Affecting approximately 50% of individuals who are critically ill, intensive care unit-acquired weakness (ICU-AW) is a common and serious consequence of critical illness (1, 2). ICU-AW can originate from critical illness myopathy, critical illness polyneuropathy, or critical illness neuromyopathy (3) and typically manifests as a symmetric, widespread weakness affecting the respiratory and limb muscles, but sparing the face and ocular muscles (4). Muscle tone is almost non-invasively reduced, and deep tendon reflexes may be diminished or normal (4). ICU-AW, also known as the syndrome of global weakness, is estimated to affect one million patients globally, including 75,000 patients in the United States (5). Severe functional impairment, extended mechanical ventilation, increased healthcare expenses, longer hospital stays, and higher mortality rates connected to the intensive care unit and hospitalization are all linked to ICU-AW (6, 7). The causes and mechanisms of ICU-AW remain poorly understood, resulting in an absence of targeted treatment alternatives for ICU-AW. Managing risk factors and hindering the initial advancement of the condition is essential, as the irregularities present in this phase may be reversible (8, 9). Early diagnosis necessitates early intervention; however, detecting ICU-AW in a timely manner is often difficult owing to states of unawareness in patients or the administration of sedatives, which can lead to delays in diagnosis and treatment (5, 10).
There is no “gold standard” diagnostic test for ICU-AW, even in the latest clinical practice guidelines (5). A diagnosis of ICU-AW can be accomplished through four approaches: using manual muscle testing with the Medical Research Council (MRC) score, conducting electrophysiological assessments (including electromyography and nerve conduction studies), performing muscle ultrasound, and examining muscle or nerve tissue pathology (11). However, these four methods have limited application in clinical practice. Manual muscle testing has considerable constraints as patients must be sufficiently alert and cooperative for the tests. Other diagnostic techniques—particularly muscle electrophysiological assessments and muscle ultrasound—are technically challenging and not commonly accessible in the ICU (12). In the past decade, serum biomarkers have been increasingly studied for early diagnosis of ICU-AW. Growth differentiation factor-15 (GDF-15) is one of the potential biomarkers that received great attention due to its close relationship with muscle wasting and decline in muscle mass.
GDF-15, first recognized as macrophage inhibitory cytokine-1 in 1997, is a stress-responsive component of the transforming growth factor-beta (TGF-β) cytokine superfamily (13). GDF-15 is predominantly expressed in myocardial cells, adipocytes, macrophages, endothelial cells, and vascular smooth muscle cells under pathological circumstances such as inflammation, insulin resistance, and oxidative stress (14). In addition, skeletal muscle cells produce GDF-15 in response to age-related stress, mitochondrial dysfunction, and mitochondrial proteotoxic stress, which may be essential in the progression of sarcopenia (15, 16). In healthy people, serum GDF-15 levels range from 200 to 1,200 pg./mL. Elevated GDF-15 levels upon ICU admission have been shown to be predictive of short-term and long-term mortality risk, particularly in patients with sepsis (17). Recently, multiple studies (18–23) have established a connection between GDF-15 and ICU-AW, and the association between elevated plasma GDF-15 levels and reduced expression of muscle microRNAs may explain the muscle atrophy observed in ICU-AW. Furthermore, the increased expression of GDF-15 in ICU patients may indicate macrophage activation and proinflammatory activities (20). Interest in utilizing GDF-15 as a biomarker for the diagnosis, prognosis, and/or risk stratification of patient populations suffering from ICU-AW is growing (24). However, the variability in findings across various studies is hindering efforts to determine the clinical significance of GDF-15 in these individuals. In this study, we collated original research articles referencing the role of GDF-15 in ICU-AW and performed a systematic evaluation to determine the value of GDF-15 as a diagnostic biomarker.
2 Materials and methods 2.1 Data sources and eligibility criteriaThis meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (25). All analyses were derived from studies that had been published previously. Consequently, obtaining ethical approval or patient consent was not applicable for this meta-analysis. Two independent reviewers conducted a selective literature search of several databases (PubMed, Cochrane, Web of Science, Embase, and CINAHL) during May 15–31, 2023. All pertinent articles were evaluated based on their titles and abstracts, and then reviewed for eligibility after being compiled and imported into NoteExpress software. MeSH terms or keywords used in the search were [(“Growth Differentiation Factor-15” OR “GDF-15” OR “Macrophage Inhibitory Cytokine-1” OR “MIC-1” OR “MIC1”) AND (“intensive care unit acquired weakness” OR “ICU-AW” OR “critical illness neuromuscular abnormality” OR “muscle weakness” OR “muscle atrophy” OR “muscle wasting and dysfunction” OR “critical illness polyneuropathy” OR “critical illness myopathy”)]. The references of the identified articles and associated reviews were searched manually to find any articles that might have been overlooked.
2.2 Study selectionThe selection process is shown schematically following the PRISMA 2020 flow diagram (Figure 1) (25). Studies in the full text were included if they satisfied the following criteria: (1) patient age ≥ 18 years; (2) proven diagnosis of ICU-AW by MRC score, electrophysiology, muscle ultrasound or muscle cell biopsy; (3) studies that documented the diagnostic characteristics of GDF-15 in ICU-AW; (4) adequate data for detailing or computing sensitivity and specificity. Exclusion criteria included: (1) studies lacking sufficient data and failed attempts to reach the authors; (2) duplicate studies; (3) studies with fewer than 10 cases; and (4) non-clinical research such as animal studies, reviews, conference abstracts, case reports, and meta-analyses.
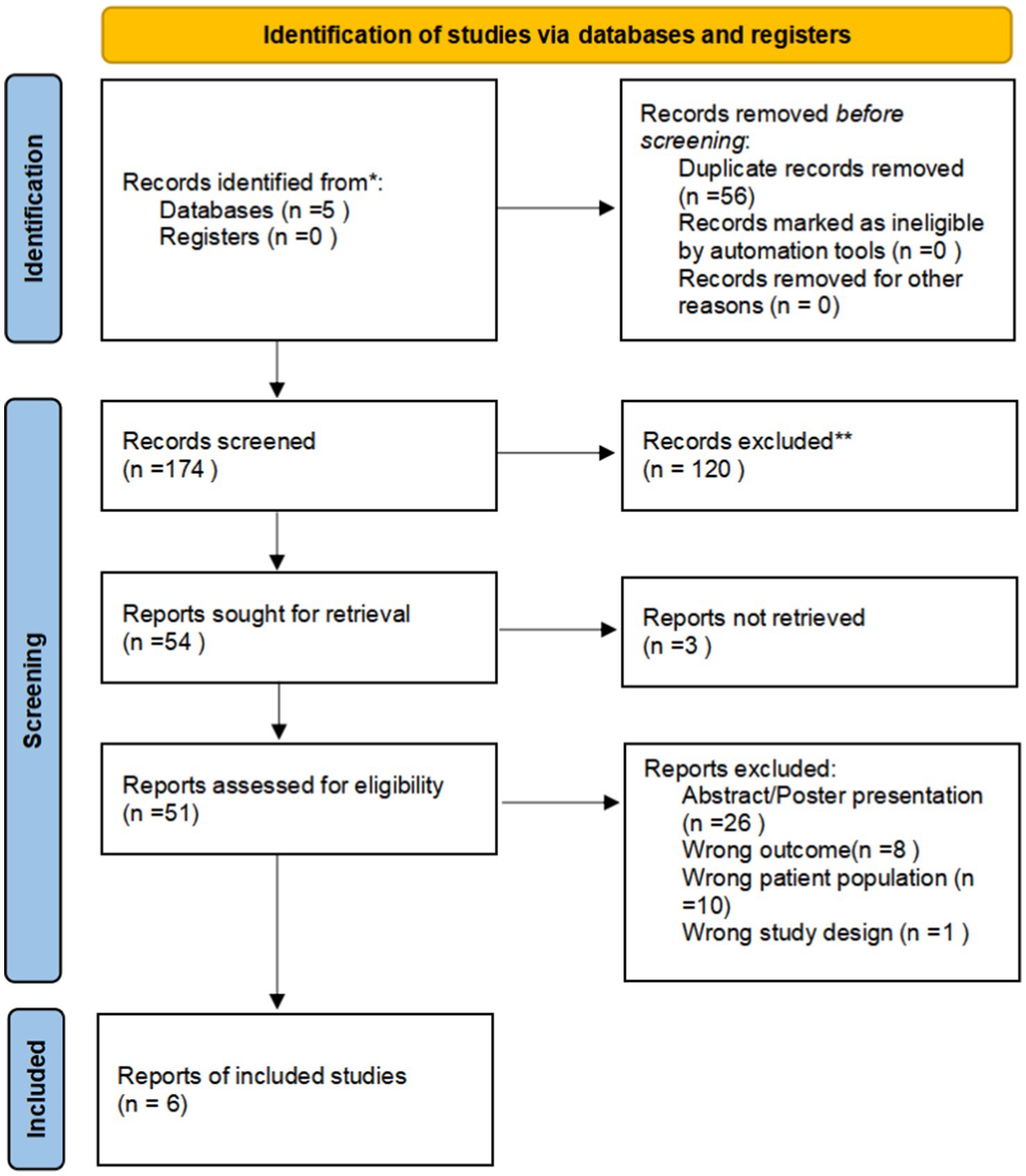
Figure 1. PRISMA 2020 flow diagram depicting the study selection procedure.
2.3 Data collectionData were extracted from articles by two independent reviewers. The collected data included the lead author’s name, publication year, country of the study population, sample type, number of patients, sensitivity, specificity, and cutoff value. In cases where sensitivity and specificity were not clearly stated in the article, these parameters were obtained from the area under the curve (AUC) using Engauge Digitizer software or manually computed from other available diagnostic accuracy metrics in the articles, or both. Attempts were made to contact authors of the original articles for the missing data, but these efforts were unsuccessful. When a study provided data for multiple comparisons, only one was selected for the final analysis based on its relevance to our research topic, concerns about heterogeneity, and to prevent a unit-of-analysis error. Any disagreements were settled through discussion or by reaching a consensus with a third reviewer.
2.4 Assessment of risk of biasThe quality and risk of bias of each study were examined using the revised Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) criteria in Review Manager 5.3 software, based on specified criteria including patient selection, index test, reference standard, and flow and timing. All pertinent evidence was incorporated into the final analysis.
2.5 Data analysisMeta-analysis was conducted using Meta Disc (C1.4) software. The primary outcomes included pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio (DOR). Stata 17.0 was utilized to generate the summary receiver operating characteristic (SROC) curve and AUC along with their corresponding 95% confidence intervals (CIs). Cochrane’s Q and I2 statistics were employed to confirm statistically significant heterogeneity among the studies, leading to the selection of a fixed-effects model. Owing to the inclusion of fewer than 10 studies, a funnel plot asymmetry analysis for assessing publication bias was not performed.
3 Results 3.1 Study selection and characteristicsOur literature search identified 261 potentially relevant studies (see Figure 1). These studies were reviewed based on titles, keywords, and abstracts, resulting in the exclusion of 210 studies due to reasons such as duplication, being outside the scope, or being basic or animal model studies. The remaining 51 studies were examined in detail, and six studies were included in the meta-analysis, which consisted of 401 patients with ICU-AW and 246 patients with non-ICU-AW based on our inclusion criteria. The features of the six included studies are summarized in Table 1. Among the six diagnostic studies, the participants comprised Chinese individuals (18, 22, 23), Americans (19), and British individuals (20, 21). All six studies were conducted prospectively. Only five of the studies reported cutoff values for GDF-15, which varied from 357.5 to 7,239 pg./mL (18–20, 22).
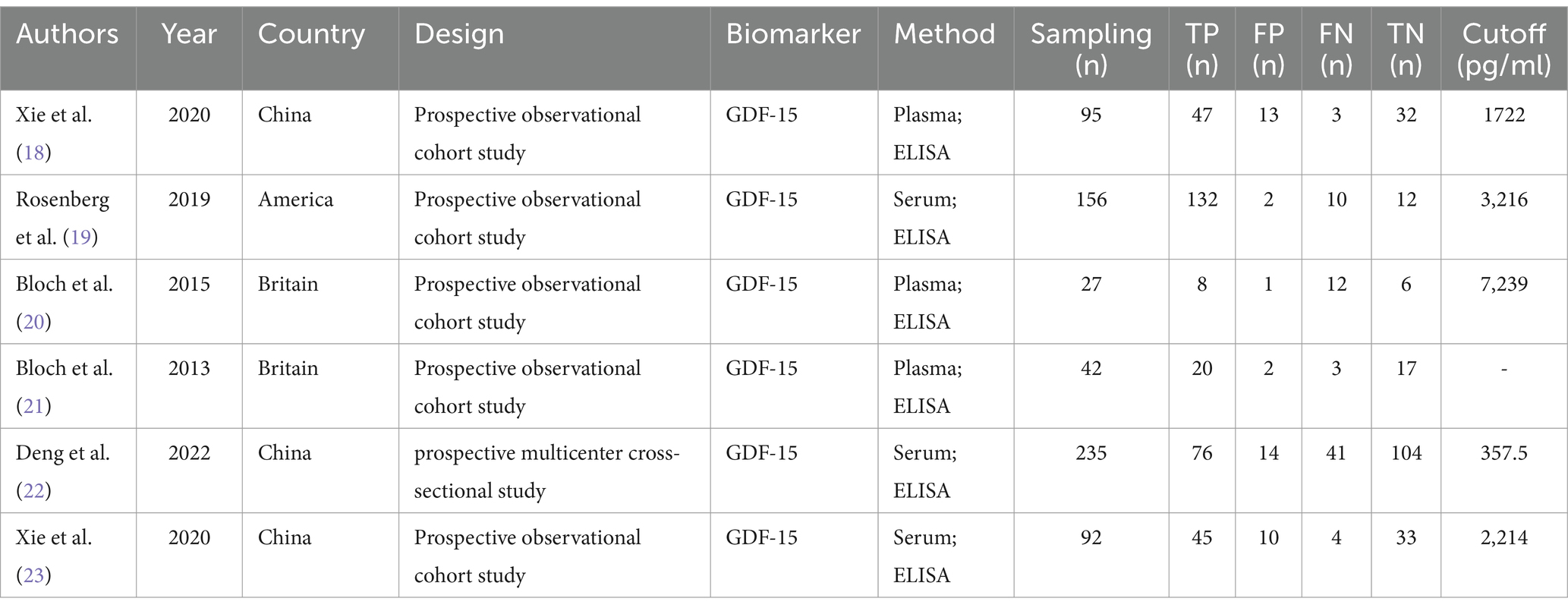
Table 1. Study characteristics of all the articles included in the diagnostic meta-analysis.
3.2 Risk of bias of included studiesThe quality of the studies was assessed using QUADAS-2 (Figure 2). According to the QUADAS-2 evaluation, three of the six studies were at risk of bias in patient selection, two of the six studies had a risk of bias in the index test, one study showed a risk of bias in the reference standard, and two studies had a risk of bias of flow and timing. Despite these potential biases, all studies were included for further statistical analysis.
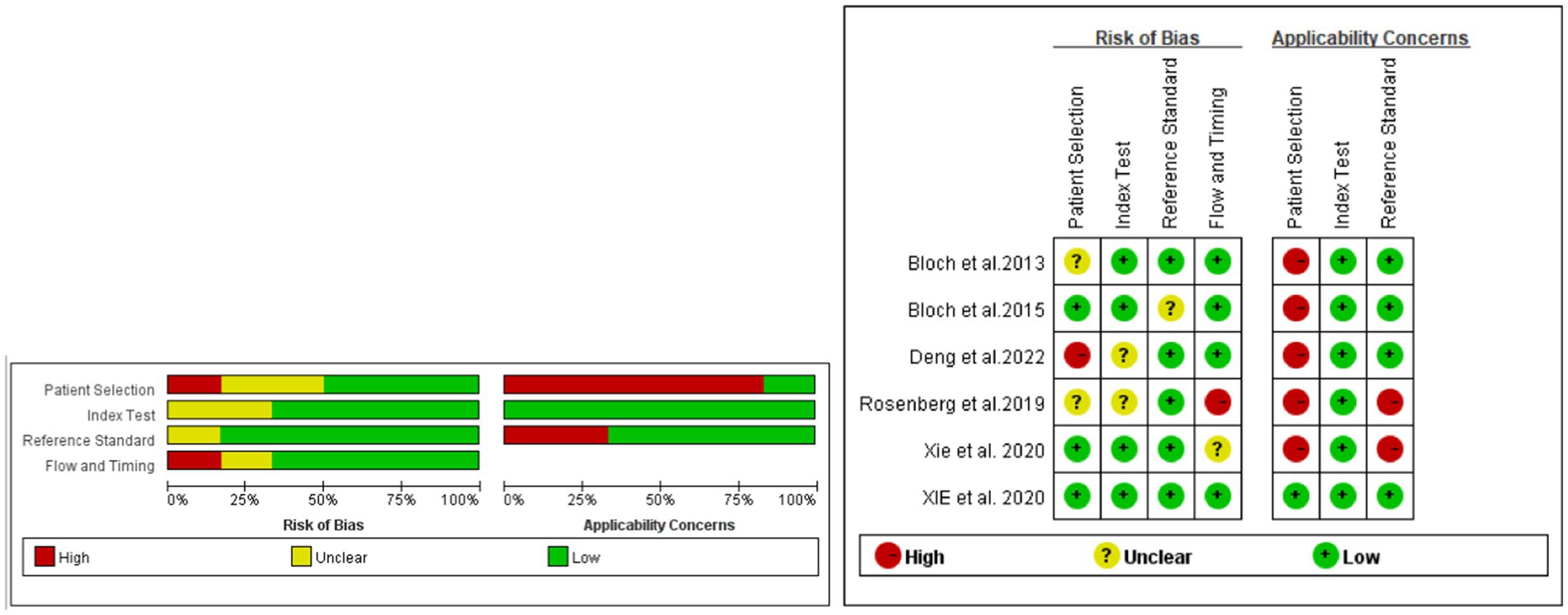
Figure 2. Evaluation of the risk of bias and applicability concerns of the included diagnostic studies using QUADAS-2.
3.3 Synthesis of resultsThe Spearman correlation coefficient between the logarithm of sensitivity and the logarithm of (1 − specificity) was 0.714 (p = 0 111), indicating that there was no heterogeneity caused by threshold effects in this study (see Figure 3). A Cochrane-Q of DOR = 8.67 (df = 5, p = 0.1228) showed that there was no heterogeneity caused by non-threshold effects in this study. The diagnostic accuracy metrics for the included studies are presented in Table 2 and Figure 3. The overall pooled sensitivity, specificity, DOR, and AUC for GDF-15 in distinguishing ICU-AW from non-ICU-AW were 0.82 (95% CI: 0.78–0.86), 0.83 (95% CI: 0.61–0.88), 21.39 (95% CI: 13.36–34.24), and 0.88 (95% CI: 0.85–0.91), respectively. These metrics correspond to a positive likelihood ratio of 4.58 (95% CI: 3.44–6.09) and a negative likelihood ratio of 0.26 (95% CI: 0.21–0.33). The SROC curve is shown in Figure 4. These findings suggest that GDF-15 levels could be a valuable alternative biomarker for diagnosing ICU-AW when compared to non-ICU-AW.
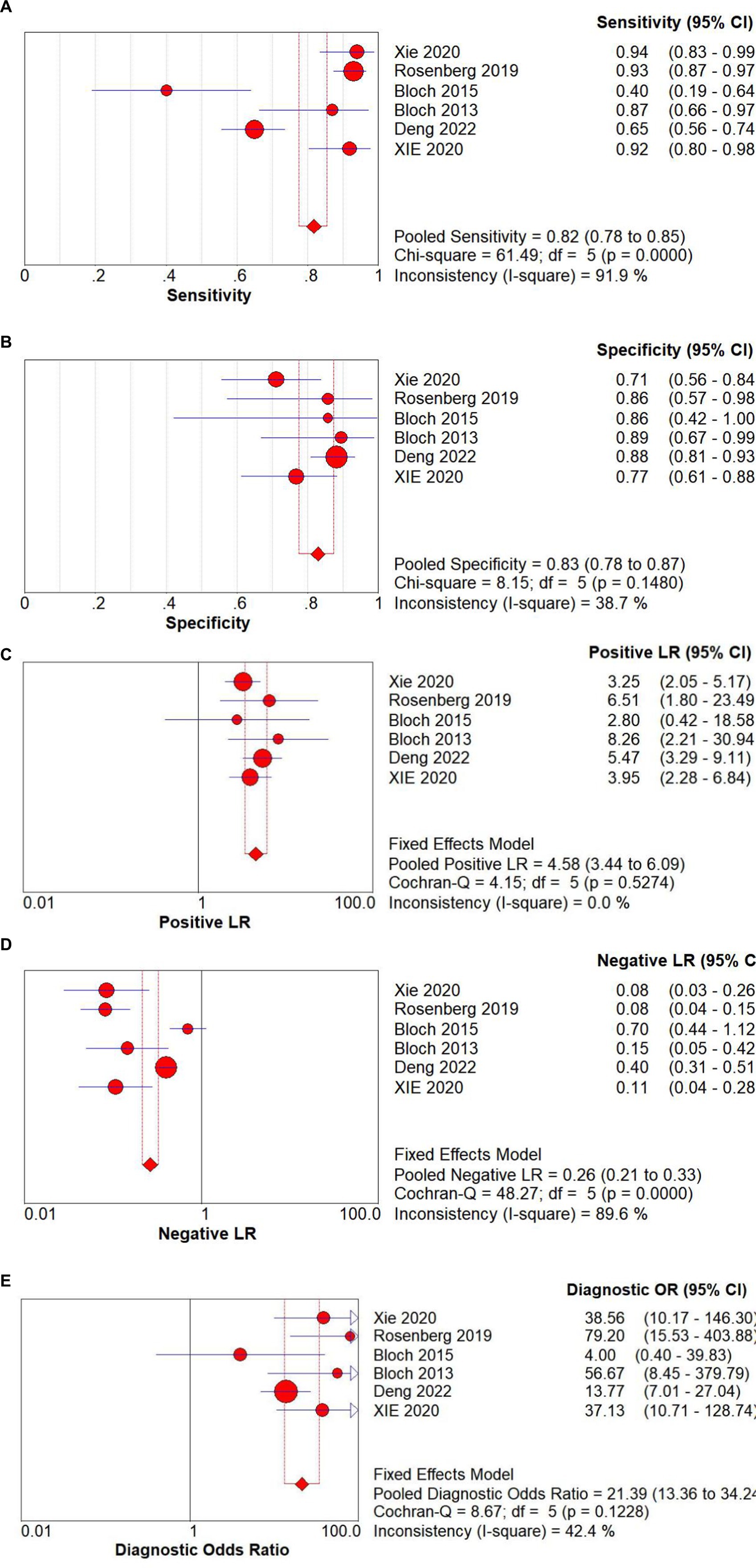
Figure 3. Forest plots of GDF-15 for the (A) sensitivity, (B) specificity, (C) positive LR, (D) negative LR, and (E) diagnostic odds ratio of the pooled data from the included studies.
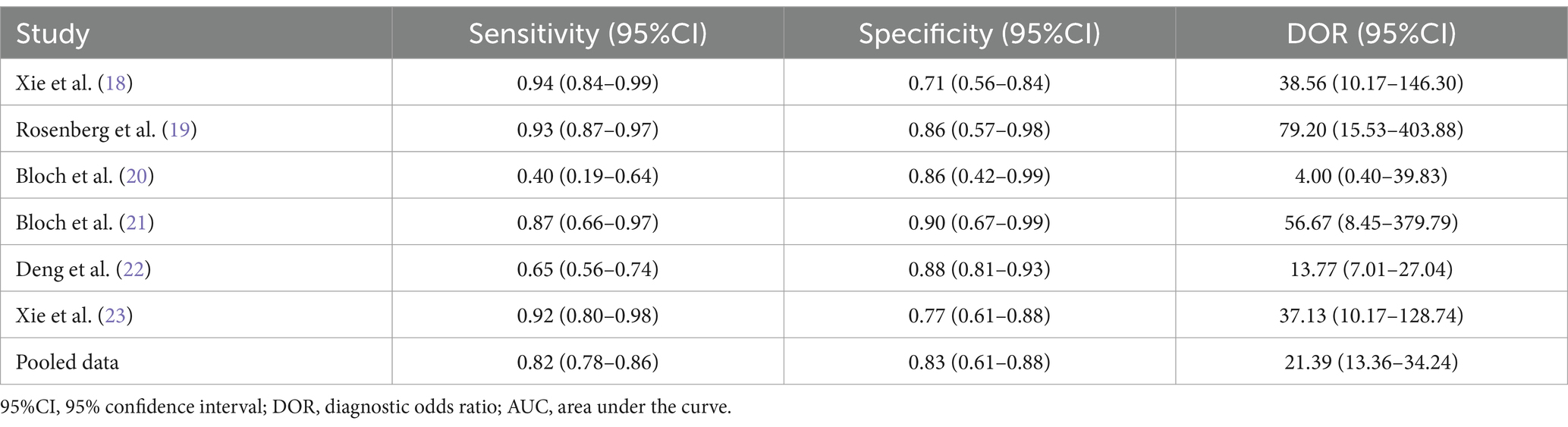
Table 2. Measures of diagnostic accuracy in the selected studies.
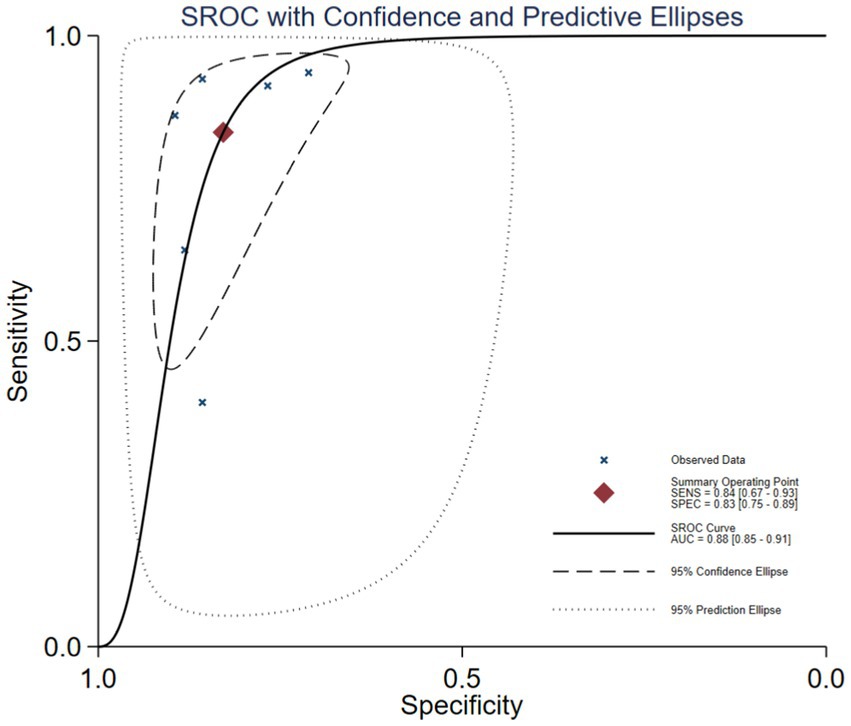
Figure 4. SROC curve of GDF-15 for the diagnosis of ICU-AW.
4 DiscussionICU-AW is a common complication in critically ill patients after prolonged stays in intensive care unit. ICU-AW can greatly impact the quality of life. Early recognization and diagnosis of ICU-AW can facilitate personalized treatments. Previous studies have reported that several biomarkers could be applied for early clinical diagnosis of ICU-AW. These biomarkers included lactate (26), neurofilament (27), urinary titin (28), MiR-181a (29), monocyte chemoattractant protein-1 (MCP-1) (30), glucose transporter type 4 (GLUT-4) (31), interleukin-6 (IL-6) (32), and growth differentiation factor-15 (GDF-15) (18–23).
Serum lactate is a valuable biomarker in critically ill individuals, but its connection to ICU-AW remains debatable (26). Plasma neurofilament level was proposed for early diagnosis of ICU-AW, but this biomarker is unable to distinguish between critical illness polyneuropathy and critical illness myopathy (33). Urinary titin levels can be an effective biomarker for diagnosing ICU-AW and are strongly linked to muscle atrophy (34), with increased urinary titin levels correlating with atrophy of the rectus femoris muscle but not with diaphragm thickness. In addition, urinary titin levels can vary by the timing of urine collection and various physiological factors (13). The increase in miR-181a shortly after ICU admission exhibited a high specificity (91%) for muscle atrophy within one week. However, its low sensitivity (56%) suggested that some patients at risk of muscle atrophy could be missed, thus this test cannot be relied upon for ruling out a diagnosis of acute muscle atrophy. It was reported that plasma MCP-1 level could be one of the risk factors for ICU-AW in patients with sepsis (29). However, studies with a larger sample size are required to confirm its diagnostic significance (30). Monitoring GLUT-4 provides some predictive value for ICU-AW in liver transplantation patients. An increased GLUT-4 level was associated with a low probability of ICU-AW, but the role in early diagnosis was limited (31). Animal studies have shown that IL-6 could contribute to increased muscle fatigue and reduced contractility of the diaphragm. Furthermore, recent clinical findings indicated a connection between IL-6 and the age-related reduction in muscle strength. This research highlighted the possible involvement of IL-6 in the formation of non-excitable muscle membranes during the early stages of critical illness, ultimately resulting in muscle weakness. The clinical implications of these findings need further investigation (32).
Recently, the clinical value of GDF-15 in the early diagnosis of ICU-AW is receiving increasing attention since GDF-15 is closely associated with muscle wasting and a decline in muscle mass. In our research, we identified six original research articles that assessed the potential of GDF-15 as a biomarker for differentiating ICU-AW from non-ICU-AW. Through a meta-analysis, we concluded that GDF-15 may be a reliable biomarker, with a pooled sensitivity of 82% and a specificity of 83%. In addition, there was no significant heterogeneity observed among the studies, suggesting that the findings were stable and reliable. Five of the six studies were encompassed within the 95% confidence interval of the SROC curve, with one study falling within the 95% prediction interval. The AUC value for the SROC curve is 0.88, indicating that GDF-15 exhibits very high diagnostic accuracy for ICU-AW.
Excessive catabolism represents a pivotal metabolic phenomenon in critically ill patients. The breakdown of muscle protein is a fundamental mechanism of catabolism, directly contributing to the development of ICU-AW (35). Degradation of muscle protein is believed to occur primarily through the ubiquitin–proteasome and autophagy–lysosome pathways. When the protein degradation pathway is aberrantly activated, protein degradation is accelerated, resulting in a reduction in muscle mass and muscle atrophy (36).
The cytokine GDF-15 is a principal regulator of the protein synthesis/catabolism balance and may be involved in the activation of the aforementioned proteolytic pathways (37). Abnormal expression of GDF-15 in the human body was found to result in a reduction in muscle protein synthesis, thereby contributing to the development of muscle atrophy. The MRC score for patients with ICU-AW decreased progressively over the course of their treatment, whereas plasma levels of GDF-15 showed a marked upward trend. By the seventh day of treatment, the GDF-15 levels in the ICU-AW group were considerably higher than those in the non-ICU-AW group (18). A study by Bloch et al. (20) demonstrated that GDF-15 may inhibit the expression of muscle microRNAs by enhancing the sensitivity of the TGF-β signaling pathway, thus contributing to muscle wasting. This conclusion resulted from observations on muscle biopsies from the rectus femoris muscle of patients with ICU-AW. Meanwhile, Xie et al. (23) observed that the loss of paraspinal muscle cross-sectional area and the rate of loss were significantly and positively associated with serum GDF-15 levels on the seventh day. This finding suggests that GDF-15, as a biomarker reflecting muscle wasting, has a strong intrinsic relationship with the objective measurement of paraspinal muscle mass through imaging. GDF-15 can be used in conjunction with the MRC score, which represents muscle function, to evaluate the degree of muscle wasting in patients. These parameters complement each other and have a certain correlation, particularly in cases where assessment of muscle strength through the MRC score is not feasible, such as with ICU patients under sedation or coma. Thus, as a biomarker, GDF-15 can assist in the timely diagnosis and assessment of patients with ICU-AW.
There are several limitations to this meta-analysis. First, the studies are confined to Chinese, American, and British populations. The small sample sizes with high selectivity of populations will require additional validation studies to determine the generalizability of our findings in clinically diagnosing ICU-AW. Second, we only included six articles after vigorous screening to select researches for meta-analysis, which might cause attrition bias with missed studies and participant drop-offs. Future prospective studies in a larger sample size and diverse patient populations are required to confirm our research findings here. In addition, before introducing GDF-15 into clinical practice, cutoff values for GDF-15 need to be established and validated internationally. In our analysis, cutoff values for GDF-15 for the difference between ICU-AW and non-ICU-AW or healthy controls were provided in five of the six studies. Consequently, large-scale prospective studies are required to identify a threshold targeted to different patient populations.
5 ConclusionThis meta-analysis suggests that GDF-15 could be a valuable biomarker for differentiating ICU-AW from non-ICU-AW. Clinicians may consider testing the GDF-15 level to early identify patients with ICU-AW. However, the small sample sizes of the studies included in the analysis indicate a requirement for further research with larger, well-designed prospective studies.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributionsB-HW: Data curation, Formal analysis, Methodology, Writing – original draft. M-YQ: Data curation, Methodology, Validation, Writing – review & editing. ZY: Methodology, Project administration, Resources, Writing – review & editing. G-LH: Conceptualization, Methodology, Resources, Supervision, Writing – review & editing. S-YM: Conceptualization, Funding acquisition, Methodology, Writing – original draft.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by Health Science and Technology program of Nanshan District, Shenzhen City (grant number: NS2023098). Nanshan District medical key discipline construction financial support project.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Stevens, RD, Dowdy, DW, Michaels, RK, Mendez-Tellez, PA, Pronovost, PJ, and Needham, DM. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med. (2007) 33:1876–91. doi: 10.1007/s00134-007-0772-2
PubMed Abstract | Crossref Full Text | Google Scholar
2. Latronico, N, and Bolton, CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. (2011) 10:931–41. doi: 10.1016/S1474-4422(11)70178-8
PubMed Abstract | Crossref Full Text | Google Scholar
3. Peñuelas, O, Muriel, A, Frutos-Vivar, F, Fan, E, Raymondos, K, Rios, F, et al. Prediction and outcome of intensive care unit-acquired paresis. J Intensive Care Med. (2018) 33:16–28. doi: 10.1177/0885066616643529
PubMed Abstract | Crossref Full Text | Google Scholar
4. Latronico, N, Herridge, M, Hopkins, RO, Angus, D, Hart, N, Hermans, G, et al. The ICM research agenda on intensive care unit-acquired weakness. Intensive Care Med. (2017) 43:1270–81. doi: 10.1007/s00134-017-4757-5
PubMed Abstract | Crossref Full Text | Google Scholar
5. Fan, E, Cheek, F, Chlan, L, Gosselink, R, Hart, N, Herridge, MS, et al. An official American Thoracic Society clinical practice guideline: the diagnosis of intensive care unit-acquired weakness in adults. Am J Respir Crit Care Med. (2014) 190:1437–46. doi: 10.1164/rccm.201411-2011ST
PubMed Abstract | Crossref Full Text | Google Scholar
8. Shepherd, SJ, Newman, R, Brett, SJ, and Griffith, DM. Pharmacological therapy for the prevention and treatment of weakness after critical illness: a systematic review. Crit Care Med. (2016) 44:1198–205. doi: 10.1097/CCM.0000000000001652
PubMed Abstract | Crossref Full Text | Google Scholar
9. Qin, ES, Hough, CL, Andrews, J, and Bunnell, AE. Intensive care unit-acquired weakness and the COVID-19 pandemic: a clinical review. PM R. (2022) 14:227–38. doi: 10.1002/pmrj.12757
Crossref Full Text | Google Scholar
10. Veldema, J, Bösl, K, Kugler, P, Ponfick, M, Gdynia, HJ, and Nowak, DA. Cycle ergometer training vs resistance training in ICU-acquired weakness. Acta Neurol Scand. (2019) 140:62–71. doi: 10.1111/ane.13102
PubMed Abstract | Crossref Full Text | Google Scholar
11. Wang, W, Xu, C, Ma, X, Zhang, X, and Xie, P. Intensive care unit-acquired weakness: a review of recent Progress with a look toward the future. Front Med (Lausanne). (2020) 7:559789. doi: 10.3389/fmed.2020.559789
PubMed Abstract | Crossref Full Text | Google Scholar
12. van, N, Meersseman, P, Debaveye, Y, Wilmer, A, Gunst, J, Casaer, MP, et al. Five-year impact of ICU-acquired neuromuscular complications: a prospective, observational study. Intensive Care Med. (2020) 46:1184–93. doi: 10.1007/s00134-020-05927-5
PubMed Abstract | Crossref Full Text | Google Scholar
13. Bootcov, MR, Bauskin, AR, Valenzuela, SM, Moore, AG, Bansal, M, He, XY, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA. (1997) 94:11514–9. doi: 10.1073/pnas.94.21.11514
PubMed Abstract | Crossref Full Text | Google Scholar
14. Adela, R, and Banerjee, SK. GDF-15 as a target and biomarker for diabetes and cardiovascular diseases: a translational prospective. J Diabetes Res. (2015) 2015:490842:1–14. doi: 10.1155/2015/490842
PubMed Abstract | Crossref Full Text | Google Scholar
15. Yatsuga, S, Fujita, Y, Ishii, A, Fukumoto, Y, Arahata, H, Kakuma, T, et al. Growth differentiation factor 15 as a useful biomarker for mitochondrial disorders. Ann Neurol. (2015) 78:814–23. doi: 10.1002/ana.24506
PubMed Abstract | Crossref Full Text | Google Scholar
16. Ito, T, Nakanishi, Y, Yamaji, N, Murakami, S, and Schaffer, SW. Induction of growth differentiation factor 15 in skeletal muscle of old taurine transporter knockout mouse. Biol Pharm Bull. (2018) 41:435–9. doi: 10.1248/bpb.b17-00969
PubMed Abstract | Crossref Full Text | Google Scholar
17. Buendgens, L, Yagmur, E, Bruensing, J, Herbers, U, Baeck, C, Trautwein, C, et al. Growth differentiation Factor-15 is a predictor of mortality in critically ill patients with Sepsis. Dis Markers. (2017) 2017:5271203–10. doi: 10.1155/2017/5271203
PubMed Abstract | Crossref Full Text | Google Scholar
18. Xie, Y, Liu, S, Zheng, H, Cao, L, Liu, K, and Li, X. Utility of plasma GDF-15 for diagnosis and prognosis assessment of ICU-acquired weakness in mechanically ventilated patients: prospective observational study. Biomed Res Int. (2020) 2020:3630568. doi: 10.1155/2020/3630568
PubMed Abstract | Crossref Full Text | Google Scholar
19. Rosenberg, BJ, Hirano, M, Quinzii, CM, Colantuoni, E, Needham, DM, Lederer, DJ, et al. Growth differentiation factor-15 as a biomarker of strength and recovery in survivors of acute respiratory failure. Thorax. (2019) 74:1099–101. doi: 10.1136/thoraxjnl-2019-213621
PubMed Abstract | Crossref Full Text | Google Scholar
20. Bloch, SA, Lee, JY, Syburra, T, Rosendahl, U, Griffiths, MJ, Kemp, PR, et al. Increased expression of GDF-15 may mediate ICU-acquired weakness by down-regulating muscle microRNAs. Thorax. (2015) 70:219–28. doi: 10.1136/thoraxjnl-2014-206225
PubMed Abstract | Crossref Full Text | Google Scholar
21. Bloch, SA, Lee, JY, Wort, SJ, Polkey, MI, Kemp, PR, and Griffiths, MJ. Sustained elevation of circulating growth and differentiation factor-15 and a dynamic imbalance in mediators of muscle homeostasis are associated with the development of acute muscle wasting following cardiac surgery. Crit Care Med. (2013) 41:982–9. doi: 10.1097/CCM.0b013e318274671b
PubMed Abstract | Crossref Full Text | Google Scholar
22. Deng, M, Bian, Y, Zhang, Q, Zhou, X, and Hou, G. Growth differentiation Factor-15 as a biomarker for sarcopenia in patients with chronic obstructive pulmonary disease. Front Nutr. (2022) 9:897097. doi: 10.3389/fnut.2022.897097
PubMed Abstract | Crossref Full Text | Google Scholar
23. Xie, Y, Qian, Y, Yuan, G, Zheng, H, Cao, L, and Li, X. Utility of cross-sectional area of erector spinae muscle combined with serum GDF-15 for the diagnosis and prognosis assessment of ICU-acquired weakness in mechanically ventilated patients, China. J Emerg Med. (2020) 29:1059–65. doi: 10.3760/cma.j.issn.1671-0282.2020.08.007
Crossref Full Text | Google Scholar
24. Desmedt, S, Desmedt, V, De Vos, L, Delanghe, JR, Speeckaert, R, and Speeckaert, MM. Growth differentiation factor 15: a novel biomarker with high clinical potential. Crit Rev Clin Lab Sci. (2019) 56:333–50. doi: 10.1080/10408363.2019.1615034
PubMed Abstract | Crossref Full Text | Google Scholar
25. Moher, D, Shamseer, L, Clarke, M, Ghersi, D, Liberati, A, Petticrew, M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
PubMed Abstract | Crossref Full Text | Google Scholar
26. Yang, T, Li, Z, Jiang, L, and Xi, X. Hyperlactacidemia as a risk factor for intensive care unit-acquired weakness in critically ill adult patients. Muscle Nerve. (2021) 64:77–82. doi: 10.1002/mus.27248
PubMed Abstract | Crossref Full Text | Google Scholar
27. Wieske, L, Witteveen, E, Petzold, A, Verhamme, C, Schultz, MJ, van Schaik, IN, et al. Neurofilaments as a plasma biomarker for ICU-acquired weakness: an observational pilot study. Crit Care. (2014) 18:R18. doi: 10.1186/cc13699
PubMed Abstract | Crossref Full Text | Google Scholar
28. Nakanishi, N, Tsutsumi, R, Hara, K, Takashima, T, Nakataki, E, Itagaki, T, et al. Urinary titin is a novel biomarker for muscle atrophy in nonsurgical critically ill patients: a two-center, Prospective Observational Study. Crit Care Med. (2020) 48:1327–33. doi: 10.1097/CCM.0000000000004486
PubMed Abstract | Crossref Full Text | Google Scholar
29. Bloch, SA, Donaldson, AV, Lewis, A, Banya, WA, Polkey, MI, Griffiths, MJ, et al. MiR-181a: a potential biomarker of acute muscle wasting following elective high-risk cardiothoracic surgery. Crit Care. (2015) 19:147. doi: 10.1186/s13054-015-0853-5
PubMed Abstract | Crossref Full Text | Google Scholar
30. Ding, M, Ren, S, Dong, X, Wang, X, Zhao, X, and Qin, B. Diagnostic accuracy of muscle ultrasound and plasma monocyte chemoattractant protein-1 for ICU-acquired weakness in patients with sepsis. Chin Crit care Med. (2022) 34:12–7. doi: 10.3760/cma.j.cn121430-20211021-01531
PubMed Abstract | Crossref Full Text | Google Scholar
31. Wang, Y, Ma, J, Zhang, R, and Li, L. Predictive value of glucose transporter type 4 for intensive care unit acquired weakness in liver transplantation recipients. Chin J Organ Transplant. (2022) 43:525–9. doi: 10.3760/cma.j.cn421203-20220609-00133
Crossref Full Text | Google Scholar
32. Weber-Carstens, S, Deja, M, Koch, S, Spranger, J, Bubser, F, Wernecke, KD, et al. Risk factors in critical illness myopathy during the early course of critical illness: a prospective observational study. Crit Care. (2010) 14:R119. doi: 10.1186/cc9074
PubMed Abstract | Crossref Full Text | Google Scholar
33. Klawitter, F, Ehler, J, Bajorat, R, and Patejdl, R. Mitochondrial dysfunction in intensive care unit-acquired weakness and critical illness myopathy: a narrative review. Int J Mol Sci. (2023) 24:5516. doi: 10.3390/ijms24065516
PubMed Abstract | Crossref Full Text | Google Scholar
34. Hadda, V, Kumar, R, Khilnani, GC, Kalaivani, M, Madan, K, Tiwari, P, et al. Trends of loss of peripheral muscle thickness on ultrasonography and its relationship with outcomes among patients with sepsis. J Intensive Care. (2018) 6:81. doi: 10.1186/s40560-018-0350-4
PubMed Abstract | Crossref Full Text | Google Scholar
35. Witteveen, E, Wieske, L, Sommers, J, Spijkstra, JJ, de Waard, MC, Endeman, H, et al. Early prediction of intensive care unit-acquired weakness: a multicenter external validation study. J Intensive Care Med. (2020) 35:595–605. doi: 10.1177/0885066618771001
PubMed Abstract | Crossref Full Text | Google Scholar
36. Jude, B, Tissier, F, Dubourg, A, Droguet, M, Castel, T, Léon, K, et al. TGF-β pathway inhibition protects the diaphragm from Sepsis-induced wasting and weakness in rat. Shock. (2020) 53:772–8. doi: 10.1097/SHK.0000000000001393
PubMed Abstract | Crossref Full Text | Google Scholar
37. Hofmann, M, Schober-Halper, B, Oesen, S, Franzke, B, Tschan, H, Bachl, N, et al. Effects of elastic band resistance training and nutritional supplementation on muscle quality and circulating muscle growth and degradation factors of institutionalized elderly women:
留言 (0)