A consensus has been reached that all bioprosthetic valves will inevitably fail over time (1). One of the life-threatening complications of surgical aortic valve replacement (SAVR) is prosthetic valve endocarditis (PVE). ESC guidelines (2) recommend early surgery in patients with PVE with heart failure (HF), severe prosthetic dysfunction, abscess, or persistent fever. ACC/AHA guidelines (3) recommend early surgery in patients with PVE who have relapsing infection, while transcatheter aortic valve replacement (TAVR) is an “off-label” treatment, because without complete removal of the infected tissue, the risk of relapse or reinfection will persist. Notably, a recent study showed that 32.5% of patients with infective endocarditis (IE) were considered high risk for surgery (4), making TAVR an effective rescue treatment in select cases or a bridge to SAVR (5–9). However, older patients receiving TAVR are more likely to have prothrombotic comorbidities, and unlike SAVR, which removes native leaflets, TAVR creates neosinuses with the implantation of a transcatheter aortic valve in situ (10). This anatomical difference may lead to blood flow stagnation and subclinical leaflet thrombosis (SLT) (11). According to ESC guidelines (12, 13), the first-line antithrombotic therapy is single antiplatelet therapy (SAPT), while oral anticoagulation (OAC) is recommended only if there is an indication such as atrial fibrillation. Furthermore, ACC/AHA guidelines recommend dual antiplatelet therapy (DAPT) or vitamin K antagonist (VKA) in patients with a low risk of bleeding (3). Some studies have reported that patients with SLT managed with anticoagulation regimes, whether with VKA or with non-vitamin K antagonist oral anticoagulants (NOAC), showed regression (14, 15).
2 Case presentationWe report the case of a 70-year-old female patient with bioprosthetic valve failure (BVF) and prior PVE complicated by an ascending aortic aneurysm and a coronary artery bypass graft (CABG). The patient underwent a valve-in-valve (ViV) procedure in June 2023. Unfortunately, she required multiple rehospitalizations due to delayed coronary obstruction (CO) and HF and subsequently died. Her medical history is as follows: She underwent SAVR and CABG in May 2006 due to aortic regurgitation (AR) and a 50% stenosis at the ostia of the first diagonal branch. Her native aortic valve was replaced by a 23-mm Medtronic Hancock II bioprosthesis, and she was prescribed oral warfarin for 6 months, followed by life-long aspirin therapy. However, the patient chose to discontinue aspirin because of subcutaneous hemorrhage. In June 2017, she was readmitted due to recurrent symptoms of fatigue, and Holter monitor showed a third-degree atrioventricular block, leading to the implantation of a pacemaker in the following month. Meanwhile, a transthoracic echocardiography (TTE) demonstrated prosthetic aortic valve stenosis with a mean pressure gradient (MPG) of 42 mmHg and a peak velocity (PV) of 4.4 m/s, and the patient declined a redo SAVR. She was followed up annually by TTE; she had an MPG of 21–41 mmHg and a PV of 3.1–4.4 m/s, and the left ventricular ejection fraction (LVEF) declined from 61% in June 2017 to 44% in April 2023. In addition, TTE showed a dilation of the ascending aorta with a stable diameter of 45 mm during follow-up. In October 2022, she presented with a fever and a body temperature of 39.0°C. A blood culture was positive for Enterococcus faecalis, and an aortic bioprosthesis vegetation was confirmed using TTE; therefore, she was diagnosed with PVE, which was treated with intravenous daptomycin and ampicillin for 8 weeks. In April 2023, she was readmitted with angina, following which she underwent coronary angiography (CAG), which showed a patency of the saphenous vein graft (SVG) and right coronary artery (RCA). Subsequently, in June, she was admitted for congestive HF symptoms such as exertional dyspnea, orthopnea, and peripheral edema, and the initial treatment plan involved a redo SAVR.
At admission, the patient's laboratory tests showed the following findings: white blood cell (WBC) count, 6.14 × 109/L; hemoglobin, 111.00 g/L; platelet count, 162.00 × 109/L; blood creatinine, 100.00 μmol/L; estimated glomerular filtration rate, 49.64; aspartate aminotransferase (AST), 13.50 U/L; alanine amino transferase (ALT), 6.90 U/L; and B-type natriuretic peptide (BNP) 469.71 pg/ml. A preoperative transesophageal echocardiogram (TEE) demonstrated thickened leaflets of the prosthetic aortic valve, mild-to-moderate transvalvular regurgitation, mild paravalvular regurgitation, and prosthetic aortic valve stenosis (Figures 1A,B) with an MPG of 23 mmHg, a PV of 3.11 m/s, an left ventricular end-diastolic diameter (LVEDD) of 56 mm, and an LVEF of 43%. TEE also showed moderate mitral regurgitation (MR), mild-to-moderate tricuspid regurgitation (TR), and mild pulmonary hypertension with an estimated pulmonary artery systolic pressure (PASP) of 38 mmHg. A computed tomography (CT) scan showed a patent SVG with a mild stenosis of the left anterior descending artery (LAD) and an ascending aortic aneurysm (Figure 2A). The distances between the annulus and the ostia of the left coronary artery (LCA) and RCA were 30.7 and 24.4 mm, respectively, and the size of the sinus of Valsalva was 40.5 mm × 51.1 mm (Figures 2B–D).
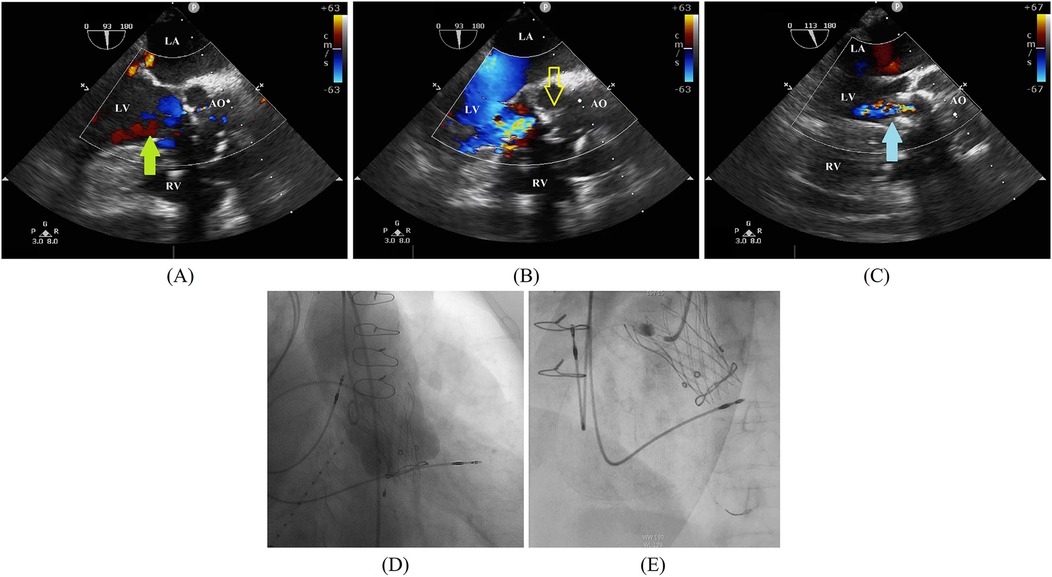
Figure 1. (A,B) A preoperative transesophageal echocardiogram demonstrates left coronary sinus perivalvular leakage (green arrow) and a thickened leaflet (yellow outlined arrow). (C) A postimplantation transesophageal echocardiogram demonstrates the absence of paravalvular regurgitation. A self-expanding valve bottom skirt fits left ventricular outflow to eliminate perivalvular regurgitation (blue arrow). (D) A final angiogram further confirms the absence of valvular or paravalvular regurgitation. (E) Coronary angiography demonstrates an obstruction of the proximal RCA. AO, ascending aorta; LA, left atrium; LV, left ventricular; RV, right ventricular.
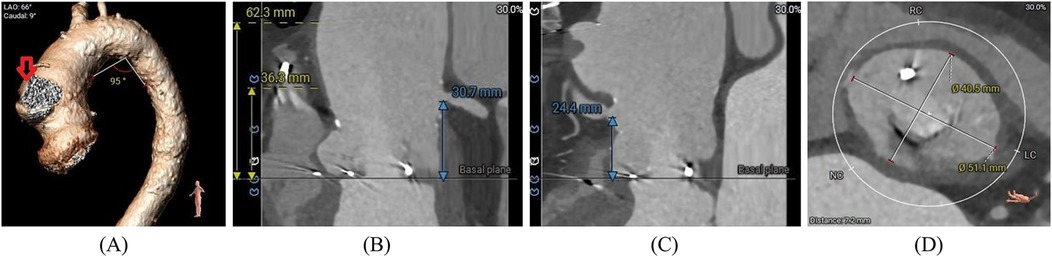
Figure 2. (A) Preoperative cardiac-gated CT images were utilized to create a 3D printed model with true-size aortic root dimensions, demonstrating a pseudoaneurysm (red arrow) of the ascending aorta. (B,C) The distance between the annulus and the ostium of the LCA and RCA. (D) The diameter of the sinus of Valsalva.
Given the complex anatomy of the aortic root and high surgical risk (STS Score: 8.09%, EuroSCORE II: 32.28%), the patient underwent a ViV procedure with the implantation of a TaurusElite 23-mm self-expandable valve under general anesthesia. A postimplantation TEE demonstrated no paravalvular regurgitation and gradient across the repaired aortic valve with a peak/mean pressure gradient of 30/15 mmHg (Figure 1C). The final angiography further confirmed no transvalvular or paravalvular regurgitation (Figure 1D). Her postoperative courses were uneventful. TTE performed 2 days postoperatively showed a well-functioning transcatheter aortic valve with no transvalvular or paravalvular regurgitation, an MPG of 18 mmHg, a PV of 3.05 m/s, and an LVEF of 47.5%. The patient was discharged after 7 days with a prescription of oral warfarin for at least 3 months. The international normalized ratio (INR) of the patient was 1.2 before discharge. However, the patient did not monitor her INR regularly after discharge, the value of which was not within the therapeutic range. She received follow-up treatment 2 weeks after discharge, and her INR increased to 2.38; subsequently, she was lost to follow-up.
In August 2023, approximately 2 months after surgery, the patient was admitted to the emergency department for angina with ECG findings of ST-segment elevation and a cardiac troponin T level of 5.530 ng/ml. The BNP level was 655.48 pg/ml and the INR was 1.31. A TTE demonstrated no dysfunction of the transcatheter aortic valve and an LVEF of 44%. Type 1 myocardial infarction (MI) was determined and an urgent CAG was performed, which showed a complete occlusion of the proximal RCA with thrombolysis in myocardial infarction (TIMI) flow grade 0 (Figure 1E). A coronary thrombus aspiration was done, and a subsequent CAG revealed an occlusion in the distal posterior left ventricular artery (PLA), which was dilated with a balloon. The CAG showed no stenosis in the RCA with TIMI flow grade 3; however, a residual lesion was observed in the distal PLA with TIMI flow grade 0. In addition, a CT scan taken before the CAG showed a patency of the left main stem and left anterior descending artery, a mild stenosis of the circumflex artery, and a patency of the SVG. Two days after the operation, the patient developed dyspnea and elevated central venous pressure; after medical treatment, her symptoms were relieved. She was discharged on day 13 with an SAPT of oral clopidogrel and anticoagulation therapy of oral warfarin. The INR before discharge was 2.89.
In September 2023, approximately 2 weeks after her last hospitalization, she was readmitted to the emergency department for acute HF. At admission, the BNP level was 2,930 pg/ml, and it rapidly increased to over 5,000 pg/ml within hours. The INR was critically high (9.74) with a worsened liver function (AST 385.50 U/L; ALT 311.90 U/L) and a low platelet count of 54.00 × 109/L. A high WBC count of 17.65 × 109/L and procalcitonin level of 6.27 ng/ml also indicated severe infection. Kidney injury was indicated by a high blood creatinine level of 268.00 μmol/L and a potassium level of 5.82 mmol/L. A TTE showed no transvalvular or paravalvular regurgitation of the transcatheter aortic valve with a moderate MR, a moderate-to-severe TR, mild pulmonary hypertension (estimated PASP 38 mmHg), and an LVEF of 38%. Following a preliminary diagnosis of acute HF with infection and multiorgan failure, she was transferred to the intensive care unit. She suddenly developed ventricular fibrillation, and resuscitation was unsuccessful; she was declared clinically dead on Day 19.
2.1 TimelineThe timeline is shown in Figure 3.
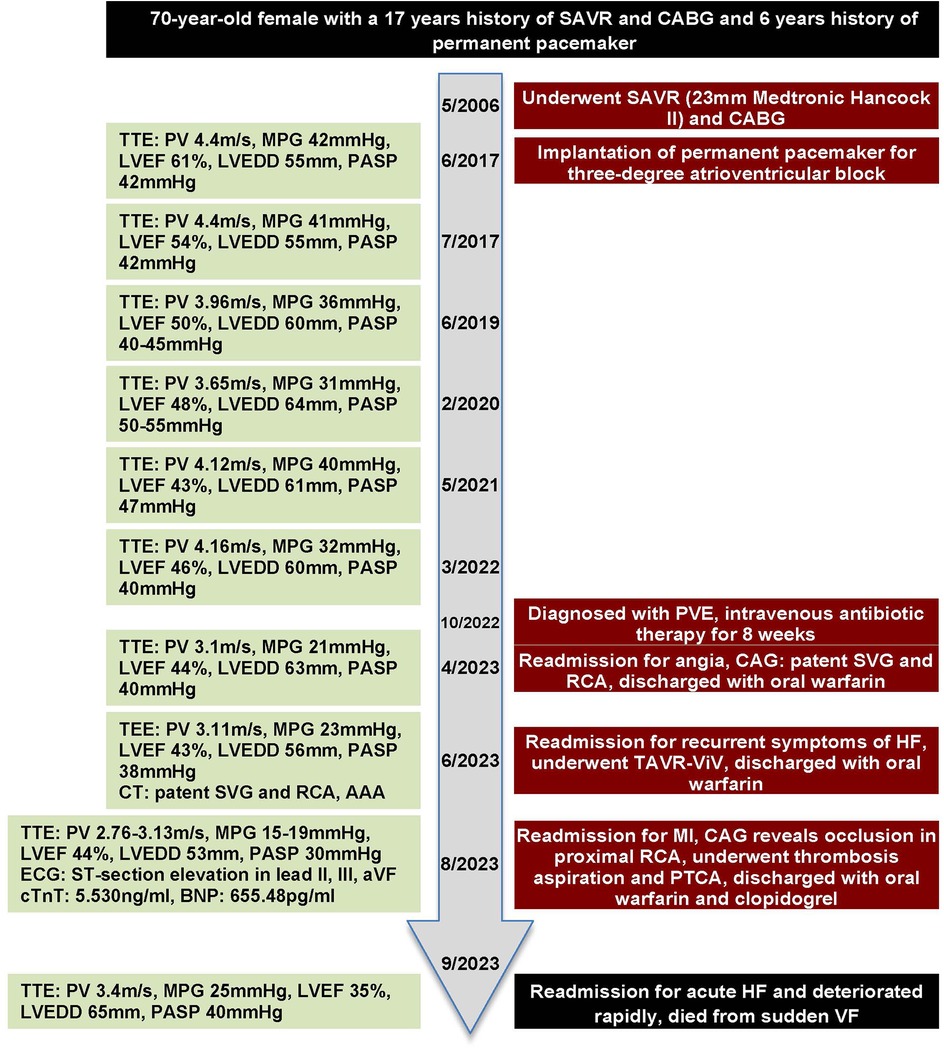
Figure 3. Timeline with key parameters of echocardiography and events throughout this case.
3 DiscussionMost patients with BVF are older and usually have comorbidities (1). With studies showing comparable early mortality compared with redo SAVR, a ViV procedure in patients with high or prohibitive surgical risk is supported by the current guidelines (2, 3).
According to the EURO-ENDO registry, only 73.9% of patients with PVE who were indicated for surgery finally underwent one (16). Several case reports have also demonstrated that TAVR can act as a rescue treatment for patients with IE having prohibitive surgical risk and presenting with cardiogenic shock or multiorgan failure (6, 8, 9, 17–20). Moreover, a recent multicenter study showed the feasibility and safety of TAVR in treating “healed” IE with residual AR (5).
Although the patient in this report had stable hemodynamic parameters and no signs of sepsis at admission, the anatomical complexity of the ascending aorta made the surgery considerably challenging; therefore, the heart team decided to perform a ViV procedure. Retrospectively speaking, the risk of CO was considered relatively low due to the fairly safe height of the coronary ostia (>12 mm) and optimal diameter of the sinus of Valsalva (>30 mm) (21) measured by the preoperative CT scan. Moreover, Medtronic Hancock II, as a stented bioprosthetic valve, is not considered a risk factor for CO according to the data from a large multicenter registry (22). However, ViV is associated with a higher risk of CO, which occurs in <1% patients postnative valve TAVR (23, 24) and in 2.3% of patients post ViV (22). Percutaneous coronary intervention (PCI) is attempted in most patients with ST-segment elevation MI after TAVR; however, self-expandable valves present a challenge in terms of coronary access for CAG and PCI. Moreover, without successful revascularization, patients have an increased risk of mortality (25). Although the cause of CO is hard to determine, we suspect a potential thrombotic origin.
SLT is indicated by CT revealing hypoattenuated leaflet thickening (HALT) and restricted leaflet motion (RLM) (26, 27). The reported rates of incidence of SLT range from 11% to 54.1% and vary across studies with different rates of time-to-first detection (14, 28–32). SLT has been a recognized event since Makkar et al. (33) first described it, and CT was mandated in clinical trials by the FDA. However, with the accumulated data from clinical trials and registries, more questions than answers have arisen. First, the time course of SLT remains unclear as its occurrence varies from weeks to months postoperatively. In most clinical trials in the past, the first CT scan was conducted as per protocol at 1 month (28, 29) or 3 months (30, 32) after TAVR; however, in real-world settings, the decision to conduct a CT scan is often left to the discretion of the treating physician. Furthermore, some patients are unsuitable for a CT scan due to comorbidities such as renal dysfunction; hence, serial CT scans after TAVR may not be an ideal modality for the detection of SLT due to repeated exposure of patients to radiation and contrast agents. Second, the clinical significance of SLT is still under debate as patients are asymptomatic. However, valve function evaluated by TTE has yielded conflicting results. For example, a recent retrospective study (34) on the natural history of patients with HALT with a median follow-up time of 4.7 years reported that no patients with HALT were initiated anticoagulation therapy. The authors reported no significant difference in the MPG between the HALT and the no-HALT group of patients, which is consistent with the results of two previous randomized controlled trials (28, 29). However, two other studies (31, 35) with long-term follow-up showed that HALT was significantly associated with increased MPG. Third, whether SLT can be prevented by OAC remains unclear. In the Global Study Comparing a rivAroxaban-based Antithrombotic Strategy to an antipLatelet-based Strategy After Transcatheter aortIc vaLve rEplacement to Optimize Clinical Outcomes (GALILEO-4D) substudy (32), the incidence of HALT was significantly lower in patients receiving an anticoagulation of rivaroxaban with aspirin than in those receiving DAPT. However, in the main trial (36), rivaroxaban-based antithrombotic strategy was associated with an increased risk of thromboembolism complications and major bleeding. In the Anti-Thrombotic Strategy After Trans-Aortic Valve Implantation for Aortic Stenosis (ATLANTIS-4D-CT) substudy (30), apixaban reduced the incidence of HALT or RLM without increasing the risk of thromboembolic or bleeding events. Some authors have suggested that SLT may be spontaneously resolved irrespective of whether OAC therapy is initiated (28, 29). In the current case, although the patient was administered warfarin after TAVR, she eventually developed leaflet thrombosis. With such unanswered questions, we conclude that it is best to personalize anticoagulation therapy and CT scans. The ongoing The Nordic Aortic Valve Intervention-4 (NOTION-4) trial (37), which will randomize patients to anticoagulation or SAPT, may provide insights on HALT after TAVR and its antithrombotic management.
4 ConclusionA ViV procedure may serve as a potential therapy in select patients with PVE; however, the prognosis worsens when leaflet thrombosis leads to CO and MI. TEE is not sensitive enough to detect HALT after TAVR, and a CT scan should be considered when TEE shows increased MPG. SAPT after TAVR is safe but may not prevent the progression of HALT. Given the limited data from long-term follow-up, whether HALT leads to hemodynamic valve deterioration remains unclear.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statementWritten informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsYZ: Writing – original draft. BF: Writing – review & editing. NJ: Writing – review & editing. ZG: Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1529523/full#supplementary-material
References1. Clark A, Malaisrie SC. Failed bioprosthetic valve approaches: transcatheter aortic valve replacement approach. J Thorac Cardiovasc Surg. (2022) 163:1795–8. doi: 10.1016/j.jtcvs.2020.12.148
PubMed Abstract | Crossref Full Text | Google Scholar
2. Delgado V, Ajmone Marsan N, de Waha S, Bonaros N, Brida M, Burri H, et al. 2023 ESC guidelines for the management of endocarditis. Eur Heart J. (2023) 44:3948–4042. doi: 10.1093/eurheartj/ehad193
PubMed Abstract | Crossref Full Text | Google Scholar
3. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation (2021) 143:e35–71. doi: 10.1161/CIR.0000000000000932
PubMed Abstract | Crossref Full Text | Google Scholar
4. Ramos-Martínez A, Calderón-Parra J, Miró JM, Muñoz P, Rodríguez-Abella H, Valerio M, et al. Effect of the type of surgical indication on mortality in patients with infective endocarditis who are rejected for surgical intervention. Int J Cardiol. (2019) 282:24–30. doi: 10.1016/j.ijcard.2019.01.014
PubMed Abstract | Crossref Full Text | Google Scholar
5. Brankovic M, Hashemi A, Ansari J, Sharma A. Transcatheter aortic valve replacement for aortic valve infective endocarditis: a systematic review and call for action. Cardiol Ther. (2023) 12:297–306. doi: 10.1007/s40119-023-00314-9
PubMed Abstract | Crossref Full Text | Google Scholar
7. Brankovic M, Ansari J, Karanam R, Waxman S. Transcatheter aortic valve replacement as a rescue treatment for prosthetic valve endocarditis. JACC Case Rep. (2022) 4:1306–10. doi: 10.1016/j.jaccas.2022.04.022
PubMed Abstract | Crossref Full Text | Google Scholar
8. Fathi AS, Ali JM, Mann S, Taghavi J, Davies WR, Sudarshan C. Emergency valve-in-valve transcatheter aortic valve implantation for endocarditis degeneration. J Card Surg. (2020) 35:713–5. doi: 10.1111/jocs.14441
PubMed Abstract | Crossref Full Text | Google Scholar
9. Naqvi SY, Salama IG, Narins C, Stuver T. Corynebacterium striatum prosthetic valve endocarditis with severe aortic regurgitation successfully treated with transcatheter aortic valve replacement. BMJ Case Rep. (2018) 11:e226881. doi: 10.1136/bcr-2018-226881
Crossref Full Text | Google Scholar
10. Ng ACT, Holmes DR, Mack MJ, Delgado V, Makkar R, Blanke P, et al. Leaflet immobility and thrombosis in transcatheter aortic valve replacement. Eur Heart J. (2020) 41:3184–97. doi: 10.1093/eurheartj/ehaa542
PubMed Abstract | Crossref Full Text | Google Scholar
11. Koren O, Patel V, Chakravarty T, Jilaihawi H, Gupta A, Sadri S, et al. Leaflet thrombosis in transcatheter aortic valve intervention: mechanisms, prevention, and treatment options. Front Cardiovasc Med. (2023) 10:1249604. doi: 10.3389/fcvm.2023.1249604
PubMed Abstract | Crossref Full Text | Google Scholar
12. Ten Berg J, Sibbing D, Rocca B, Van Belle E, Chevalier B, Collet JP, et al. Management of antithrombotic therapy in patients undergoing transcatheter aortic valve implantation: a consensus document of the esc working group on thrombosis and the European Association of Percutaneous Cardiovascular Interventions (EAPCI), in collaboration with the ESC Council on Valvular Heart Disease. Eur Heart J. (2021) 42:2265–9. doi: 10.1093/eurheartj/ehab196
PubMed Abstract | Crossref Full Text | Google Scholar
13. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43:561–632. doi: 10.1093/eurheartj/ehab395
PubMed Abstract | Crossref Full Text | Google Scholar
14. Chakravarty T, Søndergaard L, Friedman J, De Backer O, Berman D, Kofoed KF, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet. (2017) 389:2383–92. doi: 10.1016/S0140-6736(17)30757-2
PubMed Abstract | Crossref Full Text | Google Scholar
15. Sondergaard L, De Backer O, Kofoed KF, Jilaihawi H, Fuchs A, Chakravarty T, et al. Natural history of subclinical leaflet thrombosis affecting motion in bioprosthetic aortic valves. Eur Heart J. (2017) 38:2201–7. doi: 10.1093/eurheartj/ehx369
PubMed Abstract | Crossref Full Text | Google Scholar
16. Habib G, Erba PA, Iung B, Donal E, Cosyns B, Laroche C, et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Eur Heart J. (2019) 40:3222–32. doi: 10.1093/eurheartj/ehz620
PubMed Abstract | Crossref Full Text | Google Scholar
17. Kefer J, Vancraeynest D, Roelants V, Belkhir L. Valve-in-valve transcatheter aortic valve implantation (TAVI): a new valuable approach to bioprosthetic infective endocarditis? Eur Heart J Case Rep. (2024) 8:ytae384. doi: 10.1093/ehjcr/ytae384
PubMed Abstract | Crossref Full Text | Google Scholar
18. Shen CP, Munsayac MA, Robinson AA, Stinis CT. Transcatheter aortic valve replacement: a palliative approach to infective endocarditis. BMJ Case Rep. (2022) 15:e248951. doi: 10.1136/bcr-2022-248951
PubMed Abstract | Crossref Full Text | Google Scholar
19. Richards GHC, Joshi N, Turner M, Dorman S. Transcatheter aortic valve implantation in severe native pure aortic regurgitation following endocarditis with large vegetation. JACC Case Rep. (2021) 3:859–63. doi: 10.1016/j.jaccas.2021.03.020
PubMed Abstract | Crossref Full Text | Google Scholar
20. Vila-Olives R, Oristrell G, Rello P, Fernández-Hidalgo N. Transcatheter aortic valve replacement for acute aortic regurgitation due to Staphylococcus aureus infective endocarditis complicated with a perivalvular abscess: a case report. Eur Heart J Case Rep. (2023) 7:ytad166. doi: 10.1093/ehjcr/ytad166
PubMed Abstract | Crossref Full Text | Google Scholar
21. Ribeiro HB, Webb JG, Makkar RR, Cohen MG, Kapadia SR, Kodali S, et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol. (2013) 62:1552–62. doi: 10.1016/j.jacc.2013.07.040
PubMed Abstract | Crossref Full Text | Google Scholar
22. Ribeiro HB, Rodés-Cabau J, Blanke P, Leipsic J, Kwan Park J, Bapat V, et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: insights from the vivid registry. Eur Heart J. (2018) 39:687–95. doi: 10.1093/eurheartj/ehx455
PubMed Abstract | Crossref Full Text | Google Scholar
23. Jabbour RJ, Tanaka A, Finkelstein A, Mack M, Tamburino C, Van Mieghem N, et al. Delayed coronary obstruction after transcatheter aortic valve replacement. J Am Coll Cardiol. (2018) 71:1513–24. doi: 10.1016/j.jacc.2018.01.066
PubMed Abstract | Crossref Full Text | Google Scholar
24. Ojeda S, González-Manzanares R, Jiménez-Quevedo P, Piñón P, Asmarats L, Amat-Santos I, et al. Coronary obstruction after transcatheter aortic valve replacement: insights from the Spanish TAVI registry. JACC Cardiovasc Interv. (2023) 16:1208–17. doi: 10.1016/j.jcin.2023.03.024
PubMed Abstract | Crossref Full Text | Google Scholar
25. Faroux L, Lhermusier T, Vincent F, Nombela-Franco L, Tchétché D, Barbanti M, et al. ST-segment elevation myocardial infarction following transcatheter aortic valve replacement. J Am Coll Cardiol. (2021) 77:2187–99. doi: 10.1016/j.jacc.2021.03.014
PubMed Abstract | Crossref Full Text | Google Scholar
26. Blanke P, Weir-McCall JR, Achenbach S, Delgado V, Hausleiter J, Jilaihawi H, et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR): an expert consensus document of the Society of cardiovascular Computed Tomography. JACC Cardiovasc Imaging. (2019) 12:1–24. doi: 10.1016/j.jcmg.2018.12.003
PubMed Abstract | Crossref Full Text | Google Scholar
27. Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, et al. Valve academic research consortium 3. Updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol. (2021) 77:2717–46. doi: 10.1016/j.jacc.2021.02.038
Crossref Full Text | Google Scholar
28. Blanke P, Leipsic JA, Popma JJ, Yakubov SJ, Deeb GM, Gada H, et al. Bioprosthetic aortic valve leaflet thickening in the Evolut low risk sub-study. J Am Coll Cardiol. (2020) 75:2430–42. doi: 10.1016/j.jacc.2020.03.022
PubMed Abstract | Crossref Full Text | Google Scholar
29. Makkar RR, Blanke P, Leipsic J, Thourani V, Chakravarty T, Brown D, et al. Subclinical leaflet thrombosis in transcatheter and surgical bioprosthetic valves: partner 3 cardiac computed tomography substudy. J Am Coll Cardiol. (2020) 75:3003–15. doi: 10.1016/j.jacc.2020.04.043
PubMed Abstract | Crossref Full Text | Google Scholar
30. Montalescot G, Redheuil A, Vincent F, Desch S, De Benedictis M, Eltchaninoff H, et al. Apixaban and valve thrombosis after transcatheter aortic valve replacement: the Atlantis-4D-CT randomized clinical trial substudy. JACC Cardiovasc Interv. (2022) 15:1794–804. doi: 10.1016/j.jcin.2022.07.014
PubMed Abstract | Crossref Full Text | Google Scholar
31. Zhou Q, Wen J, Zhu Q, Fan J, Guan X, Chen X, et al. Long-term prosthetic-associated subclinical thrombotic events evaluation by cardiac CTA after transcatheter aortic valve implantation: incidence and outcomes. Insights Imaging. (2024) 15:125. doi: 10.1186/s13244-024-01681-0
PubMed Abstract | Crossref Full Text | Google Scholar
32. De Backer O, Dangas GD, Jilaihawi H, Leipsic JA, Terkelsen CJ, Makkar R, et al. Reduced leaflet motion after transcatheter aortic-valve replacement. N Engl J Med. (2020) 382:130–9. doi: 10.1056/NEJMoa1911426
PubMed Abstract | Crossref Full Text | Google Scholar
33. Makkar RR, Fontana G, Jilaihawi H, Chakravarty T, Kofoed KF, De Backer O, et al. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med. (2015) 373:2015–24. doi: 10.1056/NEJMoa1509233
PubMed Abstract | Crossref Full Text | Google Scholar
34. Imaeda S, Inohara T, Yoshijima N, Kobari Y, Myojin S, Ryuzaki T, et al. Natural history of leaflet thrombosis after transcatheter aortic valve replacement: a 5-year follow-up study. J Am Heart Assoc. (2022) 11:e026334. doi: 10.1161/JAHA.122.026334
PubMed Abstract | Crossref Full Text | Google Scholar
35. Hein M, Schoechlin S, Schulz U, Minners J, Breitbart P, Lehane C, et al. Long-term follow-up of hypoattenuated leaflet thickening after transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2022) 15:1113–22. doi: 10.1016/j.jcin.2022.04.018
PubMed Abstract | Crossref Full Text | Google Scholar
36. Dangas GD, Tijssen JGP, Wöhrle J, Søndergaard L, Gilard M, Möllmann H, et al. A controlled trial of rivaroxaban after transcatheter aortic-valve replacement. N Engl J Med. (2020) 382:120–9. doi: 10.1056/NEJMoa1911425
PubMed Abstract | Crossref Full Text | Google Scholar
37. Thuraiaiyah J, Jørgensen TH, Jensen JM, Fuchs A, Willemen Y, Terkelsen CJ, et al. Prospective study on the impact of different antithrombotic therapies on subclinical leaflet thickening and its temporal dynamics in transcatheter aortic valves—the NOTION-4 trial. Am Heart J. (2025) 279:1–8. doi: 10.1016/j.ahj.2024.10.002
留言 (0)