Autism Spectrum Disorder, commonly known as ASD, is a human brain disorder characterized by lack of communication skills, impairment in cognitive abilities, repetitive behavior, and restrictive social interaction (American Psychiatric Association et al., 2013). It is referred to as a “spectrum" because of the wide range of characteristics in the subjects who suffer from ASD. The global burden of ASD is also significant, as ASD has been found in 204 countries worldwide (Solmi et al., 2022) across diverse groups of communities. A comprehensive review (Qiu et al., 2020) of 12 studies consisting of more than 2.0 million people found the prevalence of ASD in Asia to be 0.36%.
1.1 Resting state functional magnetic resonance imaging (rs-fMRI)fMRI studies (Walsh et al., 2021; Zhang et al., 2020; Liu et al., 2021) on human brain disorders are getting popular recently as they are being used in a range of human brain disorders such as ASD, ADHD (Tang et al., 2018), schizophrenia (Hoptman et al., 2024) and Alzheimer's (Lajoie et al., 2017). The fundamental idea behind fMRI is to study the Blood Oxygenation Level Dependent (BOLD) signal from the voxels (the volume of the human brain is divided into smaller cubes to extract signals) of the human brain, which acts as a proxy for the original neuronal activity in a particular region. Resting-state fMRI studies (Cole et al., 2010; Santana et al., 2022; Van Den Heuvel and Pol, 2010) are different from task-based fMRI studies because in these studies, subjects did not perform any task, and the BOLD signal measured in this case extracts the intrinsic and spontaneous activities of the human brain. The raw fMRI dataset acquired from the human subject's brain is 4D, as it consists of a 3D set of images taken across the time dimension, therefore, there is a preprocessing stage that follows after acquiring the fMRI data on a particular brain disorder. These preprocessing steps include slice time correction, motion correction, normalization, co-registration, and noise removal. Some of the most crucial preprocessing steps are shown in Figure 1 where the “co-registration" step, in which the human brain is mapped to the known brain atlases and the Regions of Interests (ROIs) are selected, is the main area of focus of the current short investigation.
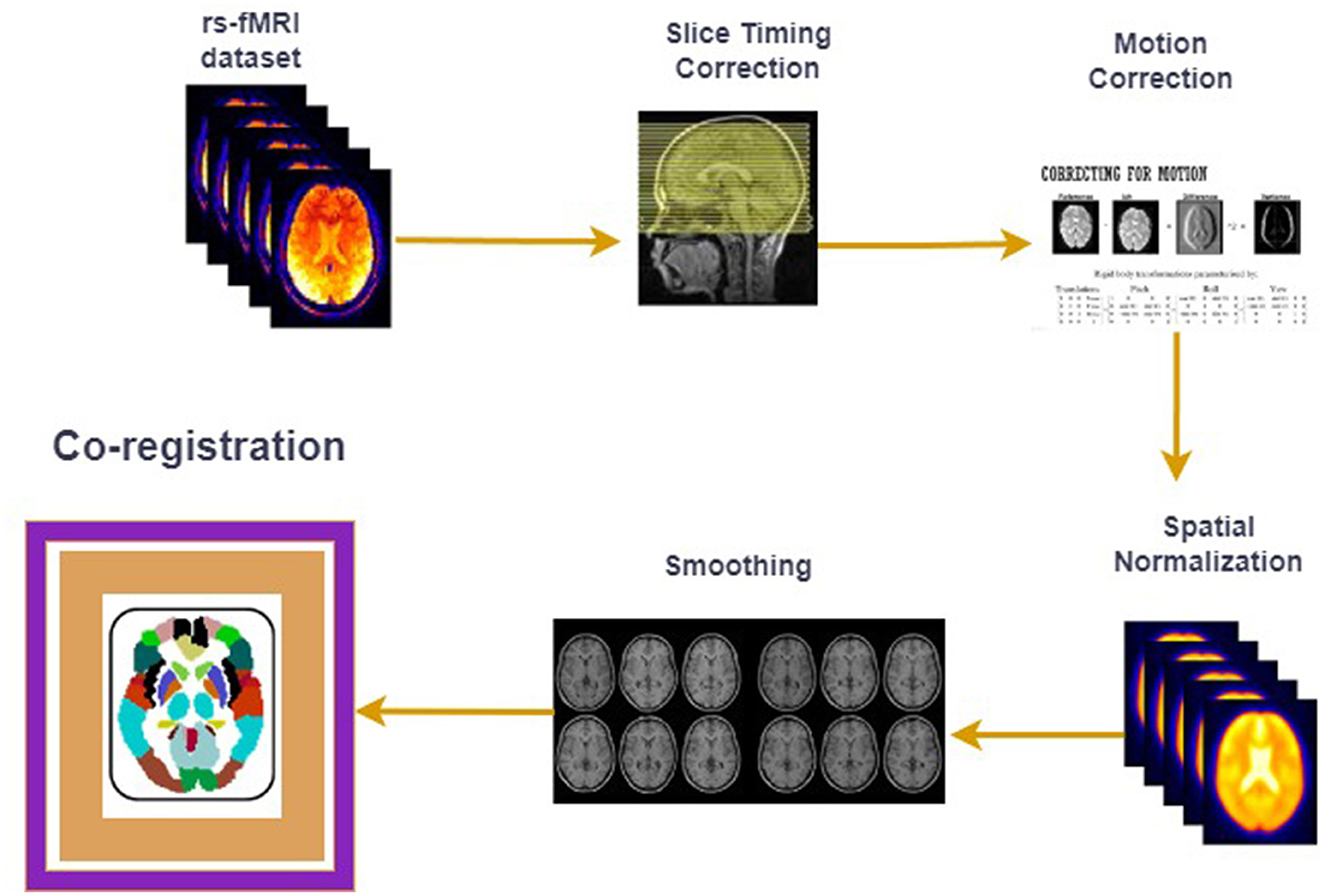
Figure 1. fMRI preprocessing pipeline.
1.2 Brain atlasesBrain atlases are essential tools in neuroimaging studies and serve as reference frameworks that divide the brain into distinct regions of interest (ROIs). These atlases facilitate the study of connectivity patterns and neural dynamics by enabling the standardized analysis of fMRI data. In the context of Autism Spectrum Disorder (ASD), brain atlases provide critical insights into underlying neural disruptions, allowing researchers to systematically examine abnormalities in brain connectivity that are characteristic of the disorder. These brain atlases are used to standardize and simplify the process of analyzing complex brain data using neuroimaging techniques, such as fMRI. Brain atlases can be categorized into two classes, “Anatomical” and “Functional” where the anatomical atlas is related to the physical structures of the brain and the functional atlas is related to the connectivity patterns and functional networks in the brain. The commonly used human brain atlases used in the fMRI prep-processing pipeline are listed below: Each human brain is anatomically different, and without an atlas, it would be difficult to compare brain regions across different individuals. These listed atlases segment the brain into regions of interest (ROIs) based on structural (e.g., AAL Atlas Tzourio-Mazoyer et al., 2002) or functional characteristics (e.g., Yeo's Networks Yeo et al., 2011), allowing researchers to consistently study specific regions across subjects. Associating an ROI with a group of voxels helps aggregate the data from small units (voxels) into meaningful brain regions, making the analysis more interpretable and reducing noise. Voxels, as already defined, represent small volumetric pixels in the brain, but by grouping them into ROIs, researchers can capture the activity or connectivity of larger, anatomically or functionally coherent brain regions, which is especially useful for understanding how different parts of the brain interact (functional connectivity) and for studying disease-related changes in specific brain areas. It also allows for reproducibility across studies, because the same ROIs can be studied using different datasets. Standardizing analyses using these atlases improves the reliability of findings in fields such as neurodevelopmental disorders, cognitive neuroscience, and brain mapping.
Mapping brain atlases in defining ASD relies on their ability to isolate and quantify alterations in brain connectivity. As discussed in Ribeiro da Costa et al. (2022), Chiong et al. (2013), and Harikumar et al. (2021), disruptions in functional networks, such as the Default Mode Network (DMN), Salience Network, and Social Brain Network, are well documented in ASD. Atlases with finer granularity, such as the CC400, provide high-resolution insights into these networks, allowing researchers to capture subtle variations in connectivity. Conversely, coarser atlases, such as AAL, offer computational efficiency by the summarizing brain activity across broader regions. Both approaches provide complementary insights, underscoring the need to select an appropriate atlas based on a specific research question. The dynamics between different atlases also play a crucial role in the understanding of ASD. Each atlas captures distinct aspects of brain organization, and their selection can significantly influence the outcomes of classification studies; coarser atlases such as AAL may miss fine-grained connectivity details but are less prone to overfitting in small datasets, and denser atlases such as CC400 offer detailed connectivity patterns but require larger datasets and more computational resources. Recent studies (Hasana et al., 2024; Heczko et al., 2023) have suggested that combining multiple atlases may yield a more comprehensive understanding of ASD by leveraging the strengths of both anatomical and functional atlases. For instance, researchers could use a coarse atlas such as AAL to identify broad connectivity disruptions and then apply a dense atlas such as CC400 to examine specific regions in detail. This layered approach could provide a deeper understanding of complex neural interactions in ASD.
Furthermore, the process of mapping brain atlases in ASD studies is closely tied to the choice of machine learning and deep learning methods. Feature-based approaches often rely on predefined connectivity metrics extracted from these atlases, whereas end-to-end learning models can directly leverage raw fMRI data, incorporating atlas-based parcellation (“parcellation" is the process of dividing the brain into smaller regions) as input layers. These methodological differences highlight the need to carefully align atlas selection with the study's objectives and the computational framework employed. The five commonly used brain atlases in the reviewed studies are as follows.
1. Automated Anatomical Labeling (AAL) (Tzourio-Mazoyer et al., 2002), which divides the brain into 116 ROIs.
2. Harvard-Oxford (Desikan et al., 2006) which is anatomical and divides the brain regions into 48 ROIs.
3. The Craddock-200 (Craddock et al., 2012) is functional and divides the brain regions into 200 ROIs.
4. CC400 (Craddock et al., 2012) is functional and divides the brain regions into 400 ROIs.
5. Yeo 7/17 (Yeo et al., 2011) is functional and divides the brain regions into 114 ROIs.
1.3 Role of atlasesWe now briefly explain the state-of-the-art approaches from the literature, the choice of atlas, and the performance of the proposed approach in relation to the selected atlases. An enumeration of various studies, their methodologies, selection of atlas, results, and limitations are also mentioned in Table 1.
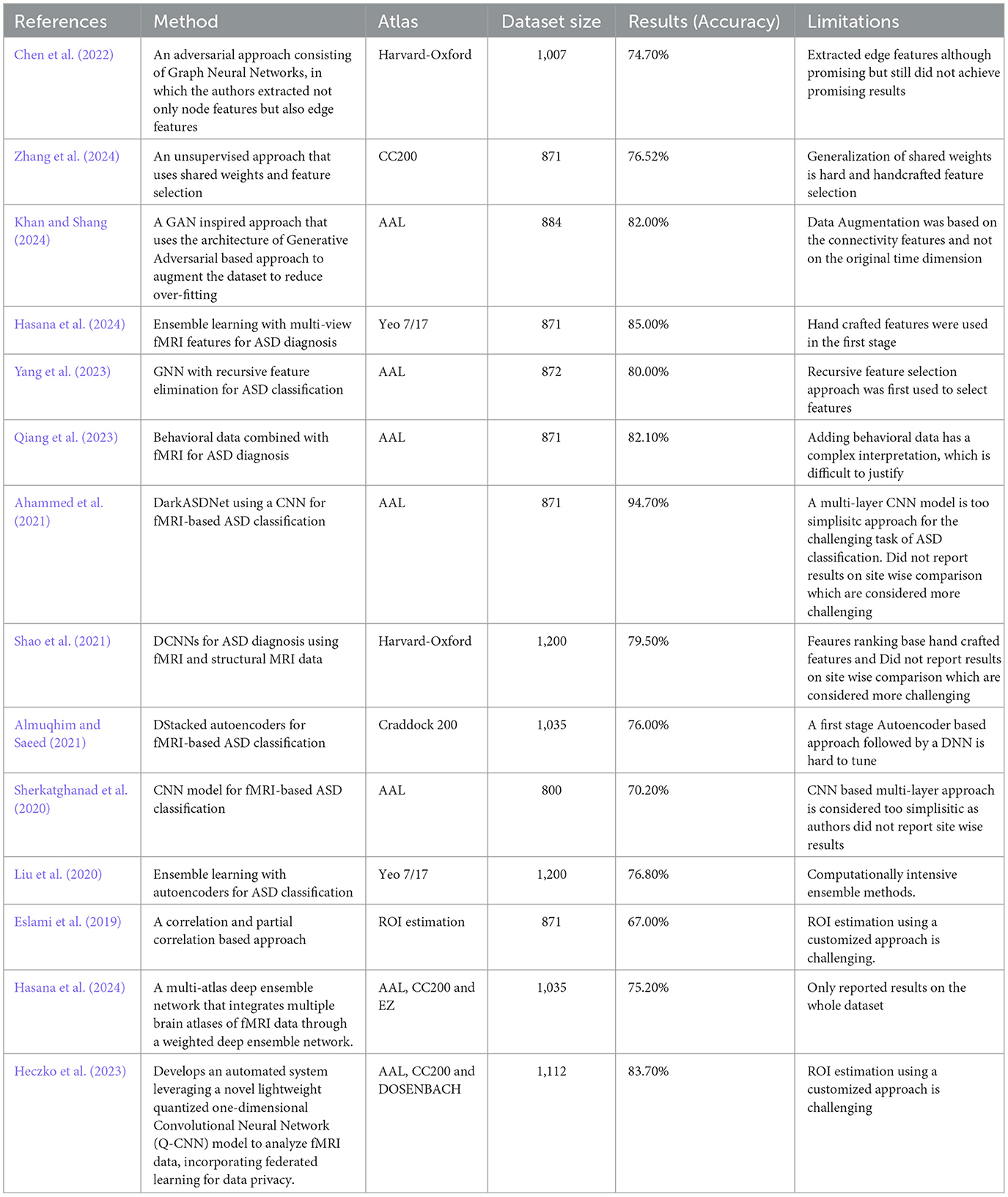
Table 1. Related studies on the classification of ASD.
In Chen et al. (2022), the authors used adversarial graph neural networks for fMRI feature extraction and ASD classification. They applied the Harvard-Oxford atlas to 900 subjects from the ABIDE and HCP datasets and achieved 83.1% accuracy. The approach was based on feature selection, with a focus on robust fMRI feature extraction. One limitation is that adversarial methods may introduce instability in training, require hand-crafted features (hand-crafted features are manually chosen by the researchers, like picking the best), and require careful balancing during optimization.
ASD-SWNet (Zhang et al., 2024) proposed a shared-weight feature extraction and CNN-based classification model for ASD diagnosis. Using the CC200 atlas of the ABIDE dataset (871 subjects), an accuracy of 76.52% was achieved. The approach was end-to-end; however, a limitation is the challenge of generalizing shared-weight networks to other neuroimaging datasets.
ASD-GANNET (Khan and Shang, 2024), study used a GAN-inspired model with multi-head attention for ASD classification, based on 884 ABIDE subjects. The AAL atlas was used for parcellation of brain regions. The method was feature based and achieved an accuracy of 82%. One limitation is the complexity of the GAN architecture, which can make training difficult, especially with smaller datasets and augmentation of subjects based on fixed-size connectivity features.
In a previous study (Hasana et al., 2024), the authors applied ensemble learning techniques with multi-view fMRI features to ASD diagnosis using 871 subjects from ABIDE. The Yeo 7/17 Network Atlas was used for segmentation. The approach is feature-based, combining multiple views of the data and achieving 85% accuracy. The main limitation of ensemble methods is that they can be computationally intensive and require large datasets to perform better.
In a previous study (Yang et al., 2023), the authors used a Graph Neural Networks (GNN) with Recursive Feature Elimination (RFE) for ASD classification, using fMRI data from 872 subjects (ABIDE). The AAL atlas was employed, and the approach involved feature selection before classification, achieving 80% accuracy. One limitation is that GNN models tend to be sensitive to the quality of feature selection and require careful tuning to achieve optimal performance.
In a previous study (Qiang et al., 2023), the authors combined behavioral data with fMRI data for ASD diagnosis using 871 subjects (ABIDE). The AAL atlas was used to define these regions. This approach included feature extraction from behavioral data before classification and, improved the overall model accuracy. One limitation is that integrating behavioral data adds complexity to the model, requiring careful feature engineering.
In (Ahammed et al., 2021), the authors developed the DarkASDNet framework, a deep learning approach using CNNs for fMRI-based ASD classification on 871 subjects (ABIDE). The AAL atlas was used for the ROI selection. The method is end-to-end and focuses on the extraction of key features directly from the data. The model achieved 94.7% accuracy, although one limitation was the high computational cost of CNN-based models.
In a previous study (Shao et al., 2021), the authors applied CNNs to ASD diagnosis using both fMRI and structural MRI data. The Harvard-Oxford atlas was used to define the brain regions. The dataset included 1,200 subjects (ABIDE), and the approach was end-to-end. The method achieved high classification accuracy, but a limitation is that multimodal approaches tend to be computationally intensive and require significant data preprocessing.
In Almuqhim and Saeed (2021), the authors developed a stacked autoencoder (SAE) for ASD classification using fMRI data from 1,035 subjects (ABIDE). The Craddock 200 atlas was used for the ROI selection. This approach is feature-based, extracting low-dimensional representations before classification. The method achieved a classification accuracy of 76%. A limitation of autoencoders is that they often require large datasets and careful tuning to avoid overfitting.
In a previous study (Liu et al., 2020), the authors used an ensemble learning method combined with autoencoders for ASD classification from 1,200 ABIDE subjects. The Yeo 7/17 Network Atlas was used for brain region segmentation. This approach involved feature extraction before classification and achieved state-of-the-art accuracy. However, the complexity of ensemble models can lead to overfitting, particularly when the dataset is small.
In a previous study (Eslami et al., 2019), the authors utilized an autoencoder for feature extraction and classification on the ABIDE dataset (871 subjects). The Craddock 400 parcellation atlas was used for the ROI definition. The approach is feature-based, as the autoencoder reduces the dimensionality of the input before classification. It showed a 1% improvement over the baseline accuracy, but a limitation was the computational cost associated with training the autoencoder.
In (Sherkatghanad et al., 2020), the authors used a CNN model for ASD classification from 800 subjects in the ABIDE dataset. The AAL atlas was employed to define the ROIs, and the proposed approach was end-to-end without explicit feature selection. The model achieved 70.2% accuracy. One limitation is that the CNN architecture is extremely simplistic and requires more data for optimal generalization and feature learning.
In Hasana et al. (2024), authors introduced a multi-atlas deep ensemble network that integrates multiple brain atlases of fMRI data through a weighted deep ensemble network. On the ABIDE I dataset, comprising resting-state fMRI data from 17 international research sites, their approach achieved 75.20%. But a major limitation of their approach is lack of results reporting on the site wise comparison.
In Heczko et al. (2023), the authors proposed a novel Multi-Atlas Enhanced Transformer framework (METAFormer) for ASD classification, employing a multi-atlas approach with self-supervised pretraining. On the ABIDE I dataset, including 406 ASD and 476 typical control subjects, they achieved an average accuracy of 83.7% and an AUC score of 0.832. However, a major limitation of their approach was the lack of on-site comparisons.
2 Future directionsBased on the short review discussed in the previous sections, the following areas need to be explored by the research community. A brain atlas like AAL can also be referred to as coarser than the brain atlas CC400 because the former has only 116 ROIs as compared to the 400 in the latter case.
1. The performance of the proposed approach should be verified with denser and coarser atlases. If the selection of a coarser atlas such as AAL-116 has better classification performance, then the reasons and the significance of this should be analyzed.
2. The relationship between different atlases should also be explored; for example, the AAL-116 and C200 atlases are different atlases, but if combining these atlases results in better classification performance, the dynamics and significance of this should also be further analyzed. It is highly possible that some regions of the brain require coarser parcellation, such as AAL-116, but other regions need denser parcellation atlases, such as CC400.
3. The effects of parcellation atlases across various sites should also be explored because the ABIDE dataset on ASD is pooled over 17 sites globally. It is highly possible that some sites provide better classification performance when the AAL-116 atlas is selected and some sites might result in better classification performance when the CC400 atlas is selected. If this association is established, then it would be interesting to determine the reasons for these dynamics.
4. The association of atlas selection and dataset size should also be explored not only on the combined dataset but also on the site-wise dataset. This analysis would provide interesting insights into why the selection of the brain atlas is not consistent in these two comparisons.
5. The relationship between the time dimension and the selected atlas should also be explored; for example, the effect of the time dimension and coarser atlas, such as AAL-116, on classification accuracy. If a smaller time dimension with a coarser atlas, such as AAL-116, results in poorer accuracy as compared to the selection of a denser atlas, CC400, with smaller time dimensions, then potential reasons should be investigated from this analysis.
6. Various data augmentation techniques, and a selected brain atlas are related to the classification accuracy of the proposed approach. If data augmentation techniques work better only when a denser atlas such as CC400 is selected, then what can be inferred from this observation?
7. The effect of the selection of the brain atlases on the handcrafted features-based approaches and the end-to-end approaches should also be explored. If the denser atlas such as CC400 results in better classification performance only when the handcrafted features selection approach is used, then what are the potential reasons for this observation?
8. It would also be very interesting to explore how exactly atlases should be combined, should they be stacked, and then the features should be simultaneously selected from different atlases, or should the feature selection process be sequential, that is, first selecting features from AAL-116 and then from the CC400.
9. It should also be interesting to analyze whether there are recent updates in the development of atlases in other brain disorders like Alzheimer's which resulted in better classification performance, which could motivate the research community to come up with some updated atlases ASD.
10. Finally, and most importantly, there should be a genuine effort in developing a web-based tools that researchers can use to map the selected features to the different brain atlases for interpretability. For example, if someone has used an AAL-116-based dataset and got obtained classification results and now wanted to see the interpretation of the brain regions for the selected atlas, the web-based tools should annotate those features on the selected atlas to interpret those features on brain lobes and hemispheres.
3 ConclusionsThese investigations delineate the importance of the selection of different kinds of human brain atlase effects on the classification accuracy of various proposed algorithms for human ASD. We believe that we have raised some important questions regarding the selection of commonly used brain atlases. Future studies need to analyze not only the classification accuracy of the proposed algorithm but also the intrinsic nature of the parcellation atlases, and there is a need for a dedicated sections in future studies on the ASD classification problem regarding the various dynamics of the chosen brain atlases.
Author contributionsNK: Conceptualization, Investigation, Methodology, Writing – original draft. XS: Funding acquisition, Project administration, Supervision, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Innovation Capability Support Program of Shaanxi (No. 2021TD-06).
AcknowledgmentsWe are extremely grateful to the China Scholarship Council, Shaanxi Province Government, and Northwestern Polytechnical University for giving us moral, administrative, and financial support for conducting this study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesAhammed, M. S., Niu, S., Ahmed, M. R., Dong, J., Gao, X., Chen, Y., et al. (2021). Darkasdnet: classification of ASD on functional MRI using deep neural network. Front. Neuroinform. 15:635657. doi: 10.3389/fninf.2021.635657
PubMed Abstract | Crossref Full Text | Google Scholar
Almuqhim, F., and Saeed, F. (2021). ASD-saenet: a sparse autoencoder, and deep-neural network model for detecting autism spectrum disorder (ASD) using fMRI data. Front. Comput. Neurosci. 15:654315. doi: 10.3389/fncom.2021.654315
PubMed Abstract | Crossref Full Text | Google Scholar
American Psychiatric Association, D.American Psychiatric Association, D., et al. (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM-5, Volume 5. Washington, DC: American Psychiatric Association. doi: 10.1176/appi.books.9780890425596
Crossref Full Text | Google Scholar
Chen, Y., Yan, J., Jiang, M., Zhang, T., Zhao, Z., Zhao, W., et al. (2022). Adversarial learning based node-edge graph attention networks for autism spectrum disorder identification. IEEE Trans.Neural Netw. Learn. Syst. 35, 7275–7286. doi: 10.1109/TNNLS.2022.3154755
PubMed Abstract | Crossref Full Text | Google Scholar
Chiong, W., Wilson, S. M., D'Esposito, M., Kayser, A. S., Grossman, S. N., Poorzand, P., et al. (2013). The salience network causally influences default mode network activity during moral reasoning. Brain 136, 1929–1941. doi: 10.1093/brain/awt066
PubMed Abstract | Crossref Full Text | Google Scholar
Cole, D. M., Smith, S. M., and Beckmann, C. F. (2010). Advances and pitfalls in the analysis and interpretation of resting-state fMRI data. Front. Syst. Neurosci. 4:1459. doi: 10.3389/fnsys.2010.00008
PubMed Abstract | Crossref Full Text | Google Scholar
Craddock, R. C., James, G. A., Holtzheimer, I. I. I., Hu, P. E., and Mayberg, X. P. H. S. (2012). A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum. Brain Mapp. 33, 1914–1928. doi: 10.1002/hbm.21333
PubMed Abstract | Crossref Full Text | Google Scholar
Desikan, R. S., Ségonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into Gyral based regions of interest. Neuroimage 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021
PubMed Abstract | Crossref Full Text | Google Scholar
Eslami, T., Mirjalili, V., Fong, A., Laird, A. R., and Saeed, F. (2019). Asd-diagnet: a hybrid learning approach for detection of autism spectrum disorder using fMRI data. Front. Neuroinform. 13:70. doi: 10.3389/fninf.2019.00070
PubMed Abstract | Crossref Full Text | Google Scholar
Harikumar, A., Evans, D. W., Dougherty, C. C., Carpenter, K. L., and Michael, A. M. (2021). A review of the default mode network in autism spectrum disorders and attention deficit hyperactivity disorder. Brain Connect. 11, 253–263. doi: 10.1089/brain.2020.0865
PubMed Abstract | Crossref Full Text | Google Scholar
Hasana, M. R., Liuc, X., Gedeona, T., and Hossaina, M. Z. (2024). Made-for-ASD: A multi-atlas deep ensemble network for diagnosing autism spectrum disorder. Comput. Biol. Med. 182. doi: 10.1016/j.compbiomed.2024.109083
PubMed Abstract | Crossref Full Text | Google Scholar
Heczko, S., Scheffler, K., and Lohmann, G. (2023). “Pretraining is all you need: a multi-atlas enhanced transformer framework for autism spectrum disorder classification," in Machine Learning in Clinical Neuroimaging: 6th International Workshop, MLCN 2023, Held in Conjunction with MICCAI 2023, Vancouver, BC, Canada, October 8, 2023, Proceedings, Volume 14312 (Cham: Springer Nature), 123. doi: 10.1007/978-3-031-44858-4_12
Crossref Full Text | Google Scholar
Hoptman, M. J., Evans, K. T., Parincu, Z., Sparpana, A. M., Sullivan, E. F., Ahmed, A. O., et al. (2024). Emotion-related impulsivity and suicidal ideation and behavior in schizophrenia spectrum disorder: a pilot fMRI study. Front. Psychiatry 15:1408083. doi: 10.3389/fpsyt.2024.1408083
PubMed Abstract | Crossref Full Text | Google Scholar
Khan, N. A., and Shang, X. (2024). ASD-gannet: a generative adversarial network-inspired deep learning approach for the classification of autism brain disorder. Brain Sci. 14:766. doi: 10.3390/brainsci14080766
PubMed Abstract | Crossref Full Text | Google Scholar
Lajoie, I., Nugent, S., Debacker, C., Dyson, K., Tancredi, F. B., Badhwar, A., et al. (2017). Application of calibrated fMRI in Alzheimer's disease. NeuroImage: Clin. 15, 348–358. doi: 10.1016/j.nicl.2017.05.009
PubMed Abstract | Crossref Full Text | Google Scholar
Liu, J., Sheng, Y., Lan, W., Guo, R., Wang, Y., Wang, J., et al. (2020). Improved ASD classification using dynamic functional connectivity and multi-task feature selection. Pattern Recognit. Lett. 138, 82–87. doi: 10.1016/j.patrec.2020.07.005
PubMed Abstract | Crossref Full Text | Google Scholar
Qiang, N., Gao, J., Dong, Q., Li, J., Zhang, S., Liang, H., et al. (2023). A deep learning method for autism spectrum disorder identification based on interactions of hierarchical brain networks. Behav. Brain Res. 452:114603. doi: 10.1016/j.bbr.2023.114603
PubMed Abstract | Crossref Full Text | Google Scholar
Qiu, S., Lu, Y., Li, Y., Shi, J., Cui, H., Gu, Y., et al. (2020). Prevalence of autism spectrum disorder in Asia: a systematic review and meta-analysis. Psychiatry Res. 284:112679. doi: 10.1016/j.psychres.2019.112679
PubMed Abstract | Crossref Full Text | Google Scholar
Ribeiro da Costa, C., Soares, J. M., Oliveira-Silva, P., Sampaio, A., and Coutinho, J. F. (2022). Interplay between the salience and the default mode network in a social-cognitive task toward a close other. Front. Psychiatry 12:718400. doi: 10.3389/fpsyt.2021.718400
PubMed Abstract | Crossref Full Text | Google Scholar
Santana, C. P., de Carvalho, E. A., Rodrigues, I. D., Bastos, G. S., de Souza, A. D., and de Brito, L. L. (2022). rs-fMRI and machine learning for ASD diagnosis: a systematic review and meta-analysis. Sci. Rep. 12:6030. doi: 10.1038/s41598-022-09821-6
PubMed Abstract | Crossref Full Text | Google Scholar
Sherkatghanad, Z., Akhondzadeh, M., Salari, S., Zomorodi-Moghadam, M., Abdar, M., Acharya, U. R., et al. (2020). Automated detection of autism spectrum disorder using a convolutional neural network. Front. Neurosci. 13:1325. doi: 10.3389/fnins.2019.01325
PubMed Abstract | Crossref Full Text | Google Scholar
Solmi, M., Song, M., Yon, D. K., Lee, S. W., Fombonne, E., Kim, M. S., et al. (2022). Incidence, prevalence, and global burden of autism spectrum disorder from 1990 to 2019 across 204 countries. Mol. Psychiatry 27, 4172–4180. doi: 10.1038/s41380-022-01630-7
PubMed Abstract | Crossref Full Text | Google Scholar
Tang, C., Wei, Y., Zhao, J., and Nie, J. (2018). Different developmental pattern of brain activities in ADHD: a study of resting-state fMRI. Dev. Neurosci. 40, 246–257. doi: 10.1159/000490289
PubMed Abstract | Crossref Full Text | Google Scholar
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. doi: 10.1006/nimg.2001.0978
PubMed Abstract | Crossref Full Text | Google Scholar
Van Den Heuvel, M. P., and Pol, H. E. H. (2010). Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 20, 519–534. doi: 10.1016/j.euroneuro.2010.03.008
PubMed Abstract | Crossref Full Text | Google Scholar
Walsh, M. J., Wallace, G. L., Gallegos, S. M., and Braden, B. B. (2021). Brain-based sex differences in autism spectrum disorder across the lifespan: a systematic review of structural MRI, fMRI, and DTI findings. NeuroImage Clin. 31:102719. doi: 10.1016/j.nicl.2021.102719
PubMed Abstract | Crossref Full Text | Google Scholar
Yang, J., Hu, M., Hu, Y., Zhang, Z., and Zhong, J. (2023). Diagnosis of autism spectrum disorder (ASD) using recursive feature elimination-graph neural network (RFE-GNN) and phenotypic feature extractor (PFE). Sensors 23:9647. doi: 10.3390/s23249647
PubMed Abstract | Crossref Full Text | Google Scholar
Yeo, B. T., Krienen, F. M., Sepulcre, J., Sabuncu, M. R., Lashkari, D., Hollinshead, M., et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165. doi: 10.1152/jn.00338.2011
PubMed Abstract | Crossref Full Text | Google Scholar
Zhang, J., Guo, J., Lu, D., and Cao, Y. (2024). ASD-Swnet: a novel shared-weight feature extraction and classification network for autism spectrum disorder diagnosis. Sci. Rep. 14:13696. doi: 10.1038/s41598-024-64299-8
PubMed Abstract | Crossref Full Text | Google Scholar
Zhang, Z., Peng, P., and Zhang, D. (2020). Executive function in high-functioning autism spectrum disorder: a meta-analysis of fMRI studies. J. Autism Dev. Disord. 50, 4022–4038. doi: 10.1007/s10803-020-04461-z
留言 (0)