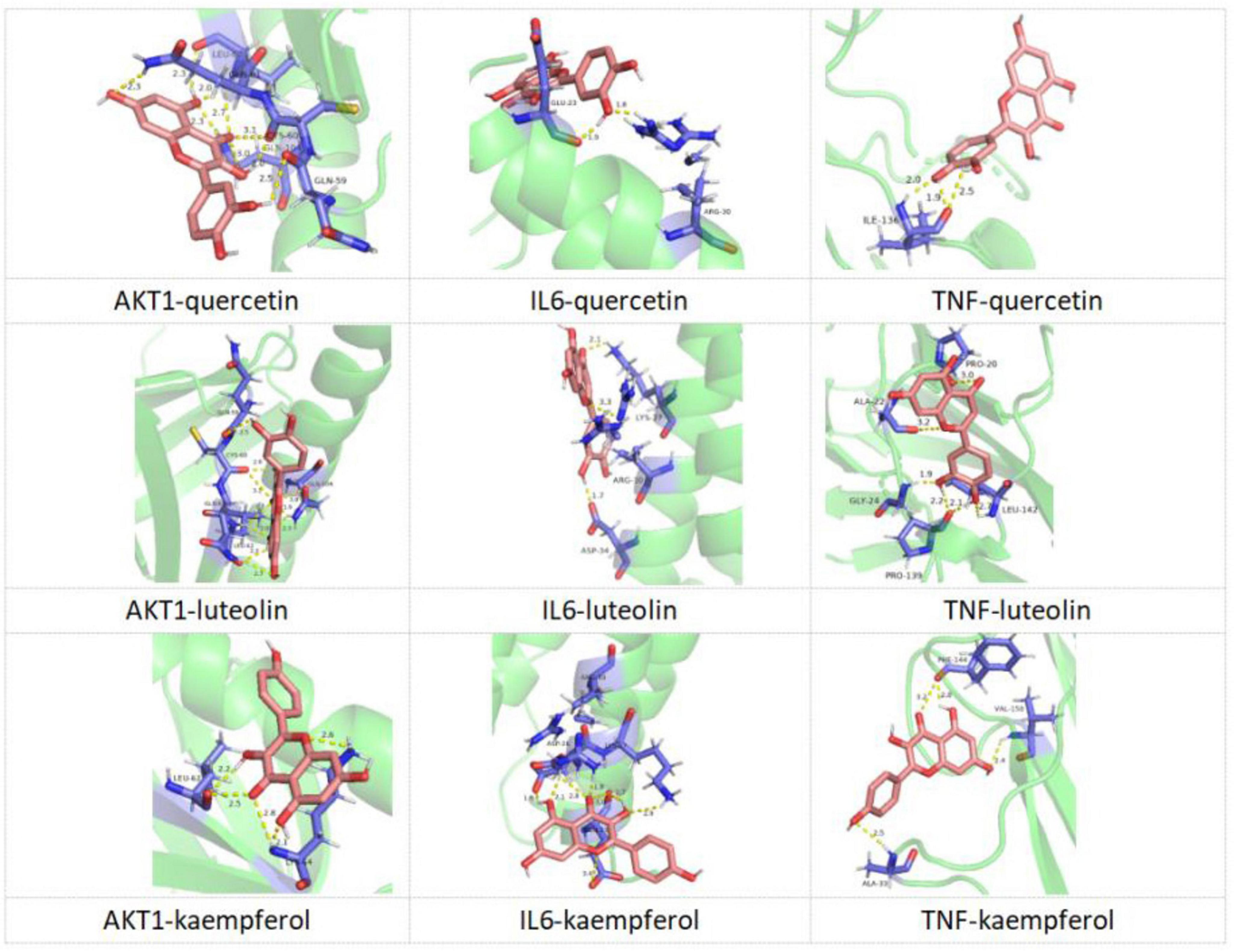
Figure 8. Molecular docking diagram of KX docking with core targets.

Table 2. Molecular docking results of KX docking with core targets.
4 DiscussionThe treatment of IMN has long been a challenge. Although the Kidney Disease: Improving KDIGO guidelines recommend the use of glucocorticoids, cyclophosphamide (CTX), cyclosporin A (CsA), tacrolimus (TAC), and RTX as standard treatments for IMN, some studies have shown that the efficacy and safety of these drugs are unsatisfactory. For instance, glucocorticoids and CTX together have good clinical efficacy, but the frequency of non-fatal side effects is significant (16). Although calcineurin inhibitors can quickly produce remission, they have a considerable risk of relapse and nephrotoxicity (17, 18). As a new biologic drug, RTX has the potential to address disease etiology, cause illness remission, and stabilize and enhance renal function (19). However, RTX therapy is expensive, and approximately 40% of patients do not respond to it (20). Treatment has become much more challenging for some individuals who do not respond to these drugs and eventually develop refractory IMN. Therefore, further research is required to investigate IMN treatment alternatives.
Traditional Chinese medicine has been used in China to treat kidney diseases clinically, and it has demonstrated promising application possibilities with good efficacy and few side effects (21). Tripterygium wilfordii preparations are currently well-studied traditional Chinese medicine immunosuppressive agents and are widely used for the treatment of various autoimmune diseases because of their prominent anti-inflammatory and immunosuppressive effects (22, 23). KX is a new type of Tripterygium wilfordii preparation and has a good effect in the treatment of IgA nephropathy, rheumatoid arthritis, and ankylosing spondylitis (24, 25). According to our network pharmacology-based research, quercetin, luteolin, and kaempferol were the main bioactive ingredients of KX in treating IMN. AKT1, IL6, and TNF were core targets. The main potential mechanism of KX in treating IMN were pathways involved in cancer, the AGE-RAGE signaling pathway in diabetic complications, lipid and atherosclerosis. Quercetin, a flavonoid widely found in plants, exhibits anti-inflammatory and anti-injury effects (26). It can reduce the expression and activity of matrix metalloproteinase-2, thereby inhibiting the proliferation and migration of vascular endothelial cells (27). Luteolin is a plant-derived liver protective immunomodulator with diverse antioxidant, free radical scavenging, and anti-inflammatory activities (28, 29). Kaempferol is one of the main bioactive compounds in Kaempferia rhizome, with antioxidant/anti-inflammatory effects demonstrated in various disease models (30).
We discovered that KX therapy was helpful for IMN clinical remission in our clinical study. Therefore, we conducted a retrospective analysis of patients with IMN treated with KX at our center, evaluating the efficacy and safety of KX in 39 patients with IMN over 6 months. The results demonstrated substantial clinical benefits, with significant improvements in clinical indicators and a considerable proportion of patients achieving CR or PR, highlighting the potential utility of KX as a treatment option for IMN. One of the most striking outcomes was the progressive reduction in 24 h urinary protein levels over the 6-month treatment period. Baseline urinary protein levels, which averaged 3.34 g/d, decreased significantly to 0.70 g/d at 6 months (P < 0.01). Proteinuria is a major risk factor for disease progression and long-term renal damage, and this reduction reflects a remarkable improvement in glomerular barrier function. Clinically, this indicates a reduced risk of progression to chronic kidney disease (CKD). Serum albumin increased from a baseline of (33.63 ± 5.17) g/L to (36.99 ± 6.07) g/L at six months (P < 0.05), indicating a reduction in protein loss through urine and the restoration of oncotic pressure. These changes suggest the potential of KX to improve systemic homeostasis. Furthermore, the clinical remission rates also underscore the efficacy of KX. The improvements in lipid profiles, as well as the stability of creatinine and eGFR levels following KX treatment, represent additional benefits, enhancing its clinical utility beyond managing proteinuria and serum albumin. Second, nearly half of the patients with IMN were relieved by KX after 3 months of treatment, indicating that KX has a rapid onset of action and good efficacy.
The included patients with IMN in our study consisted of those with refractory membranous nephropathy who did not achieve satisfactory outcomes with standard treatments, as well as patients with underlying conditions such as hypertension and diabetes who experienced increased proteinuria. None of these patients used immunosuppressants concurrently during KX therapy.
Based on the study results, KX effectively reduced proteinuria and is a viable therapeutic option for patients with refractory membranous nephropathy who failed to respond effectively to standard treatments and could not continue their original regimen due to drug side effects or cumulative dosage limits. Additionally, for patients with long-term hypertension and diabetes, high blood pressure and hyperglycemia can damage the kidneys, increasing the risk of disease progression. According to some studies, hypertension and diabetes are factors contributing to disease progression in patients with IMN (31, 32). When these patients show significantly increased proteinuria, renal biopsy is necessary to clarify the cause of kidney function changes. In such cases, timely treatment of elevated proteinuria is essential. The Kidney Disease: Improving KDIGO guidelines currently lack standard treatment protocols for such patients, and who also do not meet the criteria for immunosuppressant use. KX has shown the ability to reduce proteinuria and slow the progression of kidney dysfunction in these patients.
Common adverse reactions of Tripterygium wilfordii preparations are hepatorenal toxicity, gastrointestinal reactions, reproductive system damage, and hematologic toxicity (7). KX is compatible according to the traditional principle of monarchy in Chinese medicine (33). It uses Tripterygium hypoglaucum as the monarch drug, and its active ingredient has anti-inflammatory and immunosuppressive effects. Epimrdii Herba is the minister drug, and its active ingredient can regulate immunity and gonadotropic function; combined with Lycii Frucyus and Cuscutae Semen, products of tonifying kidney and benefiting essence, it antagonizes the toxic effects of Tripterygium hypoglaucum on the reproductive system, but does not affect its original anti-inflammatory and immunosuppressive effects (25, 34).
In our study, some patients developed the above adverse reactions; however, they improved after active symptomatic treatment, and no fatal adverse reactions occurred. We observed no significant differences in albumin levels at 1 and 3 months of treatment compared to those before treatment. Since hypoalbuminemia is a common clinical manifestation in patients with IMN, it can lead to a hypercoagulable state, increasing the risk of thrombosis. Therefore, in clinical practice, for patients with IMN and low serum albumin levels, we recommend oral anticoagulant therapy to prevent thrombosis, when necessary, based on their venous thromboembolism risk assessment results. Additionally, some patients exhibited elevated liver enzymes early after treatment, which was considered to be related to the hepatotoxicity of KX. For patients who developed elevated liver enzymes during KX treatment, we administered oral hepatoprotective drugs, such as glutathione or silibinin, while regularly monitoring liver function. During the 6-month observation period, liver enzyme levels in the vast majority of patients returned to normal after receiving hepatoprotective treatment. It is important to note that although the common side effect of Tripterygium wilfordii preparations is reproductive toxicity, the incidence of gonadal suppression in this study was low and is considered to be related to the small number and older age of female patients. Although KX has reduced reproductive toxicity through drug compatibility, reproductive toxicity is still one of the main factors limiting its widespread application in specific populations. The current study on the reproductive toxicity of KX remains at a theoretical level, and more in vitro tests and in-depth studies are required.
One of the limitations of this study is its single-center retrospective design. All treatment options were jointly decided based on the experience of clinicians combined with the patient’s choice, which may lead to selection deviation; secondly, the poor follow-up data of patients in this center led to a small sample size and short observation time, which may bias the evaluation of the efficacy and safety of KX; thirdly, this study was a single-center study of patients in Northeast China, and the results may not be universal for patients with IMN nationwide and even worldwide. Therefore, additional prospective RCT trials with larger sample sizes and longer follow-up times are required to demonstrate the effectiveness and safety of KX in IMN.
5 ConclusionKX is a safe and effective treatment option for IMN and can effectively improve serum albumin and 24 h urine protein levels in patients with IMN. This study preliminarily reveals the possible mechanism of KX in the treatment of IMN and provides a theoretical basis for future clinical research.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statementThe study was approved by the Ethics Committee of The Second Hospital of Jilin University (No.2023299), and adhered to the Helsinki Declaration guidelines. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because it is a retrospective clinical study.
Author contributionsJL: Data curation, Investigation, Writing – original draft, Writing – review & editing. XG: Validation, Writing – original draft. LL: Data curation, Validation, Writing – original draft. LH: Data curation, Writing – original draft. GT: Conceptualization, Supervision, Writing – review & editing. XL: Methodology, Project administration, Supervision, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Health Commission of Jilin Province (No. 2022Lc116) and the Department of Science and Technology Jilin Province (No. YDZJ202401189ZYTS).
AcknowledgmentsThe authors would like to thank the researches and staff of the above software and databases.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes References1. Zhang N, Dai H, Dong X, Liu W, Jiang H, Zhao Q, et al. Level of Interleukin-35 in patients with idiopathic membranous nephropathy and its predictive value for remission time. Front Immunol. (2022) 13:926368. doi: 10.3389/fimmu.2022.926368
PubMed Abstract | Crossref Full Text | Google Scholar
2. Wang B, Zhu Z, Huang F, Huang H, Tu L, Wang Y, et al. Anti-Phospholipase A2 receptor antibody levels at diagnosis predicts outcome of Tac-based treatment for idiopathic membranous nephropathy patients (Vol 23, 306, 2022). BMC Nephrol. (2022) 23:306. doi: 10.1186/s12882-022-02950-0
PubMed Abstract | Crossref Full Text | Google Scholar
3. McQuarrie EP, Stirling CM, Geddes CC. Idiopathic membranous nephropathy and nephrotic syndrome: Outcome in the era of evidence-based therapy. Nephrol Dial Transpl. (2012) 27:235–42. doi: 10.1093/ndt/gfr220
PubMed Abstract | Crossref Full Text | Google Scholar
4. Wang Y-N, Feng H-Y, Nie X, Zhang Y-M, Zou L, Li X, et al. Recent advances in clinical diagnosis and pharmacotherapy options of membranous nephropathy. Front Pharmacol. (2022) 13:907108. doi: 10.3389/fphar.2022.907108
PubMed Abstract | Crossref Full Text | Google Scholar
5. Feng Z, Liu W, Jiang HX, Dai H, Gao C, Dong Z, et al. How does herbal medicine treat idiopathic membranous nephropathy? Front Pharmacol. (2020) 11:994. doi: 10.3389/fphar.2020.00994
PubMed Abstract | Crossref Full Text | Google Scholar
6. Wang Z, Yu C, Zhou L-N, Chen X. Effects of tripterygium wilfordii induction therapy to Iga nephropathy patients with heavy proteinuria. Biol Pharm Bull. (2017) 40:1833–8.
7. Song C-Y, Xu Y-G, Lu Y-Q. Use of Tripterygium wilfordii hook F for immune-mediated inflammatory diseases: Progress and future prospects. J Zhejiang Univ Sci B. (2020) 21:280–90. doi: 10.1631/jzus.B1900607
PubMed Abstract | Crossref Full Text | Google Scholar
8. Liu Y-F, Zhang Z, Zhang J-J, Chen Z, Tu S-H, Xing G-L. The derivative of Tripterygium Wilfordii Hook F-Kunxian capsule, attenuated rheumatoid arthritis: A systematic review and meta- analysis. Evid Based Complement Altern Med. (2020) 2020:4178140. doi: 10.1155/2020/4178140
PubMed Abstract | Crossref Full Text | Google Scholar
9. Cheng C, Zhu R, Liu M, Yang H, Guo F, Du Q, et al. Kunxian capsule alleviates renal damage by inhibiting the Jak1/Stat1 pathway in lupus nephritis. J Ethnopharmacol. (2023) 310:116349. doi: 10.1016/j.jep.2023.116349
PubMed Abstract | Crossref Full Text | Google Scholar
10. Jin B, Liu J, Zhu Y, Lu J, Zhang Q, Liang Y, et al. Kunxian capsule alleviates podocyte injury and proteinuria by inactivating B -Catenin in Db/Db Mice. Front Med. (2023) 10:1213191. doi: 10.3389/fmed.2023.1213191
PubMed Abstract | Crossref Full Text | Google Scholar
11. Tao W, Xu X, Wang X, Li B, Wang Y, Li Y, et al. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal radix curcumae formula for application to cardiovascular disease. J Ethnopharmacol. (2013) 145:1–10. doi: 10.1016/j.jep.2012.09.051
PubMed Abstract | Crossref Full Text | Google Scholar
12. Xu X, Zhang W, Huang C, Li Y, Yu H, Wang Y, et al. A novel chemometric method for the prediction of human oral bioavailability. Int J Mol Sci. (2012) 13:6964–82. doi: 10.3390/ijms13066964
PubMed Abstract | Crossref Full Text | Google Scholar
13. Bateman A, Martin M-J, Orchard S, Magrane M, Alpi E, Bely B, et al. Uniprot: A worldwide hub of protein knowledge. Nucleic Acids Res. (2019) 47:D506–15. doi: 10.1093/nar/gky1049
PubMed Abstract | Crossref Full Text | Google Scholar
14. Amberger JS, Bocchini CA, Schiettecatte F, Scott AF, Hamosh A. Omim.Org: Online Mendelian Inheritance in Man (Omim§), an online catalog of human genes and genetic disorders. Nucleic Acids Res. (2015) 43:D789–98. doi: 10.1093/nar/gku1205
PubMed Abstract | Crossref Full Text | Google Scholar
15. Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, et al. The genecards suite: From gene data mining to disease genome sequence analyses. Curr Protoc Bioinform. (2016) 54:1.30.1–33. doi: 10.1002/cpbi.5
PubMed Abstract | Crossref Full Text | Google Scholar
16. Rojas-Rivera J, Fernandez-Juarez G, Ortiz A, Hofstra J, Gesualdo L, Tesar V, et al. A European multicentre and open-label controlled randomized trial to evaluate the efficacy of sequential treatment with tacrolimus-rituximab versus steroids plus cyclophosphamide in patients with primary membranous nephropathy: The Starmen Study. Clin Kidney J. (2015) 8:503–10. doi: 10.1093/ckj/sfv075
PubMed Abstract | Crossref Full Text | Google Scholar
17. Ramachandran R, Yadav AK, Kumar V, Pinnamaneni VST, Nada R, Ghosh R, et al. Two-year follow-up study of membranous nephropathy treated with tacrolimus and corticosteroids versus cyclical corticosteroids and cyclophosphamide. Kidney Int Rep. (2017) 2:610–6. doi: 10.1016/j.ekir.2017.02.004
PubMed Abstract | Crossref Full Text | Google Scholar
18. Qiu TT, Zhang C, Zhao HW, Zhou JW. Calcineurin inhibitors versus cyclophosphamide for idiopathic membranous nephropathy: A systematic review and meta-analysis of 21 clinical trials. Autoimmun Rev. (2017) 16:136–45. doi: 10.1016/j.autrev.2016.12.005
PubMed Abstract | Crossref Full Text | Google Scholar
19. Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. (2019) 381:36–46. doi: 10.1056/NEJMoa1814427
PubMed Abstract | Crossref Full Text | Google Scholar
20. Sethi S, Kumar S, Lim K, Jordan SC. Obinutuzumab is effective for the treatment of refractory membranous nephropathy. Kidney Int Rep. (2020) 5:1515–8. doi: 10.1016/j.ekir.2020.06.030
PubMed Abstract | Crossref Full Text | Google Scholar
21. Miao H, Zhang Y, Yu X, Zou L, Zhao Y. Membranous nephropathy: Systems biology-based novel mechanism and traditional Chinese medicine therapy. Front Pharmacol. (2022) 13:969930. doi: 10.3389/fphar.2022.969930
PubMed Abstract | Crossref Full Text | Google Scholar
22. Liu S, Li X, Li H, Liang Q, Chen J, Chen J. Comparison of Tripterygium wilfordii multiglycosides and tacrolimus in the treatment of idiopathic membranous nephropathy: A prospective cohort study. BMC Nephrol. (2015) 16:200. doi: 10.1186/s12882-015-0199-x
留言 (0)