Excessive fluoride exposure is identified by the World Health Organization as one of the top ten chemicals that pose significant public health problems (1). Long-term exposure to excessive fluoride can result in fluorosis, and it may cause systemic health problems, including skeletal damage, such as dental fluorosis and skeletal fluorosis (2), and non-skeletal damage affecting the cardiovascular system, renal function, and the nervous system, among others (3). Of particular concern is cardiovascular damage that can cause a heavy disease burden in populations with high fluoride exposure, potentially becoming a public health concern in areas endemic to fluorosis (4).
An increasing number of human epidemiological studies have linked fluoride exposure to several cardiovascular diseases. Some studies have reported a positive relationship between fluoride levels in drinking water and the prevalence of hypertension, specifically high systolic blood pressure (5–7). A systematic review reports that high fluoride exposure can increase thyroid stimulating hormone (TSH) release, which may rise the risk of cardiovascular diseases (8). A cross-sectional study reveals a significant positive relationship between excessive fluoride exposure and the prevalence of carotid artery atherosclerosis (9). Previous studies have confirmed that elastic properties of the aorta are impaired in fluorosis patients and that fluorosis patients have left ventricular diastolic and global dysfunctions despite normal left ventricular systolic function (10, 11).
A study involving 61 patients establishes a positive relationship between fluoride uptake by the coronary arteries and cardiovascular risk (12). Moreover, some studies have revealed the association between fluoride exposure and myocardial disease. A case of fluoride poisoning in children is presented as ventricular arrhythmias (13). A hospital-based study has also confirmed that fluoride could develop arrhythmias in children with fluorosis (14). However, a cohort study in Sweden investigates that long-term drinking-water fluoride exposure is not associated with myocardial infarction (15). To the best of our knowledge, no study focuses on the relationship between excessive fluoride exposure and myocardial ischemia or arrhythmias in adults.
Meanwhile, some human epidemiological studies have focused on the relationship between fluoride intake from drinking water and myocardial function biomarkers. The National Health and Nutrition Examination Survey (2013–2016) among United States adolescents finds that fluoride intake from drinking water does not associate with the serum aspartate aminotransferase (AST) level (16). An epidemiological study on children reports that serum lactate dehydrogenase (LDH) activity is associated with fluoride intake from drinking water (17). However, no human epidemiological studies have investigated the relationship between fluoride intake from drinking water and the other effective indicators of cardiac function in adults, such as creatine kinase (CK), CK isoenzyme (CK-MB), and alpha-hydroxybutyrate dehydrogenase (α-HBD).
Therefore, this cross-sectional study, conducted in areas of Shanxi Province, China, where fluoride levels in drinking water are elevated, aims to investigate the relationship between fluoride exposure and myocardial disease, specifically in relation to myocardial enzymes in adults.
2 Materials and methods 2.1 Study populationThree villages—Gaoche, Xishe, and Xihan—located in Wenshui County, Shanxi Province, China, were selected as the investigation sites based on long-term monitoring conducted by the Shanxi Institute of Endemic Disease Prevention and Control. The fluoride concentration of drinking water in Xishe village was 1.45 mg/L. In comparison, it was 1.5 mg/L in both Gaoche and Xihan villages, exceeding the Chinese government’s stipulated limit for drinking water standards (1.2 mg/L). The inclusion criteria for our study were as follows: Villagers aged 18 years or above, who were born and have lived in these three villages. A total of 1,096 villagers were included. The exclusion criteria were as follows: (i) Villagers with diseases related to the heart, liver, muscle, or bone and had taken related medications in recent weeks (n = 1); (ii) villagers who did not provide a fasting blood sample (n = 311); (iii) villagers who did not provide their urinary sample (n = 47). In total, 737 villagers were enrolled for subsequent analysis.
2.2 General and physical information collectionGeneral and physical information, including demographic data (age, sex, education, family income, alcohol consumption, and smoking), and disease history, were collected by trained doctoral and postgraduate students using face-to-face interviews. The height, weight, and waist circumference (WC) were also measured by trained doctoral and postgraduate students based on the Chinese government’s weight control healthcare service standards (GB/T 34821–2017). Blood pressure was measured thrice in the morning using an electronic sphygmomanometer. The body mass index (BMI) was calculated based on the height and weight.
2.3 Sample collection, determination, and quality controlNurses collected 5 mL of fasting peripheral blood samples from each participant. The blood sample was centrifuged at 3,000 rpm for 10 min in 2 h, and the serum was transferred into 1.5-ml Eppendorf (EP, Corning Incorporated, New York, USA) tubes to detect myocardial function biomarkers and blood glucose levels. A 5-ml morning urine sample was also collected from each participant. All serum and urine samples were stored at −80°C in a refrigerator until analysis.
Urinary fluoride, an accepted internal measurement index of fluoride exposure (18), was detected using fluoride-ion selective electrodes according to the industry-standard method in China (WS/T 89–2015, Beijing, China). Each sample was analyzed twice, and the average result was used as the final urinary fluoride concentration.
Serum CK, serum CK-MB, serum LDH, serum α-HBD, serum AST, and blood glucose were measured using an automatic biochemical analyzer 3,100 (Hitachi Hi-TECH international TRADE Co., LTD, Shanghai, China). The reagent used for the measurement was provided by MedicalSystem Biotechnology Co. Ltd. (Ningbo, China), and the tests were performed according to standard operating procedures (details are shown at https://www.nbmksw.com/). Cut-off points of elevation for each myocardial enzyme are shown in Supplementary Table S1. These points are based on the industry standard of reference intervals for common clinical biochemistry tests in China (WS/T 404.1-2012, Beijing, China) and a previous study (19).
2.4 Diagnosis of diabetes mellitus, hypertension, skeletal fluorosis, and cardiac abnormalityDiabetes mellitus and hypertension were diagnosed through fasting blood glucose measurements and blood pressure readings. The cut-off points of fasting blood glucose for diabetes mellitus was 6.1 mmol/L, which was recommended by the Guideline for the Prevention and Treatment of Type 2 Diabetes Mellitus in China (2020 edition). The cut-off points of hypertension was 140/90 mm Hg, which was recommended by the Chinese Hypertension League Guidelines on Ambulatory Blood Pressure Monitoring (2020). Standard simultaneous 12-lead electrocardiogram (ECG) examinations were recorded at a sampling rate of 10,000 Hz (MedEx-1694, Beijing Madix Technology Co. Ltd, Beijing, China) and stored for subsequent analysis. The same technician conducted all ECG examinations and the diagnoses were made by a cardiologist and a specially trained ECG healthcare professional. Myocardial ischemia and arrhythmia were diagnosed based on the previous studies (20, 21). Skeletal fluorosis was diagnosed following the Chinese Diagnostic Criteria of Endemic Skeletal Fluorosis (WS 192–2008, Beijing, China).
2.5 Statistical analysisMean ± standard deviation (SD) or median (P25–P75) was employed to describe the continuous variables, and the categorical variables were expressed as numbers (percentages). The normal distribution test for the levels of each myocardial function biomarker was conducted by P–P chart. The urinary fluoride concentration was divided into four categorical values based on quartile and was used for further statistical analyses. Based on our prior knowledge and the directed acyclic graph, age, sex, educational level, family income, alcohol consumption, smoking, BMI, WC, diabetes mellitus, and hypertension were selected as potential confounders (Supplementary Figure S1). Binary logistic regression models were used to investigate the relationship between urinary fluoride concentration and myocardial damage.
Stratified analyses by age (<60 years and ≥ 60 years), sex (male and female), BMI (normal and overweight/obesity), WC (normal and central obesity), alcohol consumption (yes and no), and smoking (yes and no) were conducted. Furthermore, sensitivity analyses were used to test the robustness of the main results, while participants who had diabetes mellitus or hypertension were excluded.
Data analyses were performed using R statistical software (version 4.2.1; R Core Team, New Jersey, USA) and Statistical Package for the Social Sciences (SPSS) version 23.0 for Windows (SPSS, Inc., Chicago, IL, United States), and two-sided p-values less than 0.05 were considered statistically significant.
3 Results 3.1 Participant characteristicsThe demographic statistics of 737 participants are presented in Table 1. The mean (±SD) age of the participants was 57.78 (±11.54) years and the age of 47.20% of them were above 60 years. There were more female individuals (67.17%) in this study than male counterparts (32.83%). The proportion of individuals with higher educational levels was low, those with primary or lower accounted for 36.50%, the junior high school graduates were 53.03%, and the senior high school or higher were only 10.47%. Approximately 19.75% of participants were alcohol consumers and smokers. In addition, the family income of 61.90% of participants was above 10,000 ¥/year.
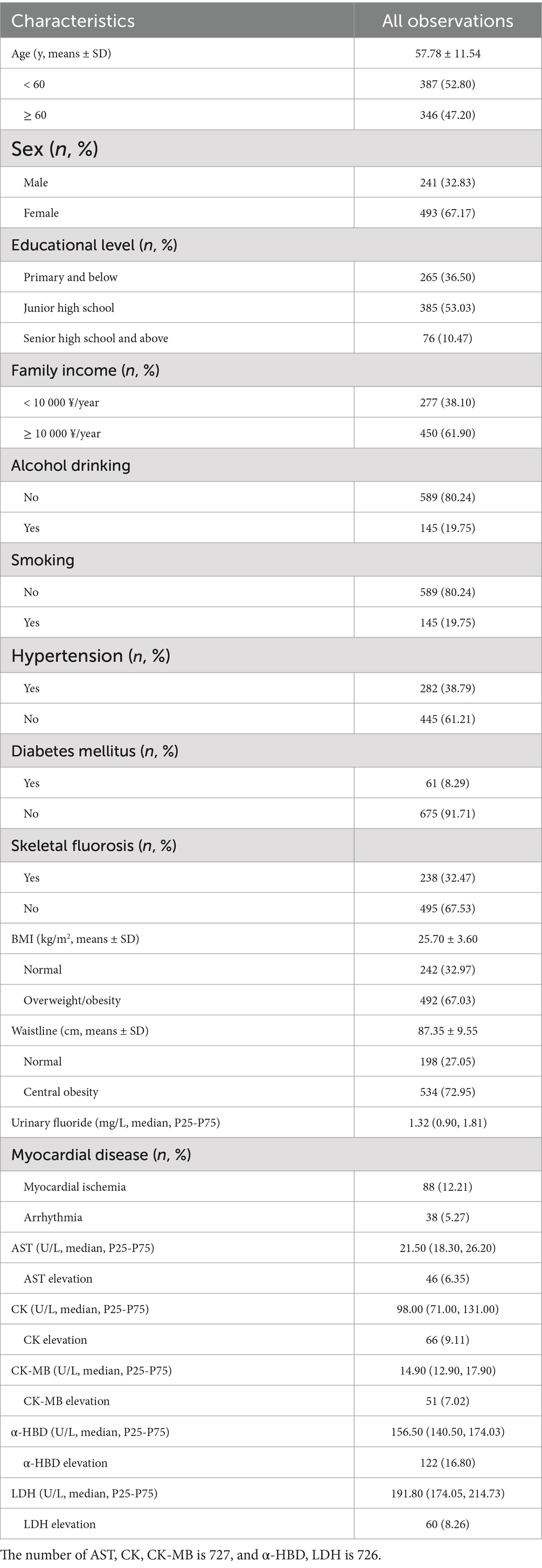
Table 1. Basic characteristics of general population (n = 737).
Physical statistics found that 38.79% of participants had hypertension, 8.29% of participants had diabetes mellitus, and 32.47% of participants had skeletal fluorosis. The mean (±SD) BMI of participants was 25.70 (±3.60) kg/m2 and 67.03% of participants were found to be overweight and obese. In addition, the mean (±SD) WC was 87.35 (±9.55) cm, and 72.95% of participants had central obesity. ECG examinations found that 88 (12.21%) participants had myocardial ischemia and 38 (5.27%) participants had arrhythmia.
Urinary fluoride, serum levels of myocardial enzymes, and the proportion of myocardial enzyme elevation were also shown in Table 1. The median (P25–P75) level of serum CK, CK-MB, LDH, α-HBD, and AST were 98.00 (71.00–131.00) U/L, 14.90 (12.90–17.90) U/L, 191.80 (174.05–214.73) U/L, 156.50 (140.50–174.03) U/L, and 21.50 (18.30–26.20) U/L. The level of urinary fluoride concentration was 1.32 (0.90–1.81) mg/L.
3.2 Association between urinary fluoride concentrations and the risk of myocardial damageThe relationship between urinary fluoride concentration and the risk of myocardial damage is shown in Table 2. Binary logistic regression analysis found that the urinary fluoride concentration was positively associated with the risk of serum CK elevation (OR = 1.39 [95% CI: 1.09–1.78]) and CK-MB elevation (OR = 1.49 [95% CI: 1.12–1.97]).
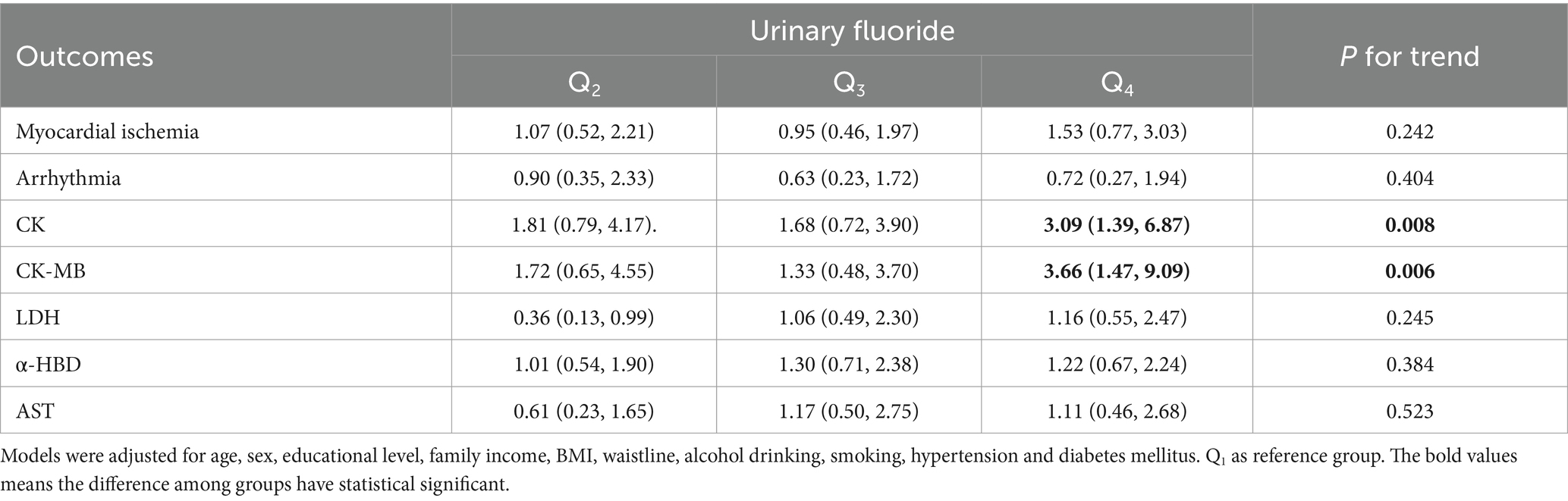
Table 2. Associations between urinary fluoride concentrations and the risk of myocardial damage.
Sensitivity analysis showed that the urinary fluoride concentration was also positively associated with the risk of serum CK elevation (OR = 1.73 [95%CI, 1.25–2.39]) and CK-MB elevation (OR = 1.80 [95% CI: 1.23–2.63]) when excluding participants with hypertension (Supplementary Table S2). In addition, when excluding participants with diabetes mellitus, we still found a positive association between urinary fluoride concentration and the risk of serum CK elevation (OR = 1.56 [95% CI: 1.20–2.02]) and CK-MB elevation (OR = 1.55 [95% CI: 1.15–2.10]) (Supplementary Table S3).
3.3 Relationship between urinary fluoride concentration and the risk of CK and CK-MB elevation in different subgroupsStratified analysis was conducted in subgroups according to age, sex, BMI, WC, alcohol consumption, and smoking, with the goal of revealing the association between urinary fluoride concentration and the risk of CK elevation, as presented in Table 3. There was a positive association between urinary fluoride concentration and the risk of serum CK elevation in participants under the age of 60 years (OR = 1.80 [95% CI: 1.26–2.59]). In addition, no interaction effect was found between urinary fluoride concentration and the remaining subgroups regarding the risk of serum CK elevation.
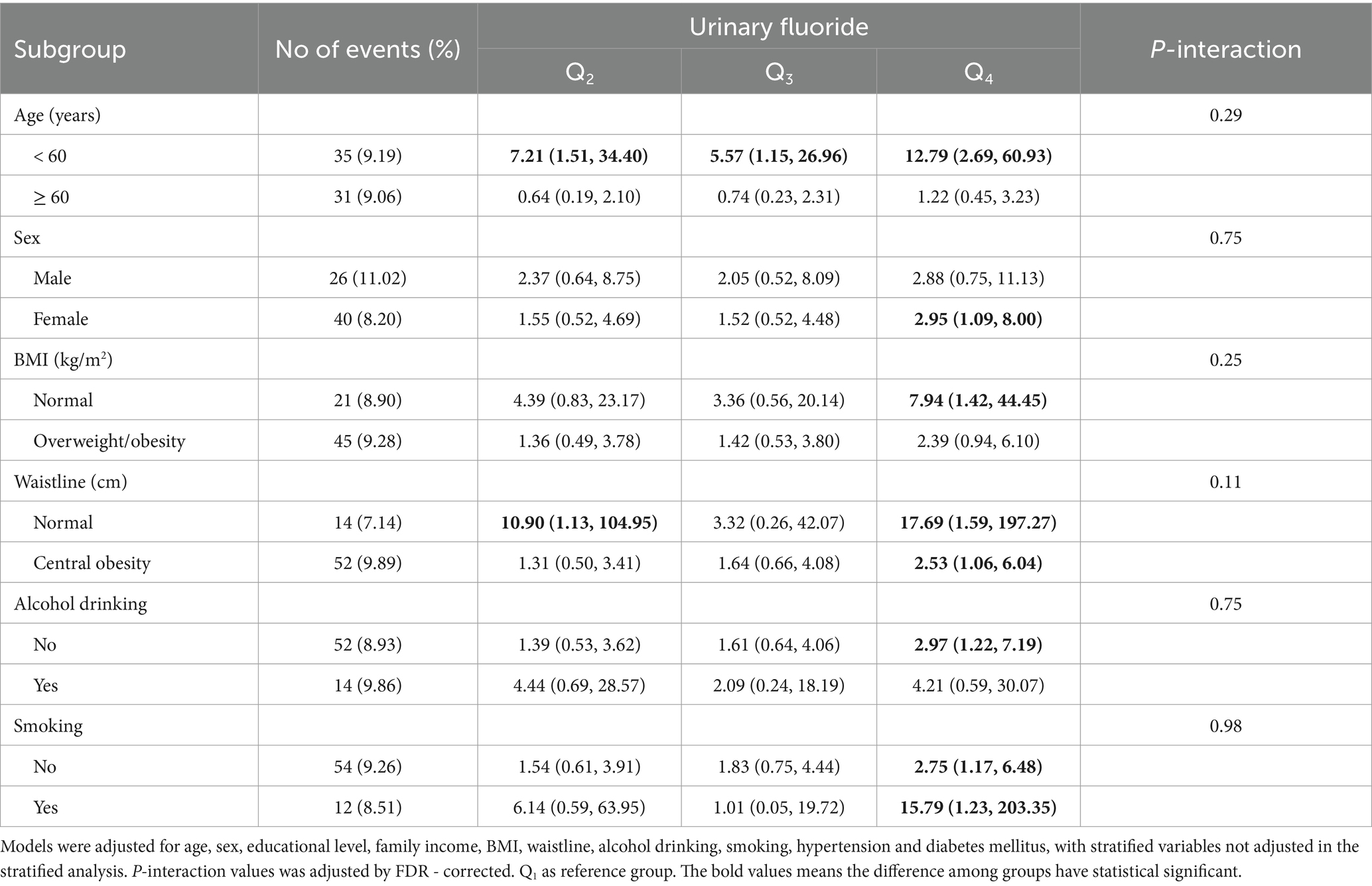
Table 3. Associations between urinary fluoride concentrations and the risk of CK elevation in subgroups.
The same stratified analysis was conducted to assess the risk of CK-MB elevation in Table 4. There was a positive association between urinary fluoride concentration and the risk of CK-MB elevation in participants under the age of 60 years (OR = 2.18 [95% CI: 1.39–3.42]), female (OR = 1.53 [95% CI: 1.07–2.19]), overweight/obesity (OR = 1.96 [95% CI: 1.28–2.99]), central obesity (OR = 1.59 [95% CI: 1.12–2.25]), alcohol consumption (OR = 1.49 [95% CI: 1.09–2.05]), and smoking (OR = 1.50 [95% CI: 1.10–2.04]). No interaction effects were observed on the risk of serum CK-MB elevation.
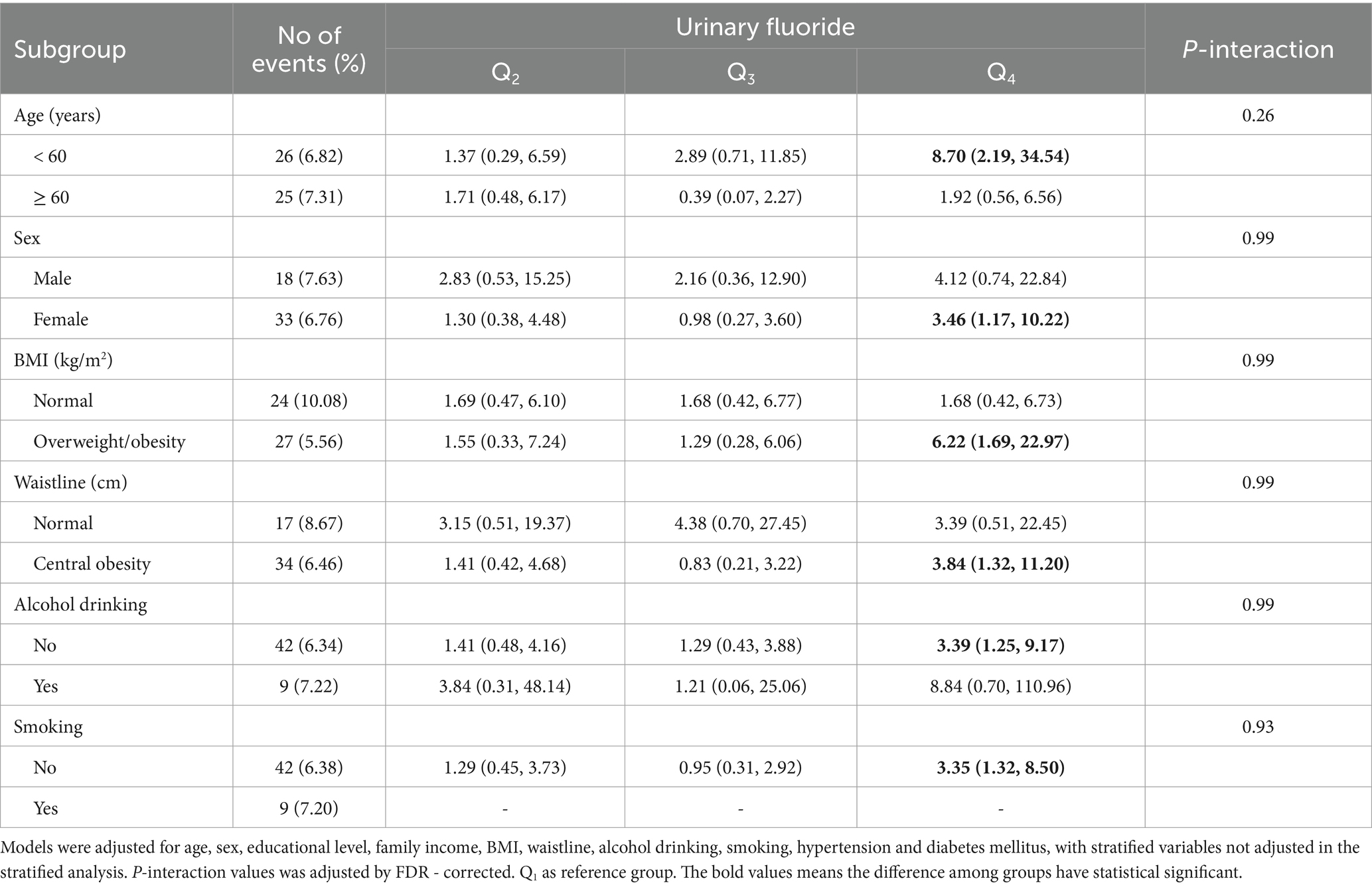
Table 4. Associations between urinary fluoride concentrations and the risk of CK-MB elevation in subgroups.
4 DiscussionCell experiments revealed that fluoride could induce damage in H9c2 cardiomyocytes (22), and myocardial damage would lead to a change in the level of myocardial enzymes. Some studies have reported that an association between fluoride exposure and myocardial infarction, serum CK, and LDH, although their conclusions remain inconsistent. In addition to CK and LDH, the most common myocardial enzyme spectrum in clinical practice include CK-MB, α-HBD, and AST (23, 24). Therefore, our study focused not only on the relationship between urinary fluoride concentration and the risk of myocardial enzymes (serum CK, serum CK-MB, serum LDH, serum α-HBD, and serum AST) but also on myocardial ischemia and arrhythmia in a population-based cross-sectional study. We found that urinary fluoride concentration was only positively associated with the risk of serum CK and CK-MB elevation; however, it was not associated with myocardial ischemia, arrhythmia, or the remaining myocardial enzymes.
Our research found the urinary fluoride concentration was not associated with myocardial ischemia, which was consistent with the study conducted in a large cohort study of 455,619 people in Sweden (15). However, our findings regarding the relationship between urinary fluoride concentration and arrhythmia were inconsistent with previous studies conducted on children with acute fluoride poisoning or chronic fluorosis (13, 14). These inconsistent findings of arrhythmia might be caused by the different doses of fluoride intake, duration of exposure, and specific age group.
There were three tissue- and compartment-specific CK isoenzymes, including CK-BB (brain), CK-MM (skeletal muscle), and CK-MB (cardiac muscle), which mainly catalyzed the reversible conversion of creatine and ATP to phosphocreatine and adenosine diphosphate (25). Previous studies have reported considerable controversy regarding the relationship between fluoride and CK. Animal experiments revealed that fluoride did not affect CK’s activity in vitro (26). However, it also found that the activity of serum CK was significantly increased with sodium fluoride in another animal experiment (27). Our study’s binary logistic regression analysis indicated that the urinary fluoride concentration was positively associated with serum CK elevation. Moreover, at the same time, this kind of positive relationship was confirmed by the sensitivity analyses in participants without diabetes or hypertension. According to these analyses, it prompted that the fluoride could be associated with estimated myocardial damage. In addition, our study found that the urinary fluoride concentration was positively associated with serum CK elevation in people under the age of 60 years. It might be caused by more physical labor and muscle in people under the age of 60 years. Hence, further research should be conducted to determine the potential underlying mechanisms of this effect.
As a type of CK isoenzyme, CK-MB had higher sensitivity in detecting acute myocardial infarction, which was close to 100% (28). Animal experiments revealed that sodium fluoride intervention (set at 300 mg/mL for 10 days) could increase serum CK-MB in male rats (29). Coincidentally, this relationship was also confirmed in a study involving a group of male rats exposed to high doses of sodium fluoride (administered at 45 and 90 mg F-/kg body weight/24 hours treated rats) (30). To the best of our knowledge, there were no studies focused on the relationship between urinary fluoride concentration and serum CK-MB elevation in a natural population. In addition, in contrast to previous studies that focused on male animals, our study found that fluoride exposure was a risk factor for CK-MB elevation in females rather than males. It might be caused by the different concentrations of calcium between males and females, and further studies on female rats should be conducted. Moreover, we also found that fluoride exposure was a risk factor for CK-MB elevation in participants without alcohol consumption or smoking, which the sex distribution might cause. Previous studies revealed that obesity was a risk factor for cardiomyopathy caused by the calcium homeostasis disequilibrium in mitochondria and oxidative stress (31). Our research also found that fluoride exposure was a risk factor for serum CK-MB elevation in obese participants, which the additive effect of fluoride exposure and obesity might cause.
Epidemiological studies in children found that serum LDH activity was associated with drinking water fluoride in children (17), and this relationship was also found in cell and animal experiments (9, 26, 32, 33). However, our study found no significant association between urinary fluoride concentration and the risk of serum LDH elevation. This inconsistent result in the population study might be due to the potential confounders we had controlled in our research, but not in those previous studies. α-HBD comprised the total activity of some LDH isoenzymes, namely LDH1 and LDH2, which were mainly found in myocardial damage (34, 35). As a serological biomarker for myocardial alteration, no studies that investigated the relationship between fluoride and α-HBD before. Hence, our study first reported that fluoride exposure in the population could not increase the risk of serum α-HBD elevation.
AST was still the most important biomarker for myocardial injury (36). Our study found there was no association between urinary fluoride concentration and the risk of AST elevation, which was consistent with a study based on 1,742 adolescents (16).
Our research had some limitations. First, our study was a natural population cross-sectional investigation, which could not determine the exact causality between urinary fluoride concentration and the risk of serum CK, CK-MB elevation. Second, some known cardiovascular-related confounders were not included in our research. Meanwhile, the participants in our research might consume fluoride in different ways than drinking water, such as food, which needs further evaluation. Third, our assessment of fluoride exposure was limited to urinary fluoride measurements, thereby excluding other potential sources of exposure, such as drinking water and dietary intake. Finally, this was a single-center study and did not include the different types of fluorosis, such as brick-tea-type fluorosis or chronic coal-burning fluorosis.
5 ConclusionIn conclusion, our study provides population-based evidence for the relationship between urinary fluoride concentration and myocardial disease related to myocardial enzymes, including CK, CK-MB, LDH, α-HBD, and AST. Notably, fluoride exposure may be associated with the risk of serum CK and CK-MB elevation in adults but not with myocardial ischemia, arrhythmia, serum LDH, serum α-HBD, and serum AST. Further investigations are needed to substantiate our findings, elucidate the potential mechanism underlying fluoride-induced elevation of CK and CK-MB, and explore how fluoride-induced changes in myocardial enzymes may affect myocardial injury.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statementThe studies involving humans were approved by Ethical Review Board of Center for Endemic Disease Control, Chinese Center for Disease Control and Prevention. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. The manuscript presents research on animals that do not require ethical approval for their study.
Author contributionsJW: Formal analysis, Writing – original draft, Writing – review & editing. MQ: Data curation, Methodology, Writing – review & editing. YuG: Data curation, Methodology, Writing – review & editing. YL: Data curation, Formal analysis, Writing – review & editing. XL: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. YJ: Investigation, Writing – review & editing. YY: Formal analysis, Investigation, Methodology, Project administration, Writing – review & editing. YaG: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Key R&D Program of China (2022YFC2503000).
AcknowledgmentsWe sincerely thank for the participants and the Institute of Endemic Disease Prevention and Control of Shanxi Province for their selfless contribution.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1410056/full#supplementary-material
References2. Veneri, F, Iamandii, I, Vinceti, M, Birnbaum, LS, Generali, L, Consolo, U, et al. Fluoride exposure and skeletal fluorosis: a systematic review and dose-response Meta-analysis. Curr Environ Health Rep. (2023) 10:417–41. doi: 10.1007/s40572-023-00412-9
PubMed Abstract | Crossref Full Text | Google Scholar
3. Zhou, J, Sun, D, and Wei, W. Necessity to pay attention to the effects of low fluoride on human health: an overview of skeletal and non-skeletal damages in epidemiologic investigations and laboratory studies. Biol Trace Elem Res. (2023) 201:1627–38. doi: 10.1007/s12011-022-03302-7
PubMed Abstract | Crossref Full Text | Google Scholar
6. Sun, L, Gao, Y, Liu, H, Zhang, W, Ding, Y, Li, B, et al. An assessment of the relationship between excess fluoride intake from drinking water and essential hypertension in adults residing in fluoride endemic areas. Sci Total Environ. (2013) 443:864–9. doi: 10.1016/j.scitotenv.2012.11.021
PubMed Abstract | Crossref Full Text | Google Scholar
7. Liu, Y, Téllez-Rojo, M, Sánchez, BN, Ettinger, AS, Osorio-Yáñez, C, Solano, M, et al. Association between fluoride exposure and cardiometabolic risk in peripubertal Mexican children. Environ Int. (2020) 134:105302. doi: 10.1016/j.envint.2019.105302
PubMed Abstract | Crossref Full Text | Google Scholar
8. Iamandii, I, De Pasquale, L, Giannone, ME, Veneri, F, Generali, L, Consolo, U, et al. Does fluoride exposure affect thyroid function? A systematic review and dose-response meta-analysis. Environ Res. (2024) 242:117759. doi: 10.1016/j.envres.2023.117759
PubMed Abstract | Crossref Full Text | Google Scholar
9. Liu, H, Zeng, Q, Cui, Y, Yu, L, Zhao, L, Hou, C, et al. The effects and underlying mechanism of excessive iodide on excessive fluoride-induced thyroid cytotoxicity. Environ Toxicol Pharmacol. (2014) 38:332–40. doi: 10.1016/j.etap.2014.06.008
PubMed Abstract | Crossref Full Text | Google Scholar
10. Varol, E, Akcay, S, Ersoy, IH, Koroglu, BK, and Varol, S. Impact of chronic fluorosis on left ventricular diastolic and global functions. Sci Total Environ. (2010) 408:2295–8. doi: 10.1016/j.scitotenv.2010.02.011
PubMed Abstract | Crossref Full Text | Google Scholar
11. Varol, E, Akcay, S, Ersoy, IH, Ozaydin, M, Koroglu, BK, and Varol, S. Aortic elasticity is impaired in patients with endemic fluorosis. Biol Trace Elem Res. (2010) 133:121–7. doi: 10.1007/s12011-009-8578-4
PubMed Abstract | Crossref Full Text | Google Scholar
12. Li, Y, Berenji, GR, Shaba, WF, Tafti, B, Yevdayev, E, and Dadparvar, S. Association of vascular fluoride uptake with vascular calcification and coronary artery disease. Nucl Med Commun. (2012) 33:14–20. doi: 10.1097/MNM.0b013e32834c187e
PubMed Abstract | Crossref Full Text | Google Scholar
13. Yolken, R, Konecny, P, and McCarthy, P. Acute fluoride poisoning. Pediatrics. (1976) 58:90–3. doi: 10.1542/peds.58.1.90
Crossref Full Text | Google Scholar
14. Karademir, S, Akçam, M, Kuybulu, AE, Olgar, S, and Oktem, F. Effects of fluorosis on QT dispersion, heart rate variability and echocardiographic parameters in children. Anadolu Kardiyol Derg. (2011) 11:150–5. doi: 10.5152/akd.2011.038
PubMed Abstract | Crossref Full Text | Google Scholar
15. Näsman, P, Granath, F, Ekstrand, J, Ekbom, A, Sandborgh-Englund, G, and Fored, CM. Natural fluoride in drinking water and myocardial infarction: a cohort study in Sweden. Sci Total Environ. (2016) 562:305–11. doi: 10.1016/j.scitotenv.2016.03.161
PubMed Abstract | Crossref Full Text | Google Scholar
16. Malin, AJ, Lesseur, C, Busgang, SA, Curtin, P, Wright, RO, and Sanders, AP. Fluoride exposure and kidney and liver function among adolescents in the United States: NHANES, 2013-2016. Environ Int. (2019) 132:105012. doi: 10.1016/j.envint.2019.105012
PubMed Abstract | Crossref Full Text | Google Scholar
17. Xiong, X, Liu, J, He, W, Xia, T, He, P, Chen, XM, et al. Dose-effect relationship between drinking water fluoride levels and damage to liver and kidney functions in children. Environ Res. (2007) 103:112–6. doi: 10.1016/j.envres.2006.05.008
PubMed Abstract | Crossref Full Text | Google Scholar
18. Zhao, L, Yu, C, Lv, J, Cui, Y, Wang, Y, Hou, C, et al. Fluoride exposure, dopamine relative gene polymorphism and intelligence: a cross-sectional study in China. Ecotoxicol Environ Saf. (2021) 209:111826. doi: 10.1016/j.ecoenv.2020.111826
PubMed Abstract | Crossref Full Text | Google Scholar
19. Huang, S, Liu, Z, Ge, X, Luo, X, Zhou, Y, Li, D, et al. Occupational exposure to manganese and risk of creatine kinase and creatine kinase-MB elevation among ferromanganese refinery workers. Am J Ind Med. (2020) 63:394–401. doi: 10.1002/ajim.23097
PubMed Abstract | Crossref Full Text | Google Scholar
20. Elhaj, FA, Salim, N, Harris, AR, Swee, TT, and Ahmed, T. Arrhythmia recognition and classification using combined linear and nonlinear features of ECG signals. Comput Methods Prog Biomed. (2016) 127:52–63. doi: 10.1016/j.cmpb.2015.12.024
PubMed Abstract | Crossref Full Text | Google Scholar
21. Ibanez, B, James, S, Agewall, S, Antunes, MJ, Bucciarelli-Ducci, C, Bueno, H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
PubMed Abstract | Crossref Full Text | Google Scholar
22. Yan, X, Wang, L, Yang, X, Qiu, Y, Tian, X, Lv, Y, et al. Fluoride induces apoptosis in H9c2 cardiomyocytes via the mitochondrial pathway. Chemosphere. (2017) 182:159–65. doi: 10.1016/j.chemosphere.2017.05.002
PubMed Abstract | Crossref Full Text | Google Scholar
23. Li, SW, Sun, X, He, Y, Guo, Y, Zhao, HJ, Hou, ZJ, et al. Assessment of arsenic trioxide in the heart of Gallus gallus: alterations of oxidative damage parameters, inflammatory cytokines, and cardiac enzymes. Environ Sci Pollut Res Int. (2017) 24:5781–90. doi: 10.1007/s11356-016-8223-7
PubMed Abstract | Crossref Full Text | Google Scholar
24. Wang, L, Zheng, M, Tang, Y, Yin, Y, Liu, Y, and Liu, G. Impact of various periods of perfusion-pause and reperfusion on the severity of myocardial injury in the langenodorff model. Perfusion. (2023) 38:1609–16. doi: 10.1177/02676591221122349
PubMed Abstract | Crossref Full Text | Google Scholar
25. Del Franco, A, Ambrosio, G, Baroncelli, L, Pizzorusso, T, Barison, A, Olivotto, I, et al. Creatine deficiency and heart failure. Heart Fail Rev. (2022) 27:1605–16. doi: 10.1007/s10741-021-10173-y
PubMed Abstract | Crossref Full Text | Google Scholar
26. Nedeljković, M, Matović, V, and Soldatović, D. Levels of lactate dehydrogenase and creatine kinase in plasma of fluoride-treated rabbits. J Appl Toxicol. (1985) 5:11–3. doi: 10.1002/jat.2550050103
PubMed Abstract | Crossref Full Text | Google Scholar
27. Hassan, HA, and Yousef, MI. Mitigating effects of antioxidant properties of black berry juice on sodium fluoride induced hepatotoxicity and oxidative stress in rats. Food Chem Toxicol. (2009) 47:2332–7. doi: 10.1016/j.fct.2009.06.023
PubMed Abstract | Crossref Full Text | Google Scholar
28. Mair, J, Morandell, D, Genser, N, Lechleitner, P, Dienstl, F, and Puschendorf, B. Equivalent early sensitivities of myoglobin, creatine kinase MB mass, creatine kinase isoform ratios, and cardiac troponins I and T for acute myocardial infarction. Clin Chem. (1995) 41:1266–72. doi: 10.1093/clinchem/41.9.1266
PubMed Abstract | Crossref Full Text | Google Scholar
29. Oyagbemi, AA, Omobowale TOAsenuga, ER, Adejumobi, AO, Ajibade TOIge, TM, et al. Sodium fluoride induces hypertension and cardiac complications through generation of reactive oxygen species and activation of nuclear factor kappa beta. Environ Toxicol. (2017) 32:1089–101. doi: 10.1002/tox.22306
PubMed Abstract | Crossref Full Text | Google Scholar
30. Panneerselvam, L, Govindarajan, V, Ameeramja, J, Nair, HR, and Perumal, E. Single oral acute fluoride exposure causes changes in cardiac expression of oxidant and antioxidant enzymes, apoptotic and necrotic markers in male rats. Biochimie. (2015) 119:27–35. doi: 10.1016/j.biochi.2015.10.002
PubMed Abstract | Crossref Full Text | Google Scholar
31. Ren, J, Wu, NN, Wang, S, Sowers, JR, and Zhang, Y. Obesity cardiomyopathy: evidence, mechanisms, and therapeutic implications. Physiol Rev. (2021) 101:1745–807. doi: 10.1152/physrev.00030.2020
PubMed Abstract | Crossref Full Text | Google Scholar
32. Chen, L, Ning, H, Yin, Z, Song, X, Feng, Y, Qin, H, et al. The effects of fluoride on neuronal function occurs via cytoskeleton damage and decreased signal transmission. Chemosphere. (2017) 185:589–94. doi: 10.1016/j.chemosphere.2017.06.128
PubMed Abstract | Crossref Full Text | Google Scholar
34. Lee, S, Koppensteiner, R, Kopp, CW, and Gremmel, T. α-Hydroxybutyrate dehydrogenase is associated with atherothrombotic events following infrainguinal angioplasty and stenting. Sci Rep. (2019) 9:18200. doi: 10.1038/s41598-019-54899-0
PubMed Abstract | Crossref Full Text | Google Scholar
36. Lofthus, DM, Stevens, SR, Armstrong, PW, Granger, CB, and Mahaffey, KW. Pattern of liver enzyme elevations in acute ST-elevation myocardial infarction. Coron Artery Dis. (2012) 23:22–30. doi: 10.1097/MCA.0b013e32834e4ef1
留言 (0)