Rapid and accurate diagnosis of plant pathogens is a cornerstone in ensuring global food security, safeguarding ecosystem integrity, and sustaining agricultural productivity. As plant diseases increasingly challenge crop yields and quality, the timely detection and identification of bacterial, viral, fungal, and other phytopathogens have become paramount in guiding efficient disease management and limiting the economic losses associated with epidemics (Savary and Willocquet, 2020; Ali et al., 2021). Traditional laboratory-based diagnostic methodologies—such as plating, microscopy, enzyme-linked immunosorbent assays (ELISAs), and polymerase chain reaction (PCR)—have undoubtedly advanced our capacity to detect pathogens at remarkable levels of accuracy and sensitivity (Boonham et al., 2014). However, these approaches often require specialized equipment, stable infrastructure, and trained personnel, creating practical bottlenecks in real-world, resource-limited scenarios.
Portable diagnostic technologies—ranging from handheld biosensors and smartphone-based platforms to lab-on-a-chip devices—are promising tools that bring the diagnostic power of advanced molecular assays directly into the field (Srinivasan and Tung, 2015). Such innovations align well with the broader paradigm shift toward precision agriculture, enabling evidence-based decision-making, early warning systems, and improved surveillance networks that integrate seamlessly into the Internet of Things (IoT) and cloud-based architectures (Srinivasan and Tung, 2015).
Despite this momentum, notable gaps remain. While several reviews discuss molecular methods and high-throughput genomics-based diagnostics (Studholme et al., 2011; Aslam et al., 2017; Hariharan and Prasannath, 2021; Ijaz et al., 2022; Chakraborty and Chakraborty, 2021), a comprehensive synthesis focusing explicitly on the evolving landscape of portable diagnostic platforms and their integration into digital agronomy frameworks has yet to be presented. Existing literature often addresses individual technologies—such as loop-mediated isothermal amplification (LAMP) devices or smartphone-based lateral flow assays—in isolation. However, a holistic review that connects these diverse technologies, elucidates their underlying principles, and critically examines their field applicability, data integration strategies, and regulatory frameworks is lacking. Moreover, as portable diagnostics move from proof-of-concept prototypes toward scalable, commercialized products, there is a pressing need to map out the challenges in validation, standardization, and quality assurance that must be surmounted for widespread adoption (Ahmad-Nejad et al., 2021). A recent review article by Nguyen et al. (2024) focuses on biosensors as one specific technology for plant disease diagnosis and highlights advances in biosensor technologies, focusing on technical and functional innovations in their design and application. Another review article provides a comprehensive overview of plant pathogen detection techniques, encompassing cultivation, PCR, sequencing-based methods, and immunoassays. It includes portable biosensors as a subsection, highlighting recent advancements and their application in point-of-care diagnostics (Venbrux et al., 2023). The above two articles provide a holistic, multidisciplinary perspective on portable plant pathogen diagnostics, while broader scope, deeper integration of digital technologies, and consideration of real-world challenges and future innovations set the present review apart as a more comprehensive resource.
This review addresses critical knowledge gaps in plant pathogen diagnostics by offering a comprehensive perspective on portable solutions. It examines the core principles and technologies behind handheld analyzers, smartphone-integrated biosensors, and microfluidic-based lab-on-a-chip systems while highlighting the transformative role of IoT, cloud-based analytics, artificial intelligence (AI), and machine learning (ML) in enhancing data interpretation, connectivity, and global disease surveillance.
The review explores applications across bacterial, viral, and fungal pathogens, emphasizing how on-site testing informs disease surveillance, management decisions, and early warning systems. It addresses performance evaluation, validation in resource-limited settings, regulatory challenges, and commercial applicability to bridge the gap between innovation and real-world implementation. Additionally, it identifies future innovations, such as scalable manufacturing, cost reduction, and stakeholder engagement, aimed at advancing accessible and effective portable diagnostics. Through this integrated approach, the review provides actionable insights to drive advancements in plant pathogen diagnostics.
By providing a comprehensive analysis of the state-of-the-art in portable phytopathogen diagnostic technologies—alongside insights into data management, standardization, and integrated decision-making—this review will empower researchers, policymakers, industry stakeholders, and practitioners. Such an integrative understanding will not only help advance this rapidly evolving field but also support evidence-driven disease management strategies, enhance global surveillance networks, and pave the way for more resilient and sustainable agricultural systems.
2 Portable diagnostic technologies for plant pathogen detection 2.1 General principles and core components of phytopathogen detection devicesPortable phytopathogen detection devices integrate actuators and sensors initially developed for consumer electronics, including smartphones and smartwatches, to enable on-site, real-time diagnostics. Actuator components, such as miniaturized light-emitting diodes (LEDs), are capable of emitting wavelengths spanning the visible range (approximately 400–700 nm) and have been employed to stimulate fluorescence or other optical responses in biochemical assays, particularly in the detection of pathogen-associated molecular markers. LEDs for portable biosensing, when coupled with smartphone imaging, have been well documented, offering a promising route toward rapid, field-based diagnostics of plant pathogens (Gudkov et al., 2023). Similarly, near-field communication (NFC) modules operating at 13.56 MHz allow short-range interactions with magnetic or resistive sensing elements, thereby enabling wireless activation of biosensors and facilitating assessments of soil moisture or other environmental parameters that influence pathogen spread (Gawade, 2022).
Display screens in smartphones and smartwatches, with resolutions often exceeding 720 × 1,280 px, can emit controlled wavelength outputs—such as red (628 nm), green (536 nm), and blue (453 nm)—to serve as dynamic light sources for colorimetric analyses of plant extracts. In addition, vibration motors providing haptic feedback in the range of 130–180 Hz can be leveraged to enhance assay kinetics by mixing reagents directly in the field, while integrated speakers emitting acoustic signals are being explored to disrupt sample matrices or stimulate particular biochemical reactions (Fan et al., 2021). The integration of thermal actuators is also critical, as these elements enable precise temperature control essential for nucleic acid amplification tests (NATs), including polymerase chain reaction (PCR) assays, thus facilitating on-the-spot genomic detection of pathogens without the need for a fully equipped laboratory.
Sensor components are equally transformative. Imaging detectors, including high-resolution smartphone cameras equipped for UV–Vis spectrometry, provide powerful means to capture fluorescence, color changes, or other optical indicators of infection. These imaging strategies have proven helpful for plant disease phenotyping and early stress detection (Zubler and Yoon, 2020). Environmental light sensors enhance sensitivity by accounting for ambient conditions and improving the reliability of fluorescence or colorimetric quantifications. NFC readers serve dual roles as communication modules and near-field sensors, enabling rapid, localized measurements vital for wearable and in-field biosensors.
Initially designed for human-device interaction, capacitive touchscreen sensors are increasingly recognized for their ability to detect subtle changes in pressure, moisture, or conductivity when brought into contact with plant tissues, thereby providing indirect indications of infection or physiological stress. Wearable photodetectors integrated into smartwatches further expand the scope for continuous, mobile monitoring of plant health markers, and microphones sensitive to acoustic signals emitted during microbial metabolism or pathogen-vector interactions offer unique, non-invasive diagnostic approaches (Xu et al., 2024). Coupling these sensory capabilities with geolocation data from GPS modules enables spatial mapping of disease outbreaks and supports the implementation of data-driven management strategies (Xin et al., 2009).
The convergence of these actuators and sensors within portable platforms is revolutionizing how researchers and field professionals monitor phytopathogens. Smartphones and smartwatches have evolved into versatile biosensing tools, providing real-time imaging, thermal regulation, optical stimulation, wireless connectivity, and acoustic or tactile feedback (Buja et al., 2021). These devices facilitate on-site nucleic acid amplification and rapid pathogen detection and enable continuous environmental and plant health monitoring. These integrated technologies can guide timely interventions by tracking chlorophyll fluorescence fluctuations indicative of early disease onset, mapping pathogen prevalence across agricultural landscapes, and detecting subtle changes in leaf temperature profiles. As a result, mobile devices equipped with tailored actuators and sensors are poised to become indispensable in modern precision agriculture, improving our capacity to detect, map, and manage phytopathogens and safeguarding global crop productivity and food security.
Figure 1 shows that portable phytopathogen detection devices integrate sensor components (such as imaging detectors, light sensors, and GPS modules) and actuator components (like LEDs and thermal actuators) to enable various applications, including phytopathogen detection, agricultural monitoring, and remote sensing for enhanced crop management.

Figure 1. Components of portable phytopathogen detection devices and their applications.
2.2 Classification and overviewFigure 2 illustrates various advanced biosensing technologies categorized based on their unique functionalities. Immunoaffinity devices provide sensitive detection using fluorescence techniques, while imaging biosensors enable label-free, multiplexed biomarker detection. Plasmonic sensors facilitate high-throughput screening of biomolecular interactions, and lab-on-a-chip systems integrate microfluidics for rapid analyte detection. Additionally, optofluidic devices offer real-time monitoring of molecular interactions, and smartphone integration enables biosensing in resource-limited settings by leveraging portable and accessible technologies. Together, these innovations highlight the convergence of biosensing technologies’ precision, portability, and scalability.
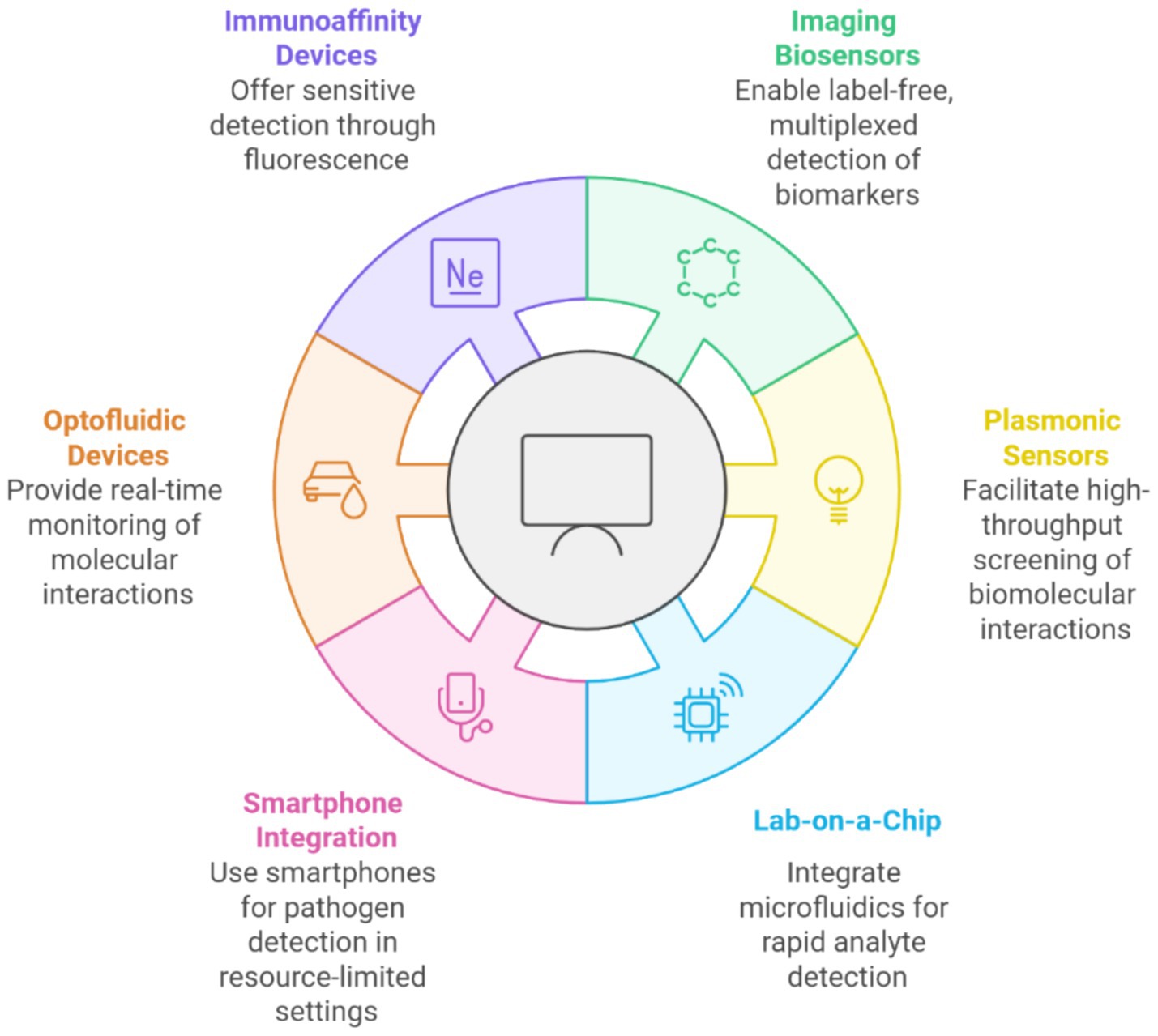
Figure 2. Emerging technologies in biosensing for rapid and sensitive detection.
2.2.1 Handheld analyzers and mobile biosensorsHandheld analyzers and mobile biosensors are essential for rapid, on-site diagnostics, especially in environments where traditional laboratory infrastructure is unavailable. These portable devices integrate advanced biosensing technologies, enabling the detection of pathogens, chemicals, and other analytes directly in the field. Their compact size, ease of use, and fast response times make them highly suited for clinical diagnostics, environmental monitoring, and resource-limited settings that require immediate results.
A significant innovation in this space is using plasmonic biosensors, which utilize nanohole arrays and CMOS camera technology for sensitive, label-free viral detection. Its adaptability lies in using a plasmonic chip surface coated with specific antibodies, which can be modified to target different viral types depending on the surface coating (Cetin et al., 2021). Similarly, portable electrochemical biosensors, like those used for detecting hepatitis C, offer Bluetooth-enabled real-time monitoring. These devices use cyclic voltammetry to identify viral markers and boast a wireless setup with over three hours of battery life, making them ideal for mobile diagnostics (de Campos da Costa et al., 2019).
Integrating smartphones into biosensing technologies further enhances these tools by providing advanced data processing, storage, and sharing capabilities. For instance, a “three-in-one” biosensor detects infections and provides diagnostic information for each stage of the infection cycle, enabling continuous patient health monitoring (Dou et al., 2022).
Moreover, microfluidic technology has been incorporated into portable analyzers, allowing rapid and multiplexed detection. A paper-based microfluidic biosensor, for instance, has been developed to detect viral pathogens in samples. This electrochemical immunosensing platform can simultaneously perform enzyme-linked immunosorbent assays (ELISA) on multiple samples, offering a low-cost and user-friendly solution for point-of-care diagnostics (Zhao and Liu, 2016).
Another valuable addition to mobile diagnostic technology is interferometric biosensors, such as the Young interferometer sensor. These highly sensitive devices combine optical sensors with antibody-antigen recognition for rapid and accurate viral detection. Their portability and ease of use make them especially useful in remote areas that lack sophisticated laboratory facilities (Ymeti et al., 2007).
Beyond medical applications, handheld analyzers have also proven valuable in agricultural settings. For example, a bioelectric recognition assay has been tested to detect plant pathogens like Potato virus Y and Cucumber mosaic virus. This technology can process up to 96 samples in approximately 70 min, making it ideal for on-site pathogen diagnostics, where quick results are critical for preventing widespread crop damage (Perdikaris et al., 2011). Another example of this advancement is depicted in Figure 3, which demonstrates the design of a wearable biosensor glove with an integrated printed circuit board for real-time pathogen detection in various field conditions.
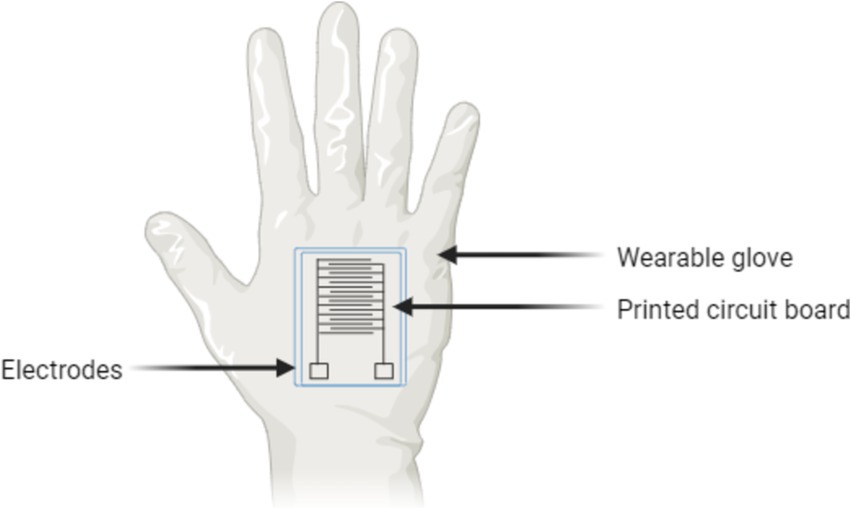
Figure 3. Wearable biosensor glove with integrated circuit for on-site pathogen detection.
2.2.2 Smartphone and smartwatch integrated diagnostic toolsSmartphone and smartwatch integrated diagnostic tools are transforming phytopathogen detection by combining molecular assays, AI-powered image analysis, and sensor technologies, providing rapid, accurate, and real-time pathogen identification in agricultural settings. Tools such as Smart LAMP use Loop-Mediated Isothermal Amplification (LAMP) to detect pathogens like Ralstonia solanacearum in crops like tomatoes and potatoes. This setup eliminates the need for complex thermocyclers operating at a single temperature. Using the smartphone camera to capture color changes in the LAMP reaction, the tool provides visual indicators of infection in real-time, making it highly accessible for remote or resource-limited environments (Ivanov et al., 2021).
The Biomeme two3 device integrates qPCR with smartphone connectivity, enabling DNA and RNA analysis for pathogens such as Fusarium oxysporum in bananas and tomatoes. This mobile platform uses fluorescence signals to quantify pathogen load in approximately 45 min, displaying results via a smartphone app. Smartwatches can extend the utility of this platform by receiving and displaying diagnostic alerts, allowing for immediate field-level responses even when the smartphone is not in use. This real-time data can be shared remotely, making it ideal for rapid, informed decision-making (Buja et al., 2021). Similarly, nano-sensor technology uses VOC detection to analyze biomarkers emitted by infected plants, allowing early disease detection even before visible symptoms appear. This smartphone-based system has proven effective in crops like wheat and potatoes, helping farmers proactively manage crop health (Li et al., 2019).
For image-based detection, ViT-SmartAgri utilizes a Vision Transformer model to classify tomato leaf images, distinguishing between healthy and diseased plants with high accuracy. By capturing and processing leaf images through the smartphone camera, ViT-SmartAgri provides instant diagnostic feedback, supporting early intervention against diseases like late blight and mosaic virus. Smartwatches can act as companion devices, offering quick access to diagnostic summaries and alerts, further improving efficiency in field operations (Alzahrani and Alsaade, 2023).
The Agdia ImmunoStrip® combines lateral flow assays with smartphone technology to detect Candidatus Liberibacter asiaticus, the pathogen behind the citrus greening disease. After the DNA amplification process, the test line’s visibility on the strip provides a straightforward readout, which can be captured and analyzed by a smartphone app. This tool has been instrumental in Florida citrus groves, allowing quick, in-field diagnostic results to mitigate disease spread (Russell et al., 2015).
2.2.3 Microfluidics and lab-on-a-chip systemsPortable microfluidic and lab-on-a-chip (LOC) systems fundamentally transform phytopathogen detection in agriculture. These compact devices integrate various functions, including sample preparation, amplification, and detection, onto a single chip, enabling quick responses to pathogen outbreaks and supporting proactive crop disease management. Examples such as the CRISPR-Cas13a-based centrifugal microfluidic system exemplifies these advancements, automating processes like nucleic acid extraction and detection within a single device, achieving sensitivity levels as low as one copy per reaction in plasmid samples, which is particularly useful for field applications requiring high sensitivity (Zhang et al., 2024).
Similarly, hand-powered microfluidic devices enable affordable sample processing by utilizing simple mechanisms, such as syringes, to facilitate digital PCR applications without external power sources, making them ideal for remote agricultural settings where cost-effective, high-throughput diagnostics are essential (Xie et al., 2021). Other LOC systems use LAMP-based technologies with electrochemical sensors capable of operating at lower temperatures (39°C), delivering real-time results within 20 min—an advantage for rapid, in-field diagnostics of viral phytopathogens (Hsieh et al., 2015).
Figure 4 illustrates a typical microfluidic LOC system for pathogen detection. The chip consists of several key components, including a collection point where the sample is introduced, a test strip for initial sample processing, and a mixing chamber that allows the sample to interact with reagents. The chip is also equipped with multiple reservoirs, which store necessary reagents and direct them through the system for diagnostic processes. This configuration enables efficient, automated pathogen detection by minimizing manual handling, integrating various analytical methods, and facilitating high-throughput testing suitable for on-field diagnostics.
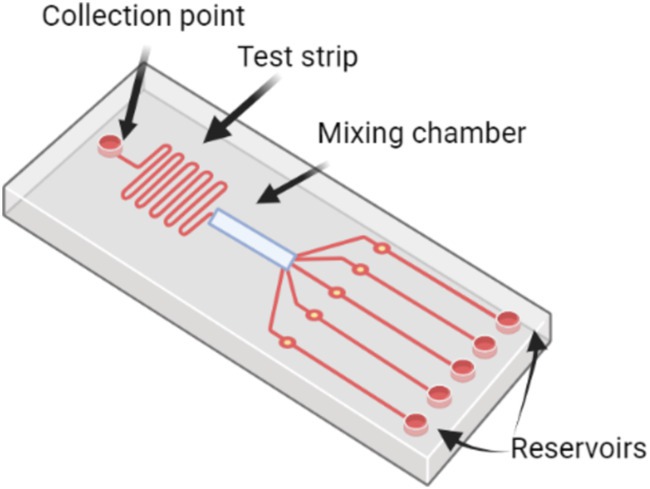
Figure 4. Schematic of a microfluidic lab-on-a-chip system for pathogen detection.
More advanced microfluidic devices, such as the mChip, expand on these capabilities with fluorescence-based detection for pathogens like Xylella fastidiosa, impacting crops such as olives. When integrated with smartphone-based fluorescence readers, this platform enables early pathogen detection with high accuracy, helping farmers in regions like Southern Italy promptly identify infections (Milson and Kevin, 2023). Similarly, multiplex platforms like PlexBio™ OptoSelect™ allow simultaneous detection of multiple pathogens. Using color-coded microbeads, PlexBio has successfully identified bacterial diseases such as spot, blight, and mosaic virus in Californian tomato farms within an hour, streamlining large-scale agricultural testing (Foudeh et al., 2012).
For simpler applications, paper-based microfluidic devices (e.g., μPADs) use capillary action for the colorimetric detection of pathogens without requiring external power. Such devices are precious in resource-limited environments across Southeast Asia, where visible color changes in response to pathogen presence enable users to interpret them more straightforwardly (Chen et al., 2021). Furthermore, ChemBio™ offers a chemiluminescent immunoassay-based solution that has proven effective in detecting pathogens associated with citrus greening disease in Chinese citrus groves, thereby allowing for tailored disease management practices (Hu et al., 2017).
Lastly, platforms like LabDisk use centrifugal forces for automated sample handling, directing fluids through preloaded reagents to reaction chambers for pathogen detection. This technology has been employed in vineyards to monitor Botrytis cinerea, responsible for grey mold, by reducing manual handling and facilitating high-throughput diagnostics suitable for large-scale field applications (Neethirajan et al., 2011).
2.3 Core technologies and methods employed in handheld analyzersPortable point-of-care (POC) devices for phytopathogen detection are essential in agricultural and environmental management, enabling on-site diagnostics and immediate decision-making to curb plant diseases. These devices’ portability and user-friendliness are designed for field deployment, allowing use by individuals without specialized training. Lightweight and compact, POC devices are optimized for rapid, on-site testing, crucial in areas lacking lab access. Their ability to deliver quick results in a few minutes to hours compared to lab tests that may take days—supports timely intervention to prevent further pathogen spread, which is especially critical in large-scale agricultural settings where delays can lead to economic losses (Buja et al., 2021; Safavieh et al., 2014). Table 1 lists the core technologies used in portable diagnostic devices.
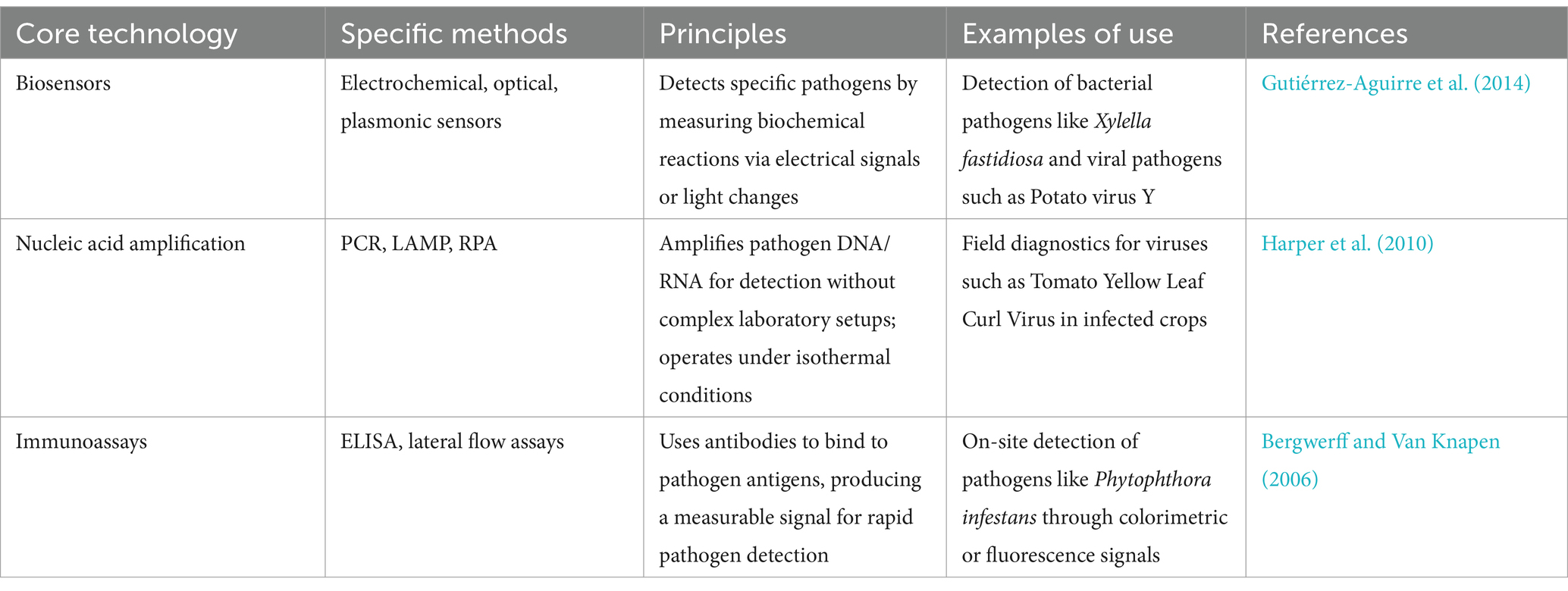
Table 1. Core technologies in portable diagnostic devices.
The latest developments integrate nucleic acid amplification and optical detection, allowing these devices to differentiate pathogenic from non-pathogenic organisms—critical for reliable diagnostics (Hsieh et al., 2015). Durability is equally important, as these devices often endure harsh conditions. Features like ruggedized casings, moisture resistance, and self-contained power sources ensure reliability and minimize maintenance in varied agricultural environments (Kou et al., 2020).
2.3.1 Biosensor technologiesPortable biosensor devices have revolutionized phytopathogen detection, enabling rapid, accurate, field-ready diagnostics supporting effective agricultural disease management. Key innovations include smartphone-based systems, LOC technology, bioluminescent cell-based sensors, and plasmonic biosensors, all offering practical alternatives to traditional laboratory methods.
Smartphone-based biosensors utilize smartphones’ cameras and processing power, often relying on colorimetric assays that change color upon pathogen detection. Custom apps then analyze these changes, providing rapid results ideal for resource-limited environments (Huang et al., 2018). LOC devices also enhance portability by miniaturizing lab processes onto a chip, allowing quick diagnostics with minimal reagents. For instance, LOC microcantilever-based biosensors can detect Salmonella at low concentrations of 103 CFU−1 mL, demonstrating their efficacy in field applications (Ricciardi et al., 2010).
Bioluminescent cell-based biosensors employ genetically engineered cells that emit light in response to specific pathogens, enabling high-intensity nanomolar detection levels suitable for direct field diagnostics (Roda et al., 2011). Enzyme-based biosensors with metal-organic frameworks (MOFs) are another portable option; these smartphone-assisted sensors provide real-time colorimetric detection, making them a cost-effective choice for agricultural pathogen monitoring (Kou et al., 2020). Additionally, nanoparticle-based biosensors enable sensitive pathogen detection by leveraging nanoparticles’ high surface area and functional versatility. Gold nanoparticles (AuNPs), known for their optical properties, produce visible color changes that assist in detecting agrichemicals, such as pesticides, within seconds, which is ideal for on-site testing in resource-limited settings (Baek et al., 2017). Magnetic nanoparticles further support pathogen detection; biosensors using Fe3O4 nanoparticles with acetylcholinesterase are highly sensitive and reusable, well-suited for repeated agricultural tests (Chauhan and Pundir, 2011).
Plasmonic biosensors amplify optical signals using metal nanoparticles, facilitating phytopathogen detection. Handheld devices with plasmonic microarrays provide quantitative data through computational imaging, offering reliable results with minimal sample prep (Cetin et al., 2014). Plant-wearable biosensors employing gold nanoparticles monitor pesticides in real-time, transmitting data to a smartphone for precision application and supporting sustainable agricultural practices (Zhao et al., 2020). Moreover, mobile apps like LAMP-CAM enhance diagnostics by utilizing colorimetric detection to identify pathogens such as Phytophthora infestans, a primary cause of late blight in crops. LAMP-CAM interprets color changes detected by a smartphone camera and translates them into diagnostic results, improving accuracy in remote areas (Raguraman et al., 2021).
Electrochemical biosensors are also noteworthy. For example, apps like PSoC Programmer and PSoC Creator connect to electrochemical sensors via USB OTG or Bluetooth, detecting pathogens like Xanthomonas oryzae in rice and enabling real-time data visualization (Currie, 2021). Meanwhile, ViT-SmartAgri uses deep learning for plant disease identification, employing a Vision Transformer (ViT) model to accurately differentiate between healthy and diseased tomato plants, providing a user-friendly disease management solution (Barman et al., 2024). Cloud-connected portable LAMP systems enhance biosensor capabilities by enabling rapid, on-site detection of Candidatus Liberibacter asiaticus, the citrus greening pathogen. These devices integrate fluorescence-based detection with smartphone apps for real-time data sharing, aiding swift management decisions (Fujikawa & Iwanami, 2012). Data is transmitted to cloud services, supporting centralized analysis and rapid outbreak response. Table 2 describes the fabrication methods used to develop sensors in phytopathogen diagnostics.
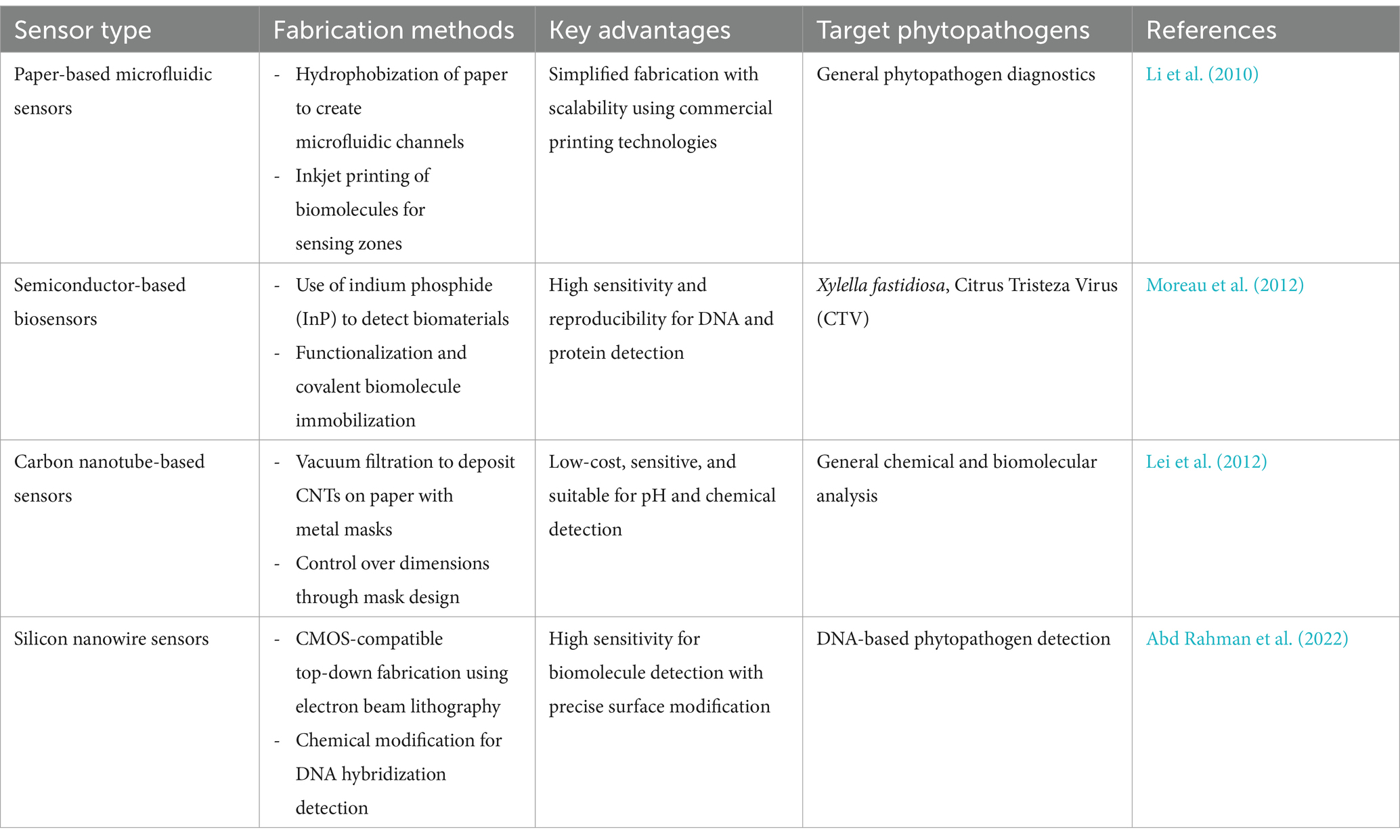
Table 2. Fabrication techniques for sensors in phytopathogen diagnostics.
Figure 5 presents two key biosensor mechanisms. In part A, the top schematic illustrates the interaction in an immunosensor where antibodies bind specifically to target proteins (analyte). This interaction is detected by a transducer, which processes the signal for data analysis. In part B, the bottom schematic shows a nucleic acid biosensor where DNA/RNA sequences interact with complementary DNA probes on the biosensor surface. The interaction generates a signal processed through the transducer, similar to immunosensors, to detect the presence of specific nucleic acids.
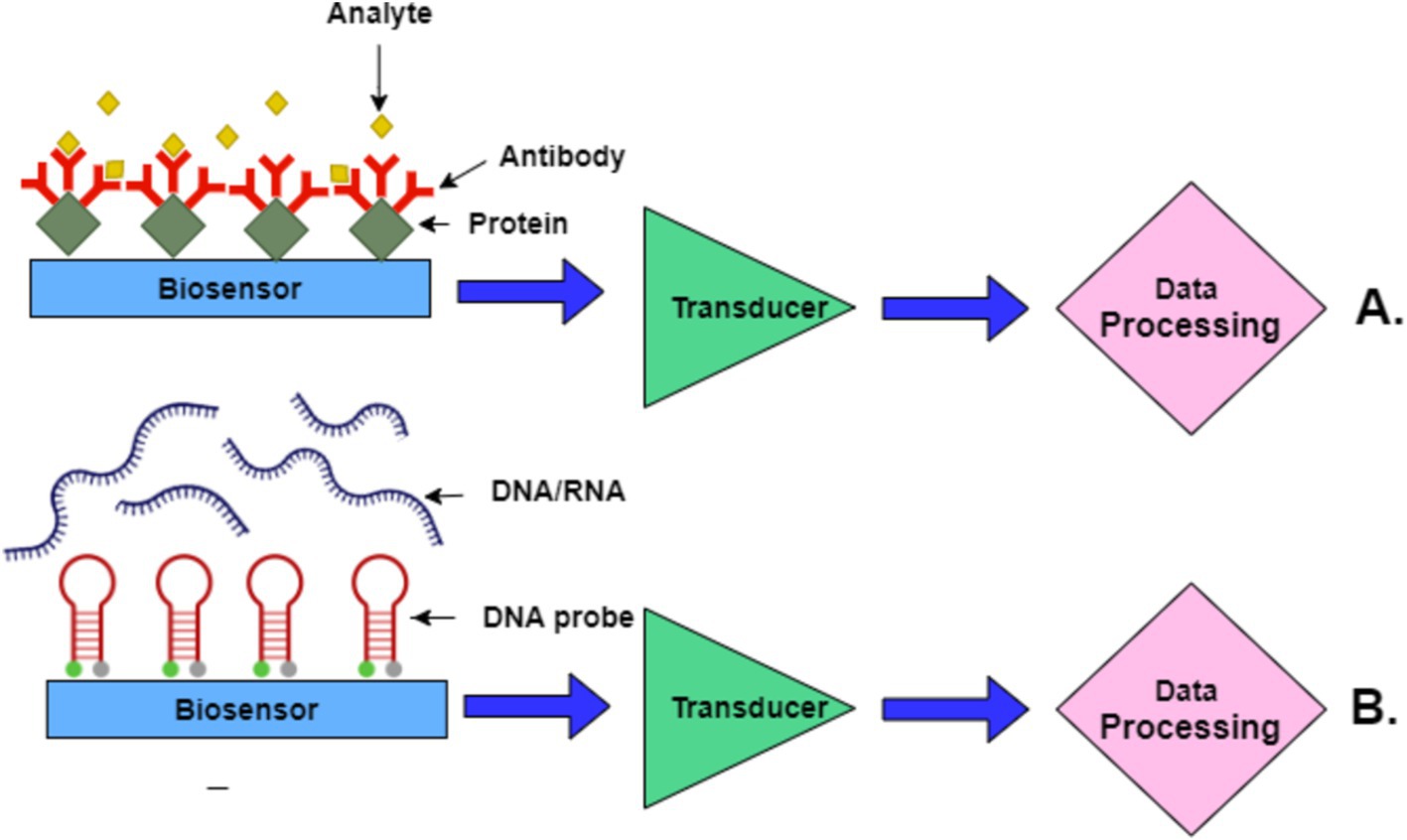
Figure 5. Schematic representation of biosensor mechanisms. (A) Immunosensor-based mechanism. (B) Nucleic acid-based biosensor mechanism.
Figure 6 illustrates the mechanism of aptamer-antigen interaction in biosensor applications. The process begins with an aptamer sequence, which forms a specific structure through folding. Once the aptamer assumes its active conformation, it selectively binds to the target antigen, creating a stable aptamer-antigen complex. This interaction is crucial in biosensor applications, as it triggers a detectable signal that can be used to identify and quantify specific pathogens or analytes in various agricultural and environmental samples.
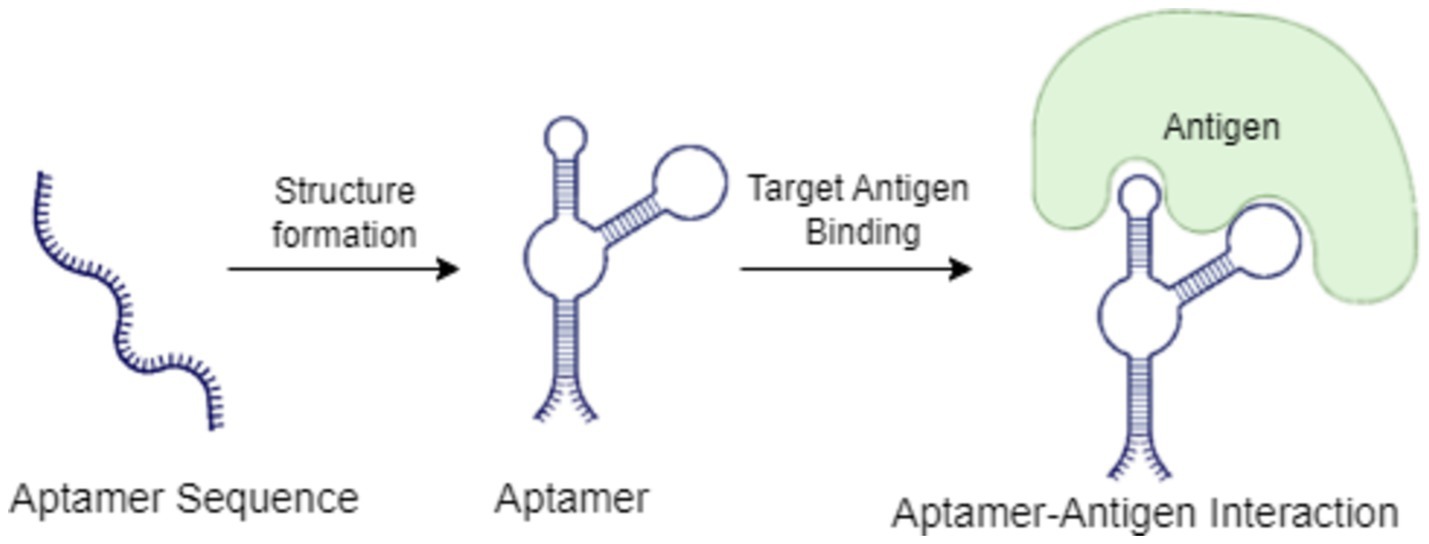
Figure 6. Mechanism of aptamer-antigen interaction in biosensor applications.
Figure 7 illustrates the operational mechanism of enzyme-based electrochemical biosensors for pathogen detection. In Pathway A, the enzyme catalyzes the conversion of an analyte into a product with the help of a mediator, which facilitates electron transfer to the electrode, generating a detectable signal. In Pathway B, the direct interaction between the enzyme and the electrode without a mediator results in the same outcome, demonstrating two common approaches used in biosensor technology.
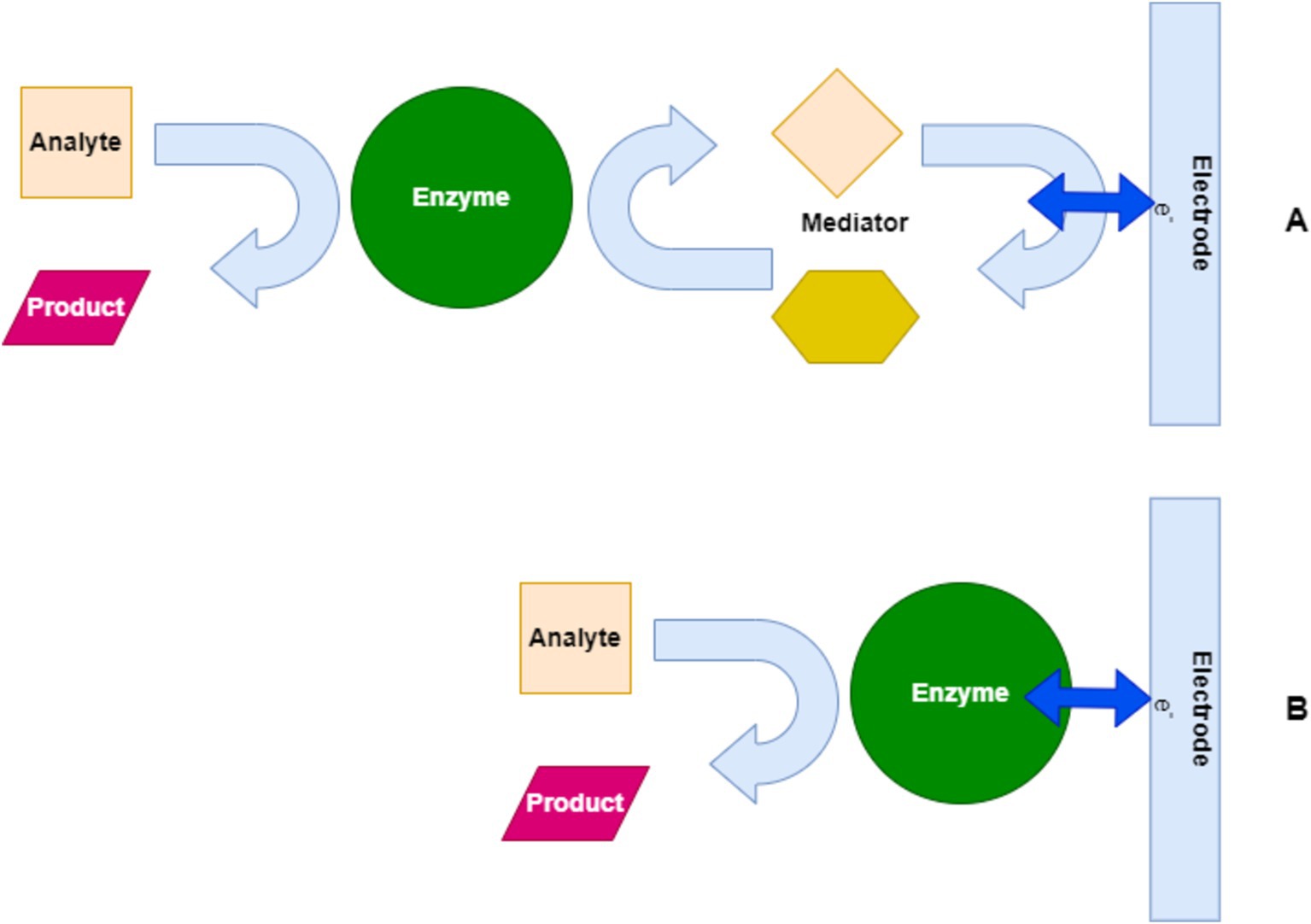
Figure 7. Mechanism of enzyme-based biosensors for pathogen detection. (A) Mediator-assisted enzyme-based biosensor. (B) Direct enzyme-based biosensor.
2.3.1.1 Optical biosensorsOptical biosensors detect changes in optical properties such as absorbance, fluorescence, or refractive index upon interaction with the target pathogen. Surface plasmon resonance (SPR) biosensors have been employed for label-free detection of plant viruses like Tobacco Mosaic Virus (TMV), offering real-time monitoring and high sensitivity (Hariharan and Prasannath, 2021). Surface SPR biosensors are increasingly used for the label-free and highly sensitive detection of various plant viruses beyond TMV. These sensors provide real-time monitoring and can be specifically tailored to detect various plant viral pathogens. An SPR biosensor has been developed to detect MCMV by utilizing a gold surface coated with 11-mercaptoundecanoic acid, which is then functionalized with anti-MCMV antibodies. This setup achieved a detection limit of around 1 part per billion (ppb), showcasing its high sensitivity compared to conventional methods like ELISA (Zeng et al., 2013). In another application, SPR biosensors were used to monitor the interaction of Potato Virus Y with monoclonal antibodies, allowing researchers to study various serotypes and their interactions with antibodies in real-time. This biosensor setup was beneficial for optimizing serologic diagnostic tools and enhancing our understanding of viral serotype variability (Gutiérrez-Aguirre et al., 2014).
Fluorescence-based sensors utilize fluorescent markers that emit signals upon binding to the target pathogen. Portable fluorescence detectors have been developed to detect pathogens like Erwinia amylovora, the causative agent of fire blight in apples and pears. Figure 8 depicts an optical sensor where an analyte interacts with a sensing element, altering light from a source, which is then detected by a photodetector to measure the analyte.
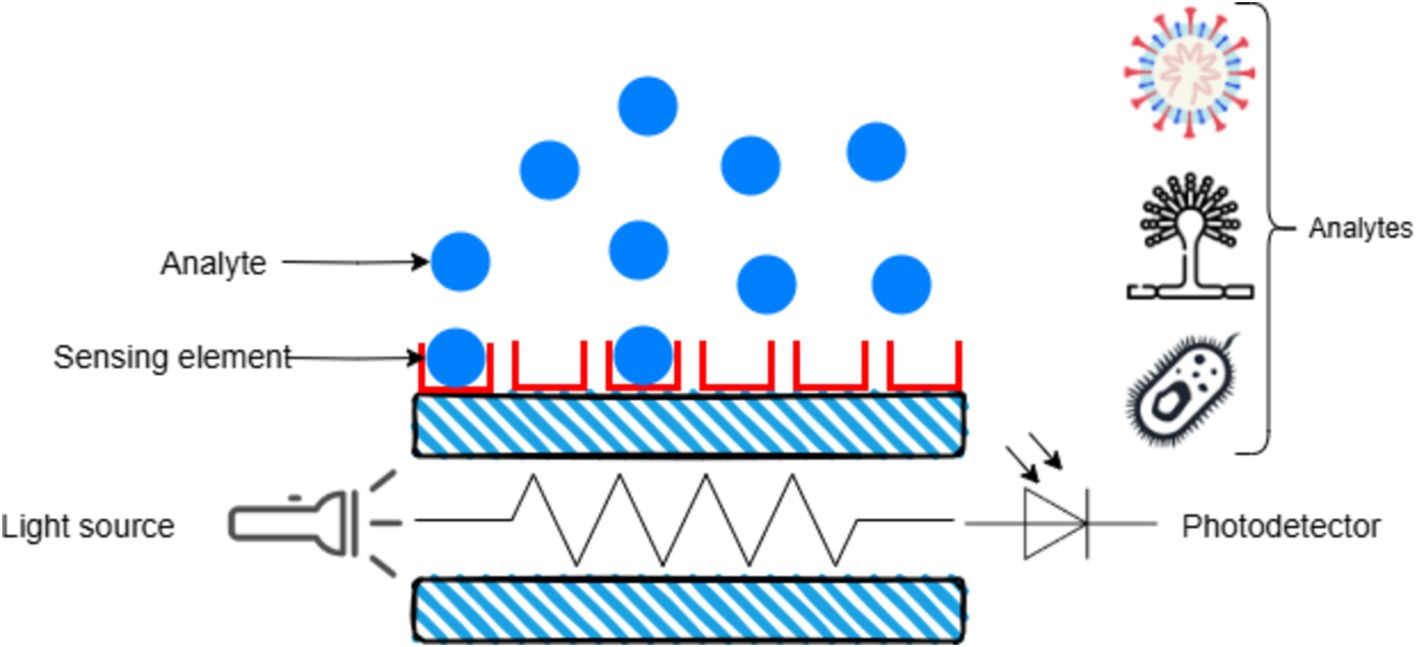
Figure 8. Working principle of optical biosensor.
2.3.1.2 Electrochemical biosensorsElectrochemical biosensors, which detect changes in electrical signals caused by biochemical reactions, have emerged as powerful tools for detecting pathogens in agriculture. By incorporating nanomaterials like gold nanoparticles, carbon nanotubes, and quantum dots, these sensors have significantly advanced in-field diagnostics, offering rapid and sensitive detection of phytopathogens.
Gold nanoparticles, widely known for their signal-amplifying properties, play a key role in lateral flow assays. These nanoparticles bind to pathogen-specific antibodies, enabling quick, on-site diagnostics without lab-based testing. For instance, they have been effectively used to detect Phytophthora infestans, a major pathogen responsible for potato blight, supporting disease management directly in the field (Bobrinetskiy et al., 2021; Kashyap et al., 2016b). Similarly, carbon nanotubes, prized for their high conductivity, enhance signal transduction in biosensors, making them particularly useful in detecting viral pathogens like the Tomato Yellow Leaf Curl Virus. These nanotubes detect even minute concentrations of viral DNA, crucial for early intervention and preventing widespread outbreaks (Rajwade et al., 2020; Attaluri and Dharavath, 2023).
Another innovative approach involves the use of quantum dots in smartphone-integrated biosensors. These quantum dots fluoresce when they interact with target genetic material, and the fluorescence is captured and analyzed by smartphone cameras, offering instant and quantitative pathogen assessments. This technology, used to detect fungal pathogens like Fusarium oxysporum, enables rapid decision-making and facilitates data sharing across networks, improving coordinated responses to agricultural threats (Rajwade et al., 2020; Attaluri and Dharavath, 2023).
Enzyme-linked biosensors are another vital tool for field diagnostics. These sensors utilize enzyme-catalyzed reactions to produce measurable signals, such as colorimetric or electrochemical outputs, allowing on-site detection without specialized lab equipment. Miniaturized versions of ELISA are widely used to detect viruses such as Plum Pox Virus in stone fruits and Maize Chlorotic Mottle Virus in maize. By linking pathogen-specific antibodies to enzymes that produce a visible color change upon pathogen detection, these devices offer real-time monitoring capabilities with sensitivity comparable to PCR, making them essential for prompt agricultural interventions (Ali et al., 2021; Ray et al., 1856).
Beyond viral detection, enzyme-linked biosensors have also proven effective in identifying bacterial and fungal pathogens, such as Fusarium oxysporum, which causes wilt disease in tomatoes. These sensors utilize peroxidase-based reactions to generate optical signals upon detecting fungal markers, making them ideal for on-site testing in remote agricultural areas where lab access is limited. Additionally, these biosensors are also capable of monitoring organophosphate pesticide residues. Acetylcholinesterase-based sensors detect pesticide residues by measuring enzyme inhibition, providing a dual benefit of pathogen and pest monitoring, which is crucial for sustainable agricultural practices (Ali et al., 2021; Kaur and Singh, 2020; Karadurmus et al., 2021).
Figure 9 illustrates an electrochemical biosensor designed for phytopathogen detection. The analyte, such as microbial pathogens or their markers, interacts with a specific receptor (antibody, DNA, lipid, or peptide) immobilized on a nanoparticle matrix. This interaction generates a signal transduced into an electrochemical output, enabling pathogen identification and quantification.
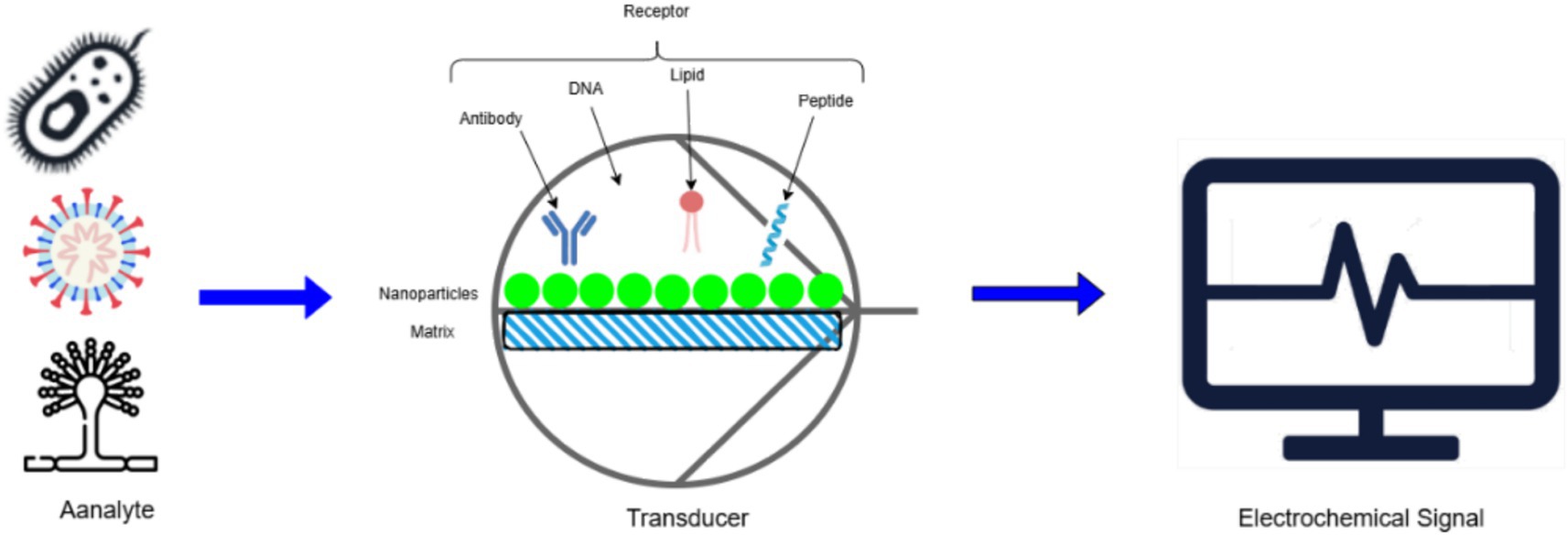
Figure 9. Schematic representation of electrochemical biosensor for phytopathogen detection.
2.3.2 Nucleic acid amplification techniques 2.3.2.1 Isothermal amplification methodsIsothermal nucleic acid amplification techniques, like LAMP and recombinase polymerase amplification (RPA), offer the advantage of operating at constant temperatures, eliminating the need for complex thermal cycling equipment, characteristic of PCR-based methods. These techniques are increasingly integrated into portable, field-deployable devices for rapid diagnostics. For instance, LAMP assays have been successfully used in portable formats to detect plant pathogens like Phytophthora infestans, the causative agent of late blight in potatoes, with amplification times usually under 30 min and colorimetric readouts for ease of interpretation (Ristaino et al., 2020). Similarly, RPA technology, operating at low temperatures (37–42°C), has been employed in handheld devices for the detection of viruses, such as the Tomato Yellow Leaf Curl Virus (TYLCV), making it highly suitable for field diagnostics (Wang and Yang, 2019). Figure 10 illustrates a detailed workflow for portable isothermal nucleic acid amplification techniques, including sample collection, preparation (involving cell lysis and nucleic acid purification), and the essential primer binding step for amplification.

Figure 10. Workflow of portable isothermal nucleic acid amplification method.
2.3.2.2 PCR-based portable devicesPCR is a critical molecular biology technique known for its high specificity and sensitivity in nucleic acid amplification and pathogen detection. Traditionally confined to centralized labs due to the complexity of thermal cycling and real-time detection, recent advancements in microfluidics, miniaturization, and power efficiency have created portable PCR devices. Portable PCR devices replicate the core functions of conventional machines. Still, they are optimized for field use through features such as battery-operated thermal cyclers and microfluidic chambers, which enable rapid temperature cycling and efficient reagent use. Integrated fluorescence detection further allows real-time monitoring of DNA amplification, making quantitative PCR (qPCR) feasible in non-laboratory environments. These innovations have made portable PCR invaluable for detecting pathogens, including plant and bacterial contaminants, especially in on-site diagnostics and environmental assessments (Koo et al., 2013).
One notable example of these advancements is the handheld qPCR device, which offers high specificity and sensitivity for detecting plant pathogens on-site. This compact and user-friendly tool is particularly beneficial in agriculture, where early detection of diseases can prevent significant crop losses (DeShields et al., 2018). Additionally, devices such as the BioFire FilmArray system integrate multiple processes, from nucleic acid extraction to real-time PCR, into a single platform, delivering results in under an hour, demonstrating the potential of portable PCR devices in decentralized pathogen detection (Pham et al., 2024).
Field-based PCR and other molecular devices have revolutionized pathogen detection by providing real-time diagnostics in agricultural settings (Figure 11). These tools allow rapid identification of diseases, enabling timely intervention. Devices like the miniPCR, a compact thermocycler, have been effectively used to detect Phytophthora infestans, the causative agent of potato late blight, providing lab-equivalent results in minutes. The miniPCR connects to smartphones or tablets for real-time result monitoring, crucial for field-based diagnostics in remote areas (Gonzalez-Gonzalez et al., 2020; Ssengo et al., 2020). Similarly, the Biomeme two3 platform integrates qPCR with smartphone technology, simultaneously supporting up to three reactions and allowing real-time pathogen detection, such as Fusarium oxysporum, within 45 min. The system’s smartphone interface facilitates remote data sharing and decision-making, enhancing its utility in the field (Buja et al., 2021; Marx, 2015).
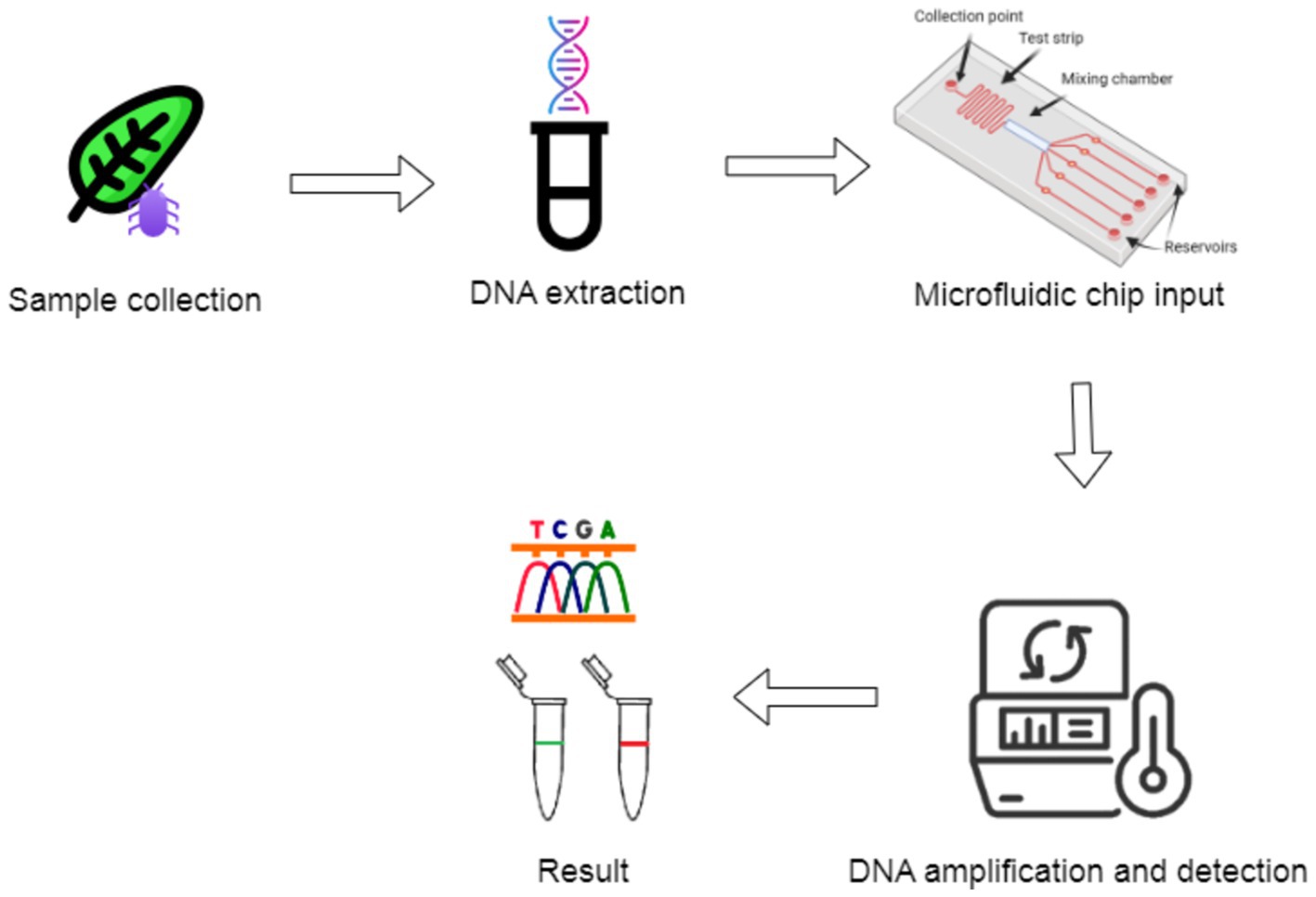
Figure 11. Portable PCR workflow with the integration of microfluidics for rapid DNA amplification and detection.
2.3.3 Immunoassay-based portable analyzersImmunoassays leverage the specificity of antigen-antibody interactions for pathogen detection. Lateral Flow Immunoassays (LFIA) are commonly used for their simplicity and rapid results. Portable devices utilizing LFIA have been developed for on-site detection of various plant pathogens, including Xanthomonas species in citrus plants (Srinivasan and Tung, 2015). These assays provide results within minutes and are user-friendly, requiring minimal training.
Figure 12 illustrates the portable ELISA process, operating on the same principle as LFIA, utilizing antigen-antibody interactions for pathogen detection. In ELISA, antibodies are first immobilized on the plate to capture target proteins, similar to how LFIA detects pathogens like Xanthomonas species in citrus plants. This cost-effective and widely used technique ensures selective binding, essential for accurate detection. The target protein binds to the immobilized antibodies, reflecting the precise interaction seen in LFIA. A secondary enzyme-conjugated antibody then binds to a different epitope, forming a sandwich structure that mirrors the detection phase of LFIA. Finally, a substrate is added, reacting with the enzyme to produce a visible color change, confirming the presence of the target. This rapid detection is akin to LFIA’s quick results in field applications. The figure effectively shows how ELISA and LFIA, though different in their settings, share the same core immunoassay principles for pathogen detection.
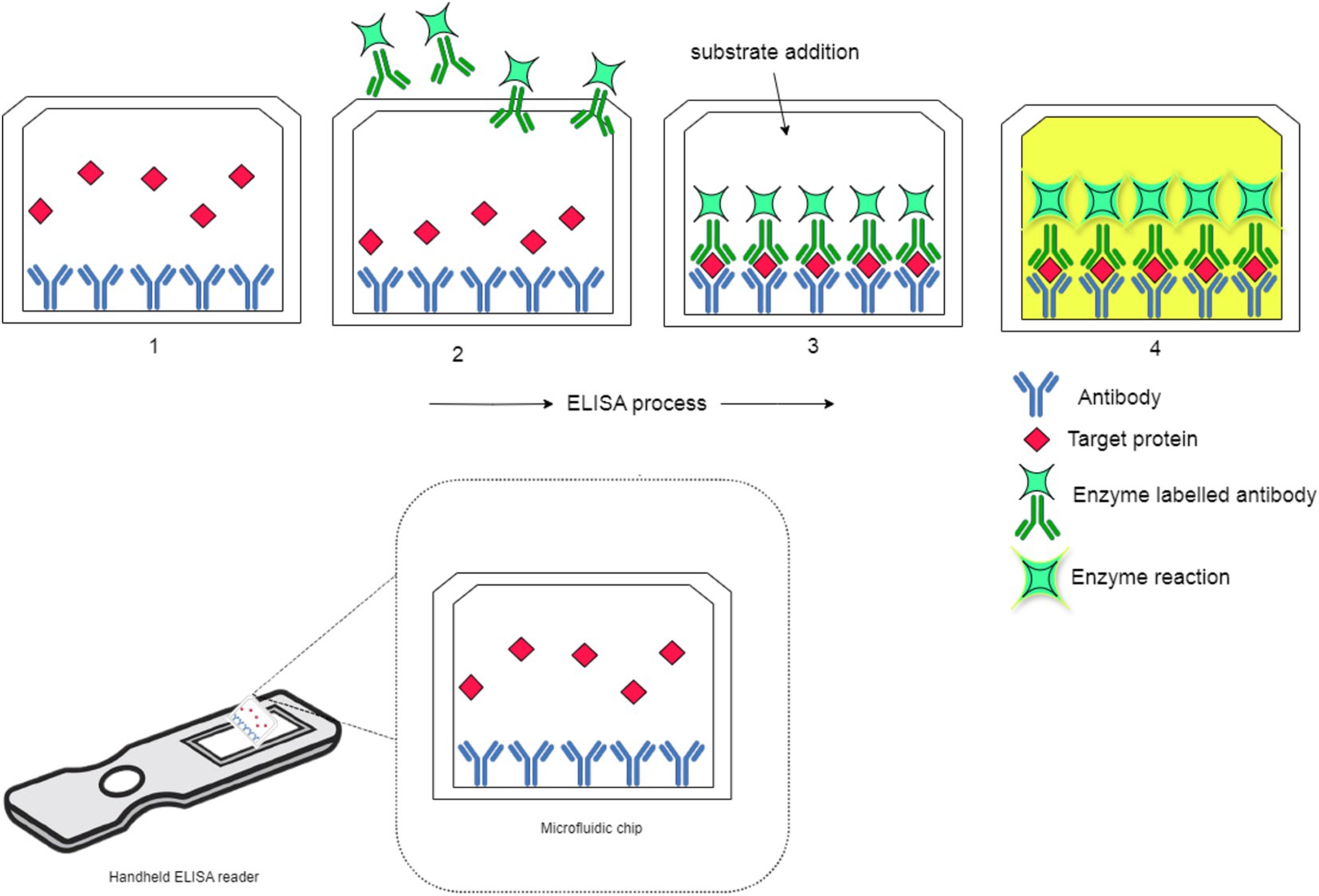
Figure 12. Schematic representation of the portable ELISA process.
Portal lateral flow assays (LFAs) and immunoassay devices are vital for rapid and cost-effective
留言 (0)