Melanoma is a melanocyte-derived malignant tumor with a generally poor prognosis. Currently, its prevalence is on the rise worldwide. From 1990 to 2019, the incidence of melanoma has surged by 170%, while deaths have increased by 90% globally (1), which is accompanied by a huge treatment-related financial burden. The 5-year overall survival (OS) rate for Chinese melanoma patients stands at 41.6%, and the median OS is 3.92 years, with the 5-year OS rate of merely 4.6% for stage IV patients (2). Acral melanoma (AM), which constitutes nearly half of Chinese melanoma cases, primarily affects palmar, plantar, and subungual skin (2, 3). Characterized by its insidious onset and advanced stage at diagnosis (4), AM is associated with an unfavorable prognosis compared to non-acral cutaneous melanoma (5, 6). In our previous retrospective study, the estimated median OS for AM patients undergoing surgery was 60 months (7).
Previous studies have investigated the link between melanoma and socioeconomic status (SES), revealing that high SES correlates with increased melanoma incidence, thinner tumor thickness, and prolonged survival (7–22). These studies have used diverse assessment measures to evaluate SES. The most commonly used SES factors impacting melanoma include educational attainment, income, occupation, medical insurance, and marital status. Evaluation indicators vary across different studies and have certain limitations, often reflecting specific aspects of SES rather than comprehensively capturing its entirety. For example, some studies have analyzed the effect of single SES factors on melanoma survival, such as educational level (16, 22), occupation (17), income (18, 19), or marital status (20, 23). Unsurprisingly, other studies evaluate SES using multiple indicators (8, 10–14, 21). Considering that different SES indicators may measure different dimensions of SES, it is necessary to comprehensively study multiple SES dimensions.
However, to date, no study has confirmed the prognostic value of SES in Chinese AM patients. Inequalities in access to treatment and care exist even within a universal healthcare system (9). Within the context of a non-universal healthcare system in China, it is particularly important to analyze the relationship between SES level, including medical insurance status, and the prognosis of AM patients.
For this purpose, we assessed the SES levels of Chinese AM patients across four dimensions in this study: educational attainment, occupation, medical insurance, and marital status. Through a multi-dimensional analysis of SES and clinical features, we identified a significant association between SES and survival. Additionally, SES was negatively correlated with Breslow thickness. Our findings seek to furnish evidence-based insights for health departments to formulate health promotion strategies and public health policies.
Methods Patient population and data collectionA total of 298 consecutive primary AM patients were collected from July 2010 to November 2021 at Drum Tower Hospital after excluding those with incomplete SES data. All patients enrolled in this study had been diagnosed by histopathology as having melanoma found on acral skin, including any histopathological subtypes such as acral lentiginous melanoma, nodular melanoma, and superficial spreading melanoma. The clinical and socioeconomic data were collected, including age, gender, stage at initial diagnosis (AJCC 8th Edition), primary tumor site, educational attainment, occupation, medical insurance, marital status, and survival status until the last follow-up or death.
Income data were not collected due to concerns about privacy, variability, and difficulty of accurate quantification, particularly for farmers. This retrospective study received approval from the Ethics Committee of Drum Tower Hospital (2024–277). Informed consent was waived due to the study’s retrospective nature and the anonymization of patient data.
Assessment of SES levelThis study categorized educational attainment among AM patients as illiteracy, nine-year compulsory education, high school, or university/college. Occupations were classified as unemployment, farmers or manual laborers, or other non-manual professions. In addition, the status of medical insurance in AM patients included uninsured status, rural or urban residents’ medical insurance, and staff medical insurance. As rural and urban residents’ medical insurance share similarities and act as the basic medical insurance system for the unemployed in China (24), they were combined into a single category termed primary medical insurance. Marital status was categorized as married and other status, including never married, divorced, and widowed.
Each indicator was assigned different weight scores based on their degrees, and detailed rules were shown in Supplementary Table S1. The sum of the scores of each indicator constituted the SES score. The optimal cut-off value of the SES score was determined using X-tile bioinformatics software (Version 3.6.1). Higher scores represented better SES levels. Patients scoring between 3 and 7 were categorized as high SES, while scores of 0 to 2 denoted low SES.
Statistical analysisThe Kaplan–Meier estimate was used to analyze the survival of various patient groups and median OS. The median follow-up time was determined according to the reverse Kaplan–Meier method. Log-rank tests and Cox proportional hazards models were utilized to identify prognostic factors and assess the risk of death for various covariates. Pearson’s chi-squared test was used for correlation analysis of categorical variables. A two-sided p-value of no more than 0.05 was considered statistically significant (*p ≤ 0.05). The R Project for Statistical Computing (version 4.3.3) and IBM SPSS Statistics (version 25.0) were used to perform these statistical analyses.
Results Patient characteristicsA total of 298 primary AM patients were included in this study. The median age of initial diagnosis was 60 years old (range: 22–87 years old). Among them, 135 (45.3%) patients were female, and 163 (54.7%) were male. Most patients presented with stage II (39.3%) or stage III (29.2%) disease, with Breslow thickness > 2.0 mm observed in the majority (54.0%) of cases. Primary lesions were predominantly located in the feet (84.6%). The median follow-up time was 36 months (4–128 months). At the last follow-up, 90 patients had died. And the estimated median OS was 60.0 months.
The AM patients in this study had a relatively low level of education, and 71.1% of patients only received or did not receive 9 years of compulsory education. Over half of the patients were farmers or manual laborers, and 35.9% were engaged in non-manual professions such as teachers, civil servants, and white-collar. In addition, 16 patients were unemployed. 30.5% of patients did not participate in any medical insurance, 26.8% of patients participated in basic medical insurance (rural or urban residents’ medical insurance), and 42.6% of patients purchased staff medical insurance. Most patients were married (95.3%), while 4.7% were in other marital status (including 2 never married, 2 divorced, and 10 widowed). The clinical and demographic characteristics are summarized in Table 1.
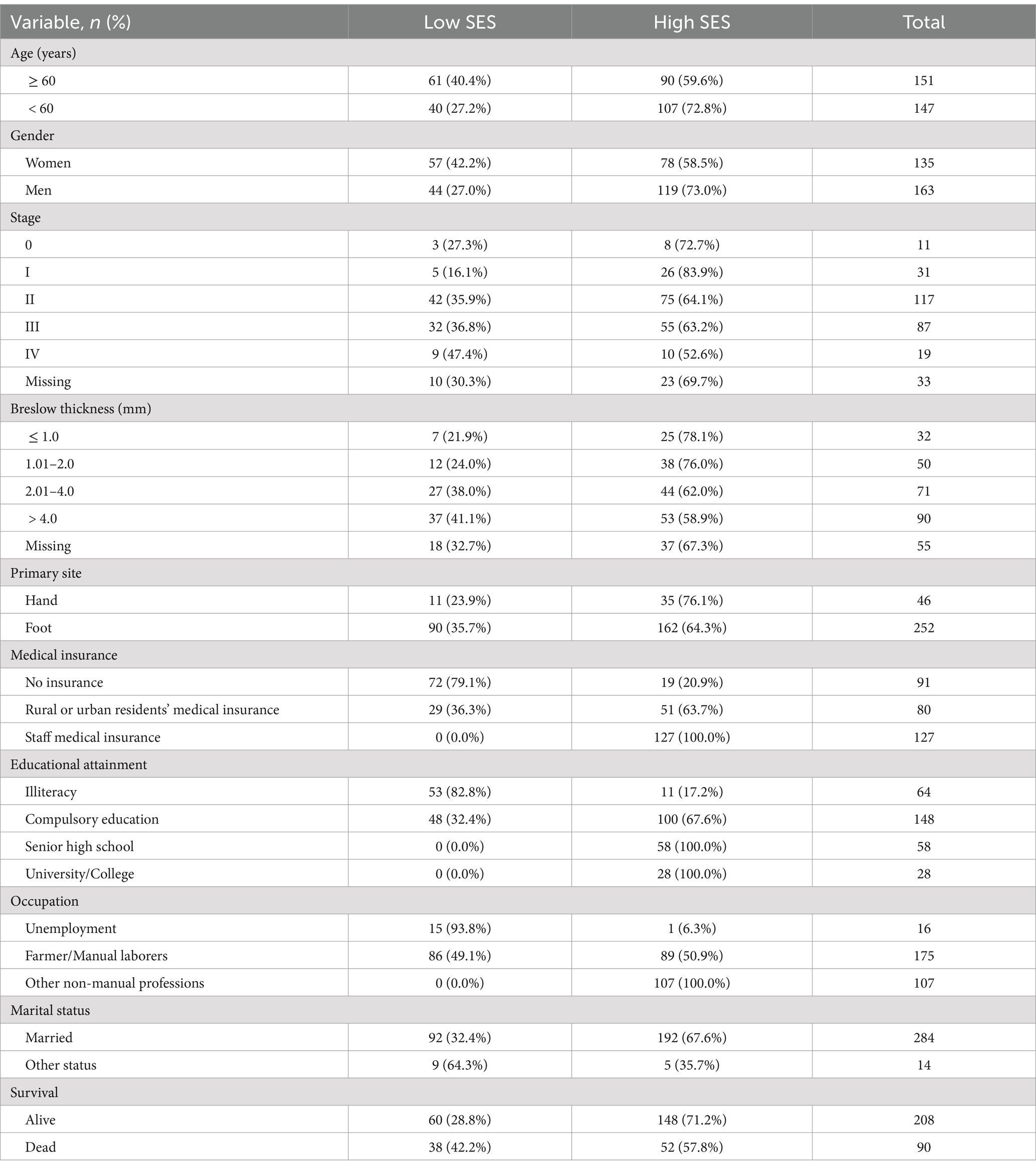
Table 1. Clinical and demographic characteristics of all patients.
Univariate survival analysisAmong these clinical and demographic characteristics, age, gender, stage, Breslow thickness, and primary site were significantly associated with AM patient survival (p < 0.01, p = 0.05, p < 0.01, p < 0.01, p < 0.01, respectively; Table 2). Educational attainment, occupation, and marital status did not significantly affect patient survival. However, the univariate COX analysis showed that medical insurance status was significantly associated with survival. Patients with staff medical insurance had longer survival times than those without any insurance (hazard ratio (HR) = 0.60, 95% confidence interval (CI) = 0.37–0.96, p = 0.03). Additionally, log-rank tests for individual socioeconomic factors did not confirm their prognostic significance (Supplementary Figure S1).
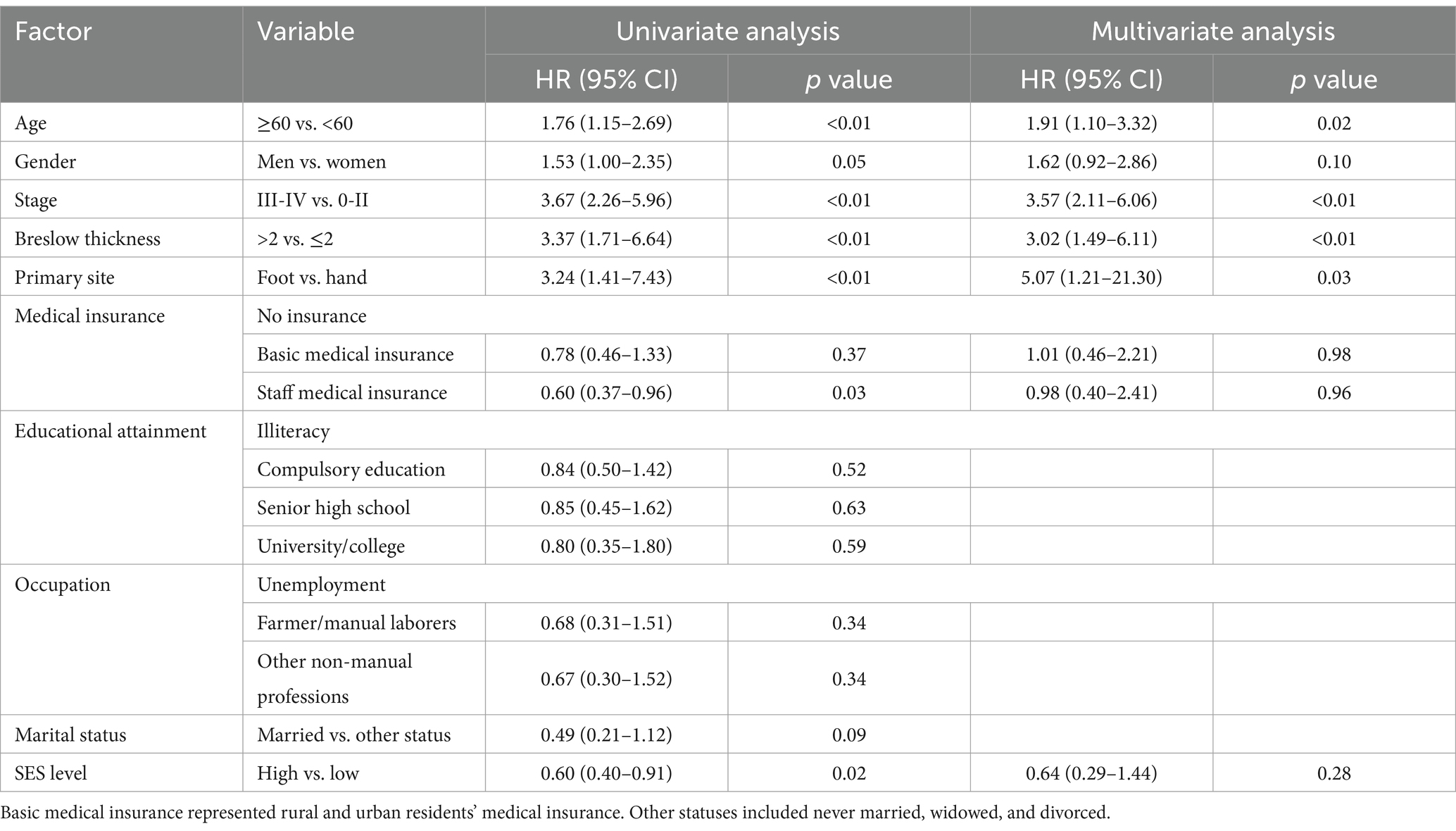
Table 2. Univariate and multivariate analyses of factors associated with overall survival.
Subsequently, we combined educational attainment, occupation, medical insurance, and marital status to obtain a SES score for each patient. Detailed scoring rules are shown in Supplementary Table S1. And the SES score showed a positive association with survival (Figure 1). Patients with higher SES scores exhibited longer survival times. Additionally, patients were categorized into two groups according to their comprehensive SES scores. We further analyzed the difference in OS between patients with high and low SES scores through the log-rank test (p = 0.015; Figure 2). It was found that the SES could predict patient survival more precisely than any single socioeconomic factor (Figures 2; Supplementary Figure S1). Patients with high SES had significantly reduced AM mortality risk (HR = 0.61, 95% CI = 0.40–0.92, p = 0.02; Table 2).
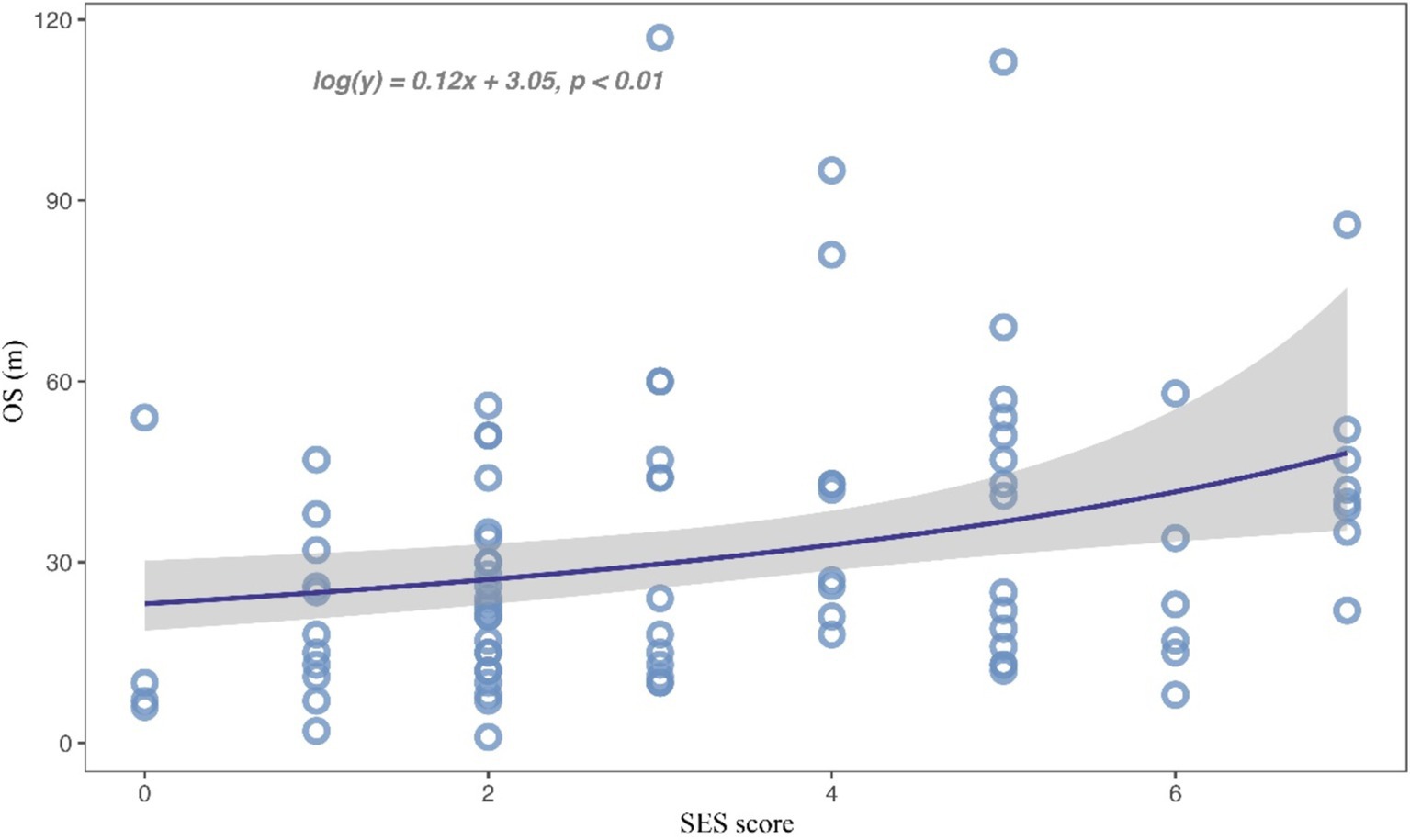
Figure 1. Scatterplot for the relationship between SES score and overall survival time.
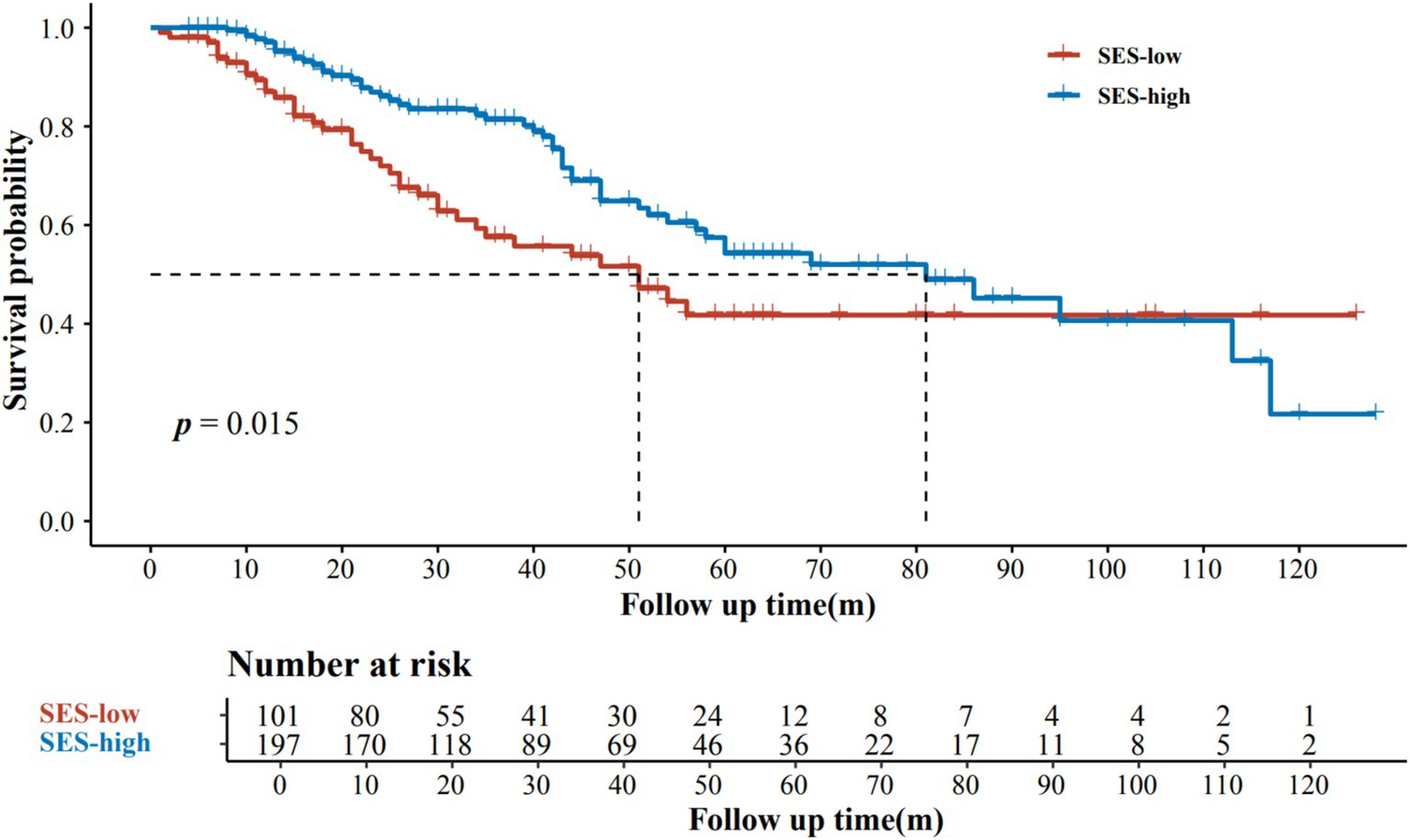
Figure 2. Kaplan–Meier estimates of overall survival according to SES levels.
Multivariate survival analysisAfter adjustment for prognostic factors such as age, gender, primary site, Breslow thickness, and stage at initial diagnosis, SES level (HR = 0.64, 95% CI = 0.29–1.44, p = 0.28) and staff medical insurance (HR = 0.98, 95% CI = 0.40–2.41, p = 0.96) were not independent predictors of AM patient prognosis. Patients with advanced stages (stage III and stage IV) had shorter OS (HR = 3.57, 95% CI =2.11–6.06, p < 0.01) compared to those with earlier stages (stage 0, stage I, and stage II). In addition, we found that AM patients with lesions located in the feet also showed a worse prognosis compared to those in the hands (HR = 5.07, 95% CI =1.21–21.30, p = 0.03), although with a wide 95% CI interval due to a small sample size of the hand subgroup. Patients with Breslow thickness > 2.0 mm had worse survival than those with Breslow thickness ≤ 2.0 mm (HR = 3.02, 95% CI 1.49–6.11, p < 0.01). Moreover, patients with older ages also had poorer survival compared with younger patients (HR = 1.91, 95% CI 1.10–3.32, p = 0.02). Multivariate Cox regression analysis for OS was summarized in Table 2 and presented as a forest plot (Figure 3).
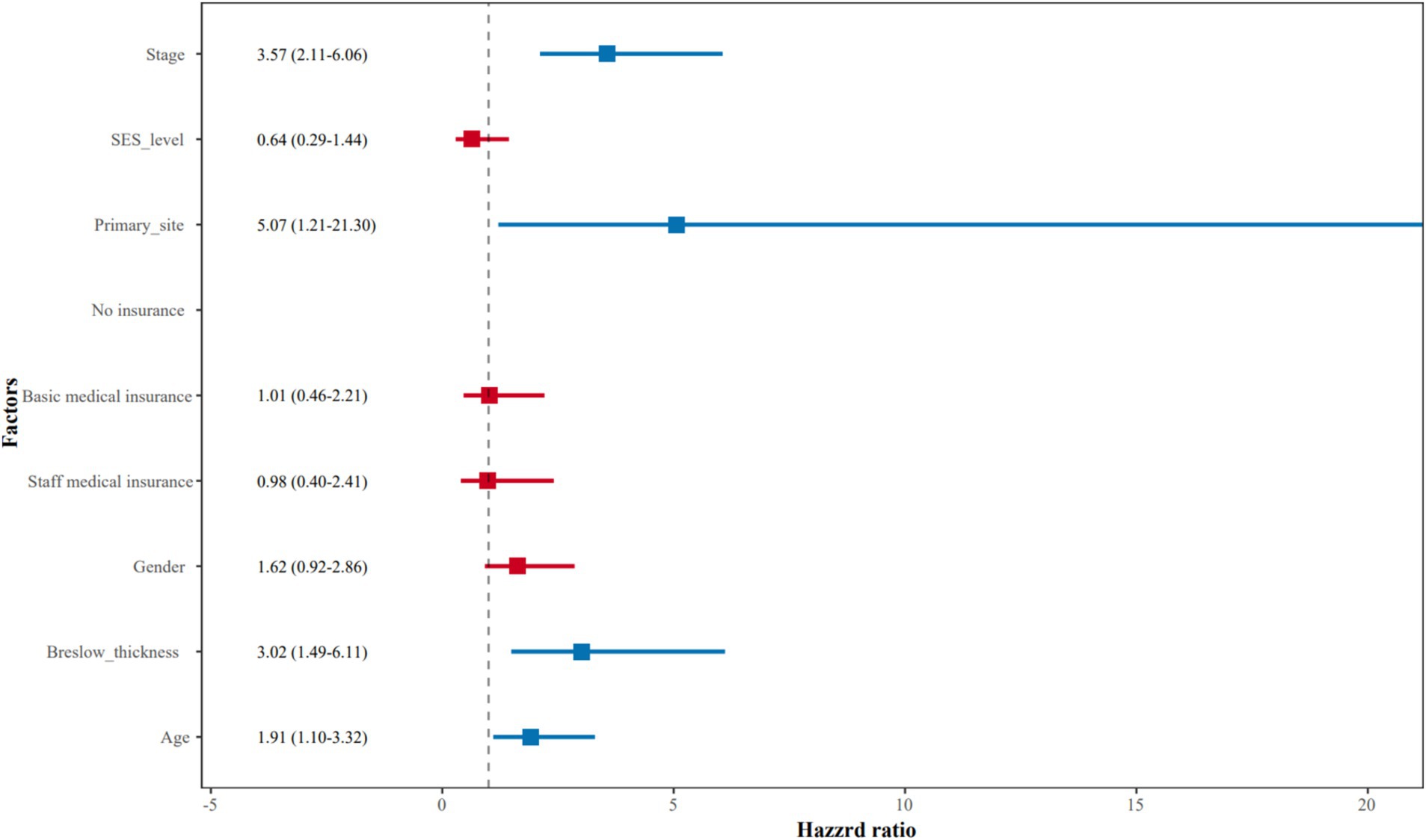
Figure 3. The forest plot of multivariate Cox regression analysis for overall survival.
Associations between SES and clinical characteristicsFurthermore, the association analysis between SES and crucial clinical characteristics was investigated. It was Breslow thickness rather than the stage at initial diagnosis that was significantly negatively associated with SES (Pearson’s chi-square = 6.64, p < 0.01; Pearson’s chi-square = 1.48, p = 0.22), as shown in Supplementary Table S2. Patients presenting with Breslow thickness ≤ 2 mm were more likely to have high SES. The stacked column chart revealed the distribution of Breslow thickness in high and low SES groups (Figure 4). Medical insurance, occupation, education, and marital status were neither associated with tumor thickness nor stage (Supplementary Table S3–S6).
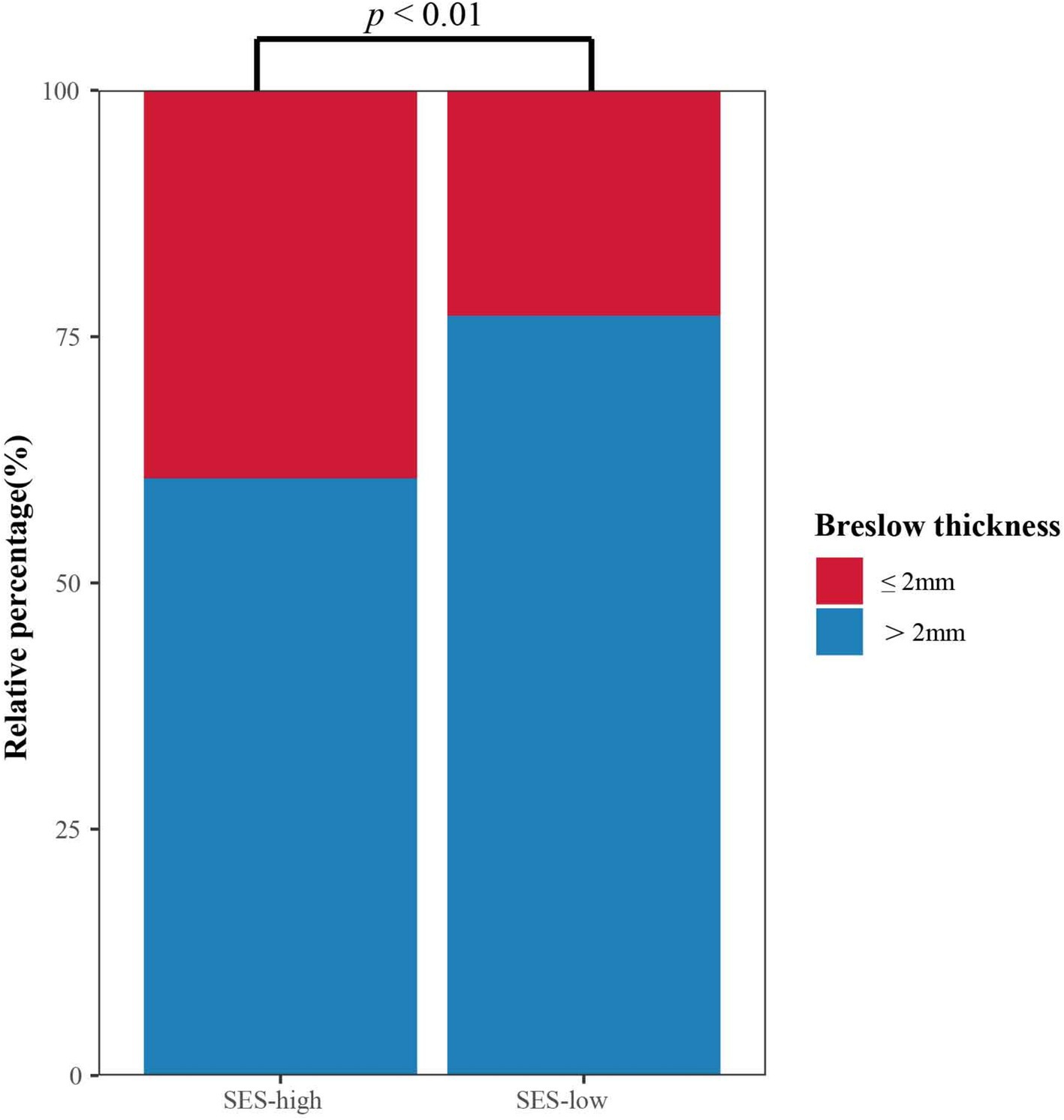
Figure 4. Stacked column chart of Breslow thickness in different SES groups.
DiscussionPrevious studies have established SES as a significant predictor of melanoma prognosis (11, 14, 15). In this study, we demonstrated the predictive role of SES in the prognosis of Chinese AM patients, finding that the combined SES level predicted survival more accurately than individual socioeconomic factors. However, SES did not act as an independent prognostic factor in AM. A negative correlation was observed between SES level and Breslow thickness, potentially explaining the better survival outcomes among patients with higher SES. To the best of our knowledge, this is the first Chinese population-based analysis evaluating the relationship between SES and AM survival and clinical characteristics.
Patients with a college education are generally more informed about melanoma (25). A stable job, medical insurance, and marriage help patients access timely anti-melanoma treatment and reduce disease-related economic burden (20, 26). A study has reported the high economic burden of melanoma patients with macroscopic lymph node metastasis or distant metastasis (27). Many patients in this study had low SES indicators, with a notable proportion being unemployed, illiterate, or medically uninsured. Single socioeconomic factors failed to serve as prognostic factors in log-rank tests. As a comprehensive measure of socioeconomic factors, SES score played a role in predicting patient survival, emphasizing the significance of evaluating SES comprehensively.
Although SES was not an independent prognostic factor in the multivariate analysis, its association with survival emphasizes its indirect impact through clinical factors such as Breslow thickness. Previous studies have consistently shown that low SES is linked to advanced stages of melanoma (15, 28, 29). The negative relationship between SES and Breslow thickness has also been revealed (8, 21). Indeed, AM often presents with thick tumors and advanced stages, especially for Asian patients (30), and these characteristics are associated with poorer survival (6, 31, 32). Our study corroborates these findings, demonstrating that patients with high SES were more likely to present with Breslow thickness ≤ 2.0 mm. This relationship likely explains the survival differences between high-and low-SES groups.
Our study has several limitations. First, this is a single-center study with a limited sample size. Second, our measures of SES were limited and may not have fully characterized an individual’s SES level. Despite these constraints, our findings suggest that AM patients with low SES levels have a higher mortality risk, which may be related to thicker tumor thickness. For AM, patients are commonly diagnosed at an advanced stage and with a thick tumor. Therefore, this study highlights that early diagnosis and treatment are crucial for AM patients, especially those with low SES levels.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by the Ethics Committee of Drum Tower Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because informed consent was waived due to the retrospective nature of the study and the anonymization of patient data.
Author contributionsRH: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing. JW: Investigation, Methodology, Resources, Writing – review & editing. KZ: Investigation, Resources, Writing – review & editing. MZ: Conceptualization, Methodology, Writing – review & editing. ZZ: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82073365 and 81872484) and the Social Development Fund of Jiangsu Province (No. BE2019605).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1496082/full#supplementary-material
AbbreviationsAM, acral melanoma; CI, confidence interval; HR, hazard ratios; OS, overall survival; SES, socioeconomic status.
References1. Li, Z, Fang, Y, Chen, H, Zhang, T, Yin, X, Man, J, et al. Spatiotemporal trends of the global burden of melanoma in 204 countries and territories from 1990 to 2019: results from the 2019 global burden of disease study. Neoplasia. (2022) 24:12–21. doi: 10.1016/j.neo.2021.11.013
PubMed Abstract | Crossref Full Text | Google Scholar
2. Chi, Z, Li, S, Sheng, X, Si, L, Cui, C, Han, M, et al. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: a study of 522 consecutive cases. BMC Cancer. (2011) 11:85. doi: 10.1186/1471-2407-11-85
PubMed Abstract | Crossref Full Text | Google Scholar
3. Bai, X, Kong, Y, Chi, Z, Sheng, X, Cui, C, Wang, X, et al. MAPK pathway and TERT promoter gene mutation pattern and its prognostic value in melanoma patients: a retrospective study of 2,793 cases. Clin Cancer Res. (2017) 23:6120–7. doi: 10.1158/1078-0432.CCR-17-0980
PubMed Abstract | Crossref Full Text | Google Scholar
5. Bello, DM, Chou, JF, Panageas, KS, Brady, MS, Coit, DG, Carvajal, RD, et al. Prognosis of acral melanoma: a series of 281 patients. Ann Surg Oncol. (2013) 20:3618–25. doi: 10.1245/s10434-013-3089-0
PubMed Abstract | Crossref Full Text | Google Scholar
6. Huang, K, Fan, J, and Misra, S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015, an analysis of the SEER registry. J Surg Res. (2020) 251:329–39. doi: 10.1016/j.jss.2020.02.010
PubMed Abstract | Crossref Full Text | Google Scholar
7. Huang, R, Shen, G, Ren, Y, Zheng, K, Wang, J, Shi, Y, et al. Prognostic value of genetic aberrations and tumor immune microenvironment in primary acral melanoma. J Transl Med. (2023) 21:78. doi: 10.1186/s12967-022-03856-z
PubMed Abstract | Crossref Full Text | Google Scholar
8. Chen, ML, de Vere Hunt, IJ, John, EM, Weinstock, MA, Swetter, SM, and Linos, E. Differences in thickness-specific incidence and factors associated with cutaneous melanoma in the US from 2010 to 2018. JAMA Oncol. (2022) 8:755–9. doi: 10.1001/jamaoncol.2022.0134
PubMed Abstract | Crossref Full Text | Google Scholar
9. Li, HO-Y, Bailey, AJM, Grose, E, McDonald, JT, Quimby, A, Johnson-Obaseki, S, et al. Socioeconomic status and melanoma in Canada: a systematic review. J Cutan Med Surg. (2021) 25:87–94. doi: 10.1177/1203475420960426
PubMed Abstract | Crossref Full Text | Google Scholar
10. JAG, G, Dobbs, TD, Griffiths, R, Song, J, Akbari, A, Whitaker, S, et al. The association of smoking and socioeconomic status on cutaneous melanoma: a population-based, data-linkage, case-control study. Br J Dermatol. (2020) 182:1136–47. doi: 10.1111/bjd.18526
PubMed Abstract | Crossref Full Text | Google Scholar
11. Rosenthal, A, Reddy, S, Cooper, R, Chung, J, Zhang, J, Haque, R, et al. Disparities in melanoma-specific mortality by race/ethnicity, socioeconomic status, and health care systems. J Am Acad Dermatol. (2023) 88:560–7. doi: 10.1016/j.jaad.2022.10.004
PubMed Abstract | Crossref Full Text | Google Scholar
12. Yan, BY, Barilla, S, Strunk, A, and Garg, A. Survival differences in acral lentiginous melanoma according to socioeconomic status and race. J Am Acad Dermatol. (2022) 86:379–86. doi: 10.1016/j.jaad.2021.07.049
PubMed Abstract | Crossref Full Text | Google Scholar
13. Steding-Jessen, M, Engberg, H, Øster, I, Jensen, JW, Hölmich, LR, Møller, H, et al. Regional and socioeconomic variation in survival of melanoma patients in Denmark. Dan Med J. (2019) 66:A5572.
PubMed Abstract | Google Scholar
14. Abdel-Rahman, O. Prognostic impact of socioeconomic status among patients with malignant melanoma of the skin: a population-based study. J Dermatol Treat. (2020) 31:571–5. doi: 10.1080/09546634.2019.1657223
PubMed Abstract | Crossref Full Text | Google Scholar
15. Sitenga, JL, Aird, G, Ahmed, A, Walters, R, and Silberstein, PT. Socioeconomic status and survival for patients with melanoma in the United States: an NCDB analysis. Int J Dermatol. (2018) 57:1149–56. doi: 10.1111/ijd.14026
PubMed Abstract | Crossref Full Text | Google Scholar
16. Eriksson, H, Lyth, J, Månsson-Brahme, E, Frohm-Nilsson, M, Ingvar, C, Lindholm, C, et al. Low level of education is associated with later stage at diagnosis and reduced survival in cutaneous malignant melanoma: a nationwide population-based study in Sweden. Eur J Cancer. (2013) 1990:2705–16. doi: 10.1016/j.ejca.2013.03.013
Crossref Full Text | Google Scholar
17. Pérez-Gómez, B, Aragonés, N, Gustavsson, P, Lope, V, López-Abente, G, and Pollán, M. Socioeconomic class, rurality and risk of cutaneous melanoma by site and gender in Sweden. BMC Public Health. (2008) 8:33. doi: 10.1186/1471-2458-8-33
PubMed Abstract | Crossref Full Text | Google Scholar
18. Salvaggio, C, Han, SW, Martires, K, Robinson, E, Madankumar, R, Gumaste, P, et al. Impact of socioeconomic status and ethnicity on melanoma presentation and recurrence in Caucasian patients. Oncology. (2016) 90:79–87. doi: 10.1159/000441524
PubMed Abstract | Crossref Full Text | Google Scholar
19. Johnson-Obaseki, SE, Labajian, V, Corsten, MJ, and McDonald, JT. Incidence of cutaneous malignant melanoma by socioeconomic status in Canada: 1992-2006. J Otolaryngol. (2015) 44:53. doi: 10.1186/s40463-015-0107-1
PubMed Abstract | Crossref Full Text | Google Scholar
20. Sharon, CE, Sinnamon, AJ, Ming, ME, Chu, EY, Fraker, DL, and Karakousis, GC. Association of Marital Status with T Stage at presentation and Management of Early-Stage Melanoma. JAMA Dermatol. (2018) 154:574–80. doi: 10.1001/jamadermatol.2018.0233
PubMed Abstract | Crossref Full Text | Google Scholar
21. Pollitt, RA, Clarke, CA, Swetter, SM, Peng, DH, Zadnick, J, and Cockburn, M. The expanding melanoma burden in California hispanics: importance of socioeconomic distribution, histologic subtype, and anatomic location. Cancer. (2011) 117:152–61. doi: 10.1002/cncr.25355
Crossref Full Text | Google Scholar
22. Quintella Mendes, GL, and Koifman, S. Socioeconomic status as a predictor of melanoma survival in a series of 1083 cases from Brazil: just a marker of health services accessibility? Melanoma Res. (2013) 23:199–205. doi: 10.1097/CMR.0b013e32835e76f8
PubMed Abstract | Crossref Full Text | Google Scholar
23. Moreau, JF, Weinstock, MA, Geller, AC, Winger, DG, and Ferris, LK. Individual and ecological factors associated with early detection of nodular melanoma in the United States. Melanoma Res. (2014) 24:165–71. doi: 10.1097/CMR.0000000000000049
PubMed Abstract | Crossref Full Text | Google Scholar
24. Xu, W, Cai, G–J, Li, G–N, Cao, J–J, Shi, Q–H, and Bai, J. Age or health status: which influences medical insurance enrollment greater? J Glob Health. (2016) 6:020801. doi: 10.7189/jogh.06.020801
PubMed Abstract | Crossref Full Text | Google Scholar
25. Sanchez, DP, Maymone, MBC, McLean, EO, Kennedy, KF, Sahni, D, Secemsky, EA, et al. Racial and ethnic disparities in melanoma awareness: a cross-sectional survey. J Am Acad Dermatol. (2020) 83:1098–103. doi: 10.1016/j.jaad.2020.04.137
PubMed Abstract | Crossref Full Text | Google Scholar
26. Tian, G, Xu, J, Zhang, T, Zhang, H, Liu, J, Shi, Q, et al. Rural residents’ willingness to participate in basic medical insurance and influential factors: a survey of three provinces in China. Front Public Health. (2022) 10:1037763. doi: 10.3389/fpubh.2022.1037763
PubMed Abstract | Crossref Full Text | Google Scholar
27. Grange, F, Mohr, P, Harries, M, Ehness, R, Benjamin, L, Siakpere, O, et al. Economic burden of advanced melanoma in France, Germany and the UK: a retrospective observational study (melanoma burden-of-illness study). Melanoma Res. (2017) 27:607–18. doi: 10.1097/CMR.0000000000000372
PubMed Abstract | Crossref Full Text | Google Scholar
28. Cortez, JL, Vasquez, J, and Wei, ML. The impact of demographics, socioeconomics, and health care access on melanoma outcomes. J Am Acad Dermatol. (2021) 84:1677–83. doi: 10.1016/j.jaad.2020.07.125
PubMed Abstract | Crossref Full Text | Google Scholar
29. Zell, JA, Cinar, P, Mobasher, M, Ziogas, A, Meyskens, FL, and Anton-Culver, H. Survival for patients with invasive cutaneous melanoma among ethnic groups: the effects of socioeconomic status and treatment. J Clin Oncol Off J Am Soc Clin Oncol. (2008) 26:66–75. doi: 10.1200/JCO.2007.12.3604
PubMed Abstract | Crossref Full Text | Google Scholar
30. Behbahani, S, Malerba, S, and Samie, FH. Racial and ethnic differences in the clinical presentation and outcomes of acral lentiginous melanoma. Br J Dermatol. (2021) 184:158–60. doi: 10.1111/bjd.19406
PubMed Abstract | Crossref Full Text | Google Scholar
31. Teramoto, Y, Keim, U, Gesierich, A, Schuler, G, Fiedler, E, Tüting, T, et al. Acral lentiginous melanoma: a skin cancer with unfavourable prognostic features. A study of the German central malignant melanoma registry (CMMR) in 2050 patients. Br J Dermatol. (2018) 178:443–51. doi: 10.1111/bjd.15803
PubMed Abstract | Crossref Full Text | Google Scholar
32. Lv, J, Dai, B, Kong, Y, Shen, X, and Kong, J. Acral melanoma in Chinese: a Clinicopathological and prognostic study of 142 cases. Sci Rep. (2016) 6:31432. doi: 10.1038/srep31432
留言 (0)