Linear IgA Bullous Dermatosis (LABD) is a rare immune-mediated bullous disease affecting both pediatric and adult populations (1). Pediatric patients frequently exhibit annular or polycyclic plaques, papules, and peripheral vesicles, known as the pearl string sign, predominantly on the face, genitals, and extremities (2). In adults, the lesions are primarily localized to the trunk, head, and extremities (3). Mucosal involvement is observed in both pediatric and adult cases, although the pearl string sign is less frequently observed in adults (2). Subjective symptoms range from mild itching to severe stinging (3, 4). Diagnosis relies on direct immunofluorescence testing of skin biopsy, showing linear immunoglobulin A (IgA) deposition along the basement membrane zone (5–7), occasionally accompanied by deposits of IgG, IgM, and C3 (5). The incidence of LABD is approximately 0.2 to 2.3 cases per million people per year (8–11).
LABD can be categorized into idiopathic and drug-induced forms. Idiopathic LABD typically occurs spontaneously, but it may also be associated with inflammatory bowel disease, malignancies, infections, and other autoimmune disorders (12). Although the majority of cases are idiopathic, various medications are known to induce LABD, with vancomycin being the most common precipitating drug (13). In addition, nonsteroidal anti-inflammatory drugs (NSAIDs), penicillins, cephalosporins, diuretics, and anticonvulsants are also common triggers for LABD (14). The clinical manifestations of both idiopathic and drug-induced LABD are diverse, with characteristics that can resemble those of pityriasis lichenoides et varioliformis acuta, bullous pemphigoid, pemphigus vulgaris, erythema multiforme, and toxic epidermal necrolysis (TEN) (5, 7, 15). Skin and mucosal symptoms in patients with drug-induced LABD are essentially similar to those with idiopathic LABD (16, 17), including vesicular rash, erythematous patches, target lesions, and string of pearls sign (17, 18). However, drug-induced LABD tends to be more severe, extensive, and atypical. The frequency of positive Nikolsky sign and large erosions is higher in drug-induced LABD, sometimes clinically resembling TEN (13). Several reports have indicated that drug-induced LABD may clinically overlap with TEN or Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) (15, 19–22), thus drug-induced LABD may pose a potential life-threatening risk. Therefore, early assessment and detection of signals for drug adverse events (AEs) are crucial for reducing the risk of drug-induced LABD.
To our knowledge, no studies have specifically utilized the FDA adverse event reporting system (FAERS) database to explore drug-induced LABD. The aim of this study is to assess the reported associations between drugs and LABD using the FAERS database.
2 Methods 2.1 Data sourceThis retrospective pharmacovigilance study utilizes the FAERS database, a global resource for post-marketing drug safety monitoring and signal detection. The database comprises reports submitted voluntarily by healthcare providers, as well as mandatory submissions from pharmaceutical companies. Information on drugs, including their names, active ingredients, routes of administration, and roles in adverse events (AEs), along with codes for different drug interactions such as primary suspect (PS), secondary suspect, interacting, and concomitant medications, is accessible within the FAERS. Each report identifies a primary suspect drug, enumerates one or more adverse drug reactions (ADRs), and may detail additional medications ingested by the patient.
2.2 Study designThis retrospective pharmacovigilance study encompassed FAERS data from January 2004 to June 2024. To account for multiple submissions with updated information, duplicate reports were identified and excluded based on case numbers, with only the most recent version retained for analysis. A case–control analysis was performed using FAERS to investigate the association between drug exposure and LABD reports. In this analysis, ‘cases’ corresponded to reports of AEs of interest, while ‘controls’ comprised all other AE reports not related to the AE under scrutiny. Classification of cases and controls was based on exposure or non-exposure to the drug in question. The reporting odds ratio (ROR) and its 95% confidence interval (CI) were calculated as a measure of association. The ROR specifically indicates whether an AE is disproportionately reported in relation to all other AEs associated with a particular exposure, thus reflecting the reporting odds of the AE of interest between those exposed and those not exposed to the drug. Additionally, the proportional reporting ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Empirical Bayes Geometric Mean (EBGM) were employed to detect drug signals.
2.3 Data exposure and adverse drug reaction definitionThis study used the preferred term ‘LINEAR IGA DISEASE’ from the Medical Dictionary for Regulatory Activities (MedDRA) to identify AEs of LABD in the REACTION (REAC) files. Given that reporters can assign various roles to the drugs in question, the assessment of drug exposure focused solely on those designated with the ‘PS’ role code. DrugBank was used to standardize different drug names in the ‘drugs’ table, such as brand names, generic names, synonyms, or abbreviations. All drugs ultimately appeared in the standardized generic name format.
2.4 Statistical analysisDisproportionate analysis is extensively applied for identifying signals of ADRs (23). In this study, we employed four analytical approaches, conducting statistical analyses based on the construction of a 2 × 2 contingency table (24). The methodologies encompassed both frequency-based metrics, namely the ROR and the PRR, and Bayesian methodologies, including the BCPNN and the EBGM. The Bayesian methods, while computationally more intensive, offer a significant advantage over the frequency-based methods by mitigating the risk of false positives associated with sparse AE reporting (25). The synergistic application of these four algorithms enhances the robustness and reliability of the findings. All data processing and statistical analyses were performed utilizing R software, version 4.4.1.
3 Results 3.1 Case characteristicsBetween the first quarter of 2004 and the second quarter of 2024, our study extracted a total of 21,433,114 AE reports from the FAERS database. After removing duplicates, we obtained 18,182,912 distinct AE reports. Ultimately, 1,394 reports related to LABD were identified. Figure 1 illustrates the annual submission of AE reports, showing a peak in 2019, potentially associated with the administration of COVID-19 vaccines or related medications (26–29). In the analyzed reports, there was a slight male predominance (44.4%) compared to females (43.2%). In terms of weight, patients weighing 50–100 kg accounted for 4.9%. Regarding age, the majority of patients were in the 66–85 age range (37.0%), followed by those aged 18–65 (34.5%). The majority of reports were submitted by Physicians (38.5%) and other healthcare professionals (32.6%). Geographically, the United States accounted for the highest proportion of reports (26.0%), followed by France (21.5%), Japan (7.7%), Great Britain (5.1%), and Spain (3.6%). Regarding outcomes, the majority of reports indicated other serious outcomes (56.0%), followed by hospitalization (33.0%) and death (5.7%). The most frequently reported indications were Pneumonia (4.3%), Infection (3.9%), Hypertension (2.9%), Endocarditis (1.9%), and Staphylococcal infection (1.9%). Table 1 presents detailed clinical characteristics of the reports associated with LABD.
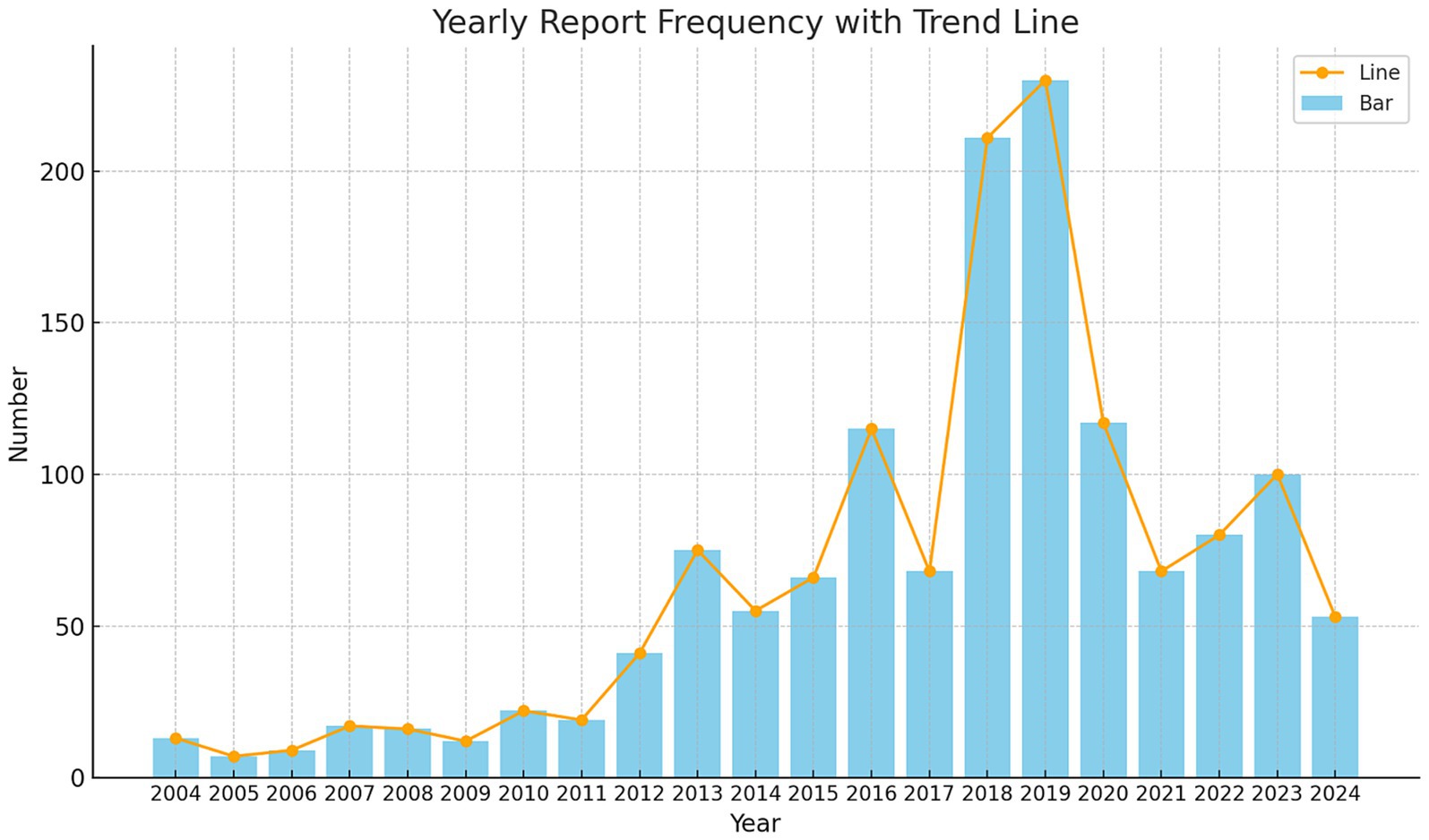
Figure 1. Annual distribution of reports associated with linear IgA bullous dermatosis collected from the FAERS database between January 2004 and June 2024.

Table 1. Clinical characteristics of linear IgA bullous dermatosis - related reports collected from the FAERS database between January 2004 and June 2024.
3.2 Medications used for LABDAmong the 1,394 AE reports associated with LABD identified in this study, a total of 353 unique drug names were listed as “PS.” After merging different names, including brand and generic names, we arrived at 175 distinct medications. Vancomycin topped the list with 559 reports, followed by Amoxicillin (n = 58), Amlodipine (n = 56), Piperacillin and Tazobactam (n = 40), and Diclofenac (n = 33) (Table 2). We performed a disproportionality analysis on the 175 medications with more than five reports and initially identified 37 drugs that met the criteria of the ROR algorithm. To more accurately elucidate the relationship between medications and AEs of LABD, we further analyzed drugs that satisfied all four disproportionality analysis methods (Table 3). Among the 34 drugs with significant associations, we found a variety of drug classes, including antibiotics, antifungal medications, analgesics, NSAIDs, cardiovascular medications, and calcium channel blockers. Notably, Ampicillin-Sulbactam and Ketoprofen had elevated RORs of 137.77 and 109.21, respectively, indicating a strong link to AEs of LABD. Vancomycin demonstrated the most significant signal with an ROR of 692.12 (95% CI 621.74–770.46), indicating a potentially strong association with AEs. Piperacillin-Tazobactam had an ROR of 73.62, emphasizing the need for safety monitoring. Although Gabapentin (n = 12, ROR 2.65) and Cyclosporine (n = 19 ROR 2.66) did not meet all four disproportionality analyses, their relatively high report numbers suggest that they warrant further clinical vigilance. While the four disproportionality analyses provide a more stable and reliable association, drugs with a high number of AE reports in the real world that meet even a single statistical method still merit further clinical attention and vigilance.
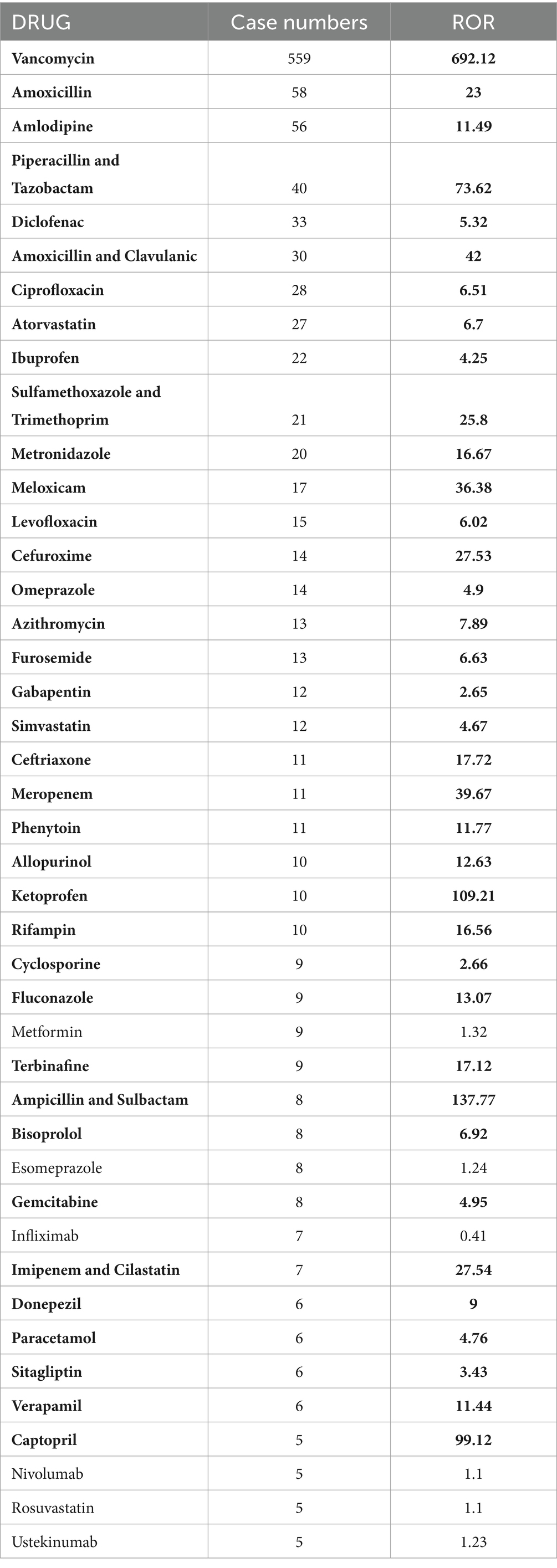
Table 2. Drugs linked to linear IgA bullous dermatosis with more than five reports (highlighting signals meeting the ROR method in bold).
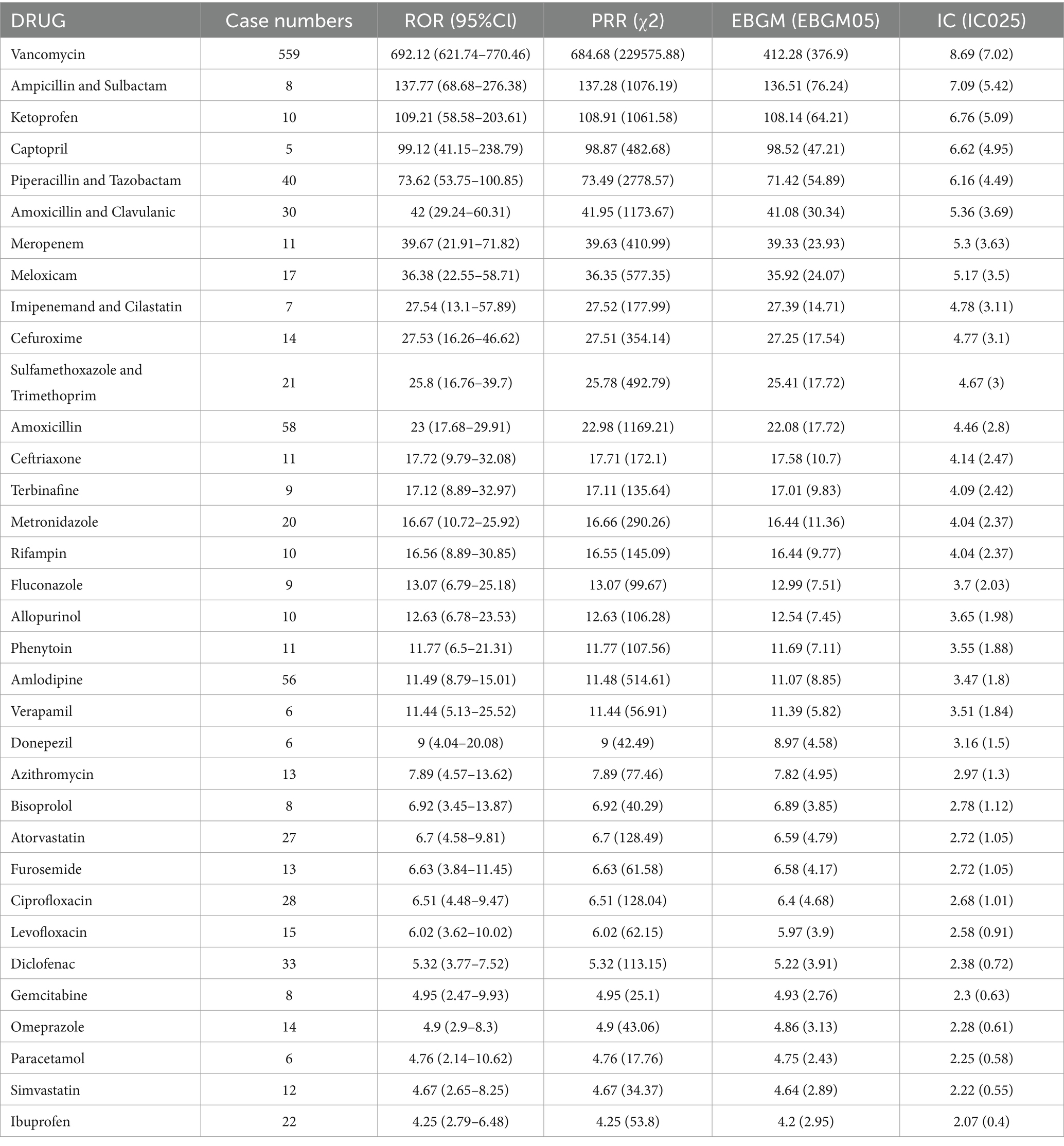
Table 3. Drugs significantly linked to linear IgA bullous dermatosis with more than five reports (meeting the criteria of all four disproportionality analysis methods).
4 DiscussionLABD is an autoimmune blistering disease with linear IgA deposits at the basement membrane. It can be categorized into idiopathic and drug-induced forms. In idiopathic LABD, LAD285, BP180, and BP230 have been identified as the primary target antigens, with the NC16A domain of BP180 being the main target for antibodies (30). In drug-induced LABD, antibodies against LAD285 and BP180 are present (17). Drugs may provoke autoimmune responses by mimicking epitopes, changing their structure, or revealing hidden antigens (31–33).
The pathogenesis of drug-induced LABD remains unclear. Drug-specific T cells and their cytokines, such as interleukins IL-4, IL-5, IL-6, IL-10, and transforming growth factor β, may augment IgA synthesis (34). Cytotoxic CD8+ T lymphocytes are thought to be central in recognizing self-antigens in drug-induced LABD (35). An increase in activated CD8+ T cells in the peripheral blood correlates with onset of LABD (35). IL-5, with elevated levels and local skin expression, is significant in drug-specific T cell responses, promoting IgA class switching and eosinophil activation, which are pivotal in tissue damage and blister formation. Elevated IFN-γ levels suggest the activation of cytotoxic T cells, potentially worsening tissue damage and triggering autoimmune processes that manifest as LABD (36). Cytotoxic mechanisms are common in drug-induced skin diseases (37, 38).
Clinical characteristic data from LABD reports highlight several key points. The gender distribution slightly favors males over females, although the difference is not significant. The significant lack of weight data limits our ability to analyze the potential link between obesity and LABD. The age distribution indicates that LABD can affect patients across all age groups but is most prevalent in adults, particularly in the 66–85 age range. This suggests a need for increased caution when treating elderly patients, considering the higher likelihood of comorbidities and polypharmacy in this age group. The diversity of reporting sources reflects a global awareness and willingness to report LABD, with physicians reporting the highest number of cases, underscoring their pivotal role in identifying and reporting adverse drug reactions. Geographically, the United States, France, Japan, Great Britain, and Spain report the most cases, which may correlate with pharmaceutical market distribution, regulatory capabilities, and demographic factors in these countries. High hospitalization rates underscore the severity of LABD, indicating the need for vigilant monitoring of LABD signs during drug use. Although rare, fatal cases remind us of the potential lethal risks associated with LABD. More than half of the patients (56.0%) reported severe clinical outcomes, further emphasizing the importance of early identification and treatment of LABD. Lastly, indications are primarily for the treatment of inflammatory diseases and infections, suggesting that certain medications used in these conditions may be associated with the occurrence of LABD. In particular, antibiotics such as Vancomycin and NSAIDs like Ketoprofen are significantly associated with LABD, and further research is needed to explore their potential mechanisms.
Drug-induced LABD is typically characterized by spontaneous resolution following drug withdrawal (39–41). The six drugs most commonly implicated in LABD in the FAERS database are Vancomycin (n = 559), followed by Amoxicillin (n = 58), Amlodipine (n = 56), Piperacillin and Tazobactam (n = 40), Diclofenac (n = 33), and Amoxicillin and Clavulanic (n = 30). The high ROR and PRR values for these drugs indicate a statistically significant association with LABD. For instance, Vancomycin, Ampicillin and Sulbactam, and Ketoprofen have ROR values of 692.12, 137.77, and 109.21, respectively, suggesting an increased risk of LABD in specific patient populations. Furthermore, the EBGM and Information Component (IC) values confirm the consistency and biological plausibility of these associations. Vancomycin, for instance, has an EBGM value of 412.28 and an IC value of 8.69, reinforcing its strong link with LABD. The findings are not surprising, as our research also indicates that antibiotics are the most representative drug class in drug-induced LABD. Vancomycin, a broad-spectrum antibiotic notably effective against methicillin-resistant Staphylococcus aureus strains, represents the most significant drug class in drug-induced LABD and is responsible for over 50% of LABD cases (42). Amoxicillin and Clavulanate, widely used antibiotics, have been implicated in multiple cases of LABD (43–47). LABD seems more related to Amoxicillin than Clavulanate, as Clavulanate combined with ticarcillin has not been associated with LABD. However, the broad use of Amoxicillin and Clavulanate poses a challenge. Fewer cases of β-lactam-induced LABD prevent a definitive conclusion about the sole effect of Amoxicillin (47). Cases of LABD induced by Piperacillin and Tazobactam have also been frequently reported (19, 36, 48, 49). Antihypertensive drugs have also been identified as potential inducing agents (50). Amlodipine-induced LABD was first reported by Low et al. (51), with 56 cases reported in the FAERS database. Therefore, clinicians should be vigilant for potential AEs, including LABD, when administering Amlodipine to treat hypertension and other conditions, especially in elderly patients. A case of captopril-induced LABD was reported by Friedman et al. (52), while another case remained indeterminate due to the presence of concomitant medications (53). Captopril has ROR and PRR values of 99.12 and 98.87, supported by five case reports in the FAERS database. Bisoprolol, with a ROR of 6.92 and eight reports, has no supporting literature for the induction of LABD. Candesartan and Eprosartan have been linked to LABD (54), yet the FAERS database lacks any corresponding case reports. Some drugs, despite few reports, show high ROR and PRR values, suggesting an increased risk of LABD in specific patient populations. For example, Ampicillin and Sulbactam have ROR and PRR values of 137.77 and 137.28, with eight reports. This indicates that even with a relatively low number of reports, the association between certain drugs and LABD remains a concern. There are reports on three Ampicillin and Sulbactam cases (55–57) and one Captopril case. Drugs used to treat coronary heart disease, hypertension, hypercholesterolemia, such as Amlodipine (ROR 11.49) and Atorvastatin (ROR 6.7), although their ROR values are not as high as Vancomycin, require a balance between therapeutic efficacy and potential AEs. Additionally, there have been reports of LABD induced by immune checkpoint inhibitors such as anti-PD1 and anti-CTLA4 (58, 59), yet the FAERS database does not contain any corresponding reports for these medications. Our findings emphasize the importance of identifying and monitoring potential risk factors for LABD, especially with drugs known to increase LABD risk. Additionally, this underscores the importance of enhanced pharmacovigilance and clinical practice in detecting and assessing AEs of drugs. These data may also inform drug development and safety regulation, suggesting further research into mechanisms of drugs associated with increased risk of LABD and strategies to prevent or mitigate LABD. Lastly, our study suggests monitoring of drug AEs should occur throughout drug development and clinical trials, not just post-marketing, to identify and mitigate potential risks early.
This study leveraged the FAERS database to retrospectively assess the reported associations between drugs and LABD using pharmacovigilance methods. To our knowledge, this is the first real-world study utilizing the FAERS database to investigate drug-induced LABD. Our findings indicate significant correlations between LABD and various drug classes, including antibiotics, antifungal agents, analgesics, NSAIDs, cardiovascular medications, and calcium channel blockers. The results underscore the necessity for close patient monitoring when these drugs are clinically prescribed to promptly detect and manage potential AEs such as LABD. This research also sets the stage for future studies to explore the specific mechanisms of drug-induced LABD and to develop strategies for prevention and management of these adverse reactions (Table 4). It is important to note that this study has certain limitations. Firstly, the present study is retrospective in nature, thereby precluding the direct inference of a causal relationship between the medication and LABD from the outcomes. Secondly, there is a potential for reporting bias in the data, as AEs are reported voluntarily, which may lead to underreporting or overreporting, significantly influencing the ROR analysis. Thirdly, the reported association between the medication and LABD is confounded by comorbidities and concomitant medications. The data analysis included only the primary suspect medications. Fourthly, the FAERS database lacks comprehensive patient-level data, which limits the ability to assess confounding factors and conduct reliable statistical analyses. Lastly, the FAERS database does not provide detailed information on the timing of drug exposure, thus precluding the calculation of incidence rates and the quantification of individual effects of multiple drug exposures. Therefore, future research should consider prospective designs and more comprehensive data collection and analysis on drug exposure and the occurrence of LABD to further validate and expand upon our findings. In summary, this study provides crucial insights into the relationship between drugs and LABD and offers valuable guidance for drug utilization and patient management in clinical practice.
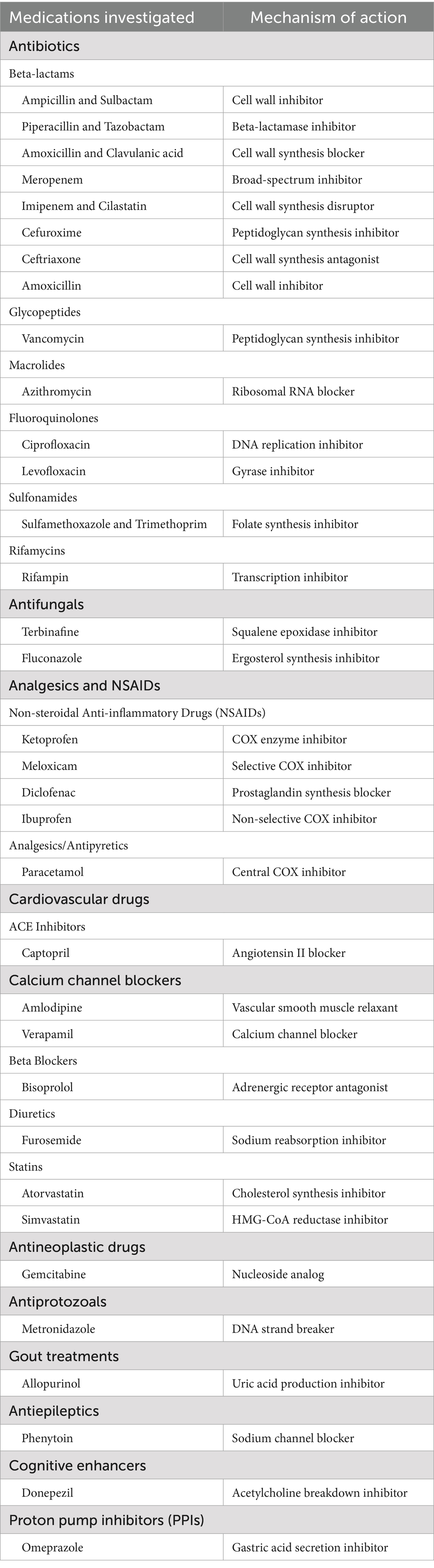
Table 4. Medications included in the present study and their mechanisms of action.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statementEthical review and approval was not required for the study on human participants, in accordance with the local legislation and institutional requirements.
Author contributionsYY: Writing – original draft. HX: Writing – original draft. SL: Writing – original draft. YJ: Writing – review & editing. BC: Writing – review & editing. ZX: Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study was supported by a grant from the High Level Chinese Medical Hospital Promotion Project (No. HLCMHPP2023027 and No. HLCMHPP2023088), the Escort Project of Guang’anmen Hospital, China Academy of Chinese Medicine Science-Backbone Talent Cultivation Project (No. GAMHH9324021) and the Scientific Research Foundation for New recruits, China Academy of Chinese Medical Sciences (No. ZZ16-XRZ-045). The funder had no role in the study design, data collection, analysis and interpretation, writing of the report, or decision to submit the paper for publication.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Genovese, G, Venegoni, L, Fanoni, D, Muratori, S, Berti, E, and Marzano, AV. Linear IgA bullous dermatosis in adults and children: a clinical and immunopathological study of 38 patients. Orphanet J Rare Dis. (2019) 14:115. doi: 10.1186/s13023-019-1089-2
Crossref Full Text | Google Scholar
3. Venning, VA, Taylor, CJ, Ting, A, and Wojnarowska, F. HLA type in bullous pemphigoid, cicatricial pemphigoid and linear IgA disease. Clin Exp Dermatol. (1989) 14:283–5. doi: 10.1111/j.1365-2230.1989.tb01980.x
Crossref Full Text | Google Scholar
4. Kelly, SE, Frith, PA, Millard, PR, Wojnarowska, F, and Black, MM. A clinicopathological study of mucosal involvement in linear IgA disease. Br J Dermatol. (1988) 119:161–70. doi: 10.1111/j.1365-2133.1988.tb03197.x
PubMed Abstract | Crossref Full Text | Google Scholar
5. Baden, LA, Apovian, C, Imber, MJ, and Dover, JS. Vancomycin-induced linear IgA bullous dermatosis. Arch Dermatol. (1988) 124:1186–8. doi: 10.1001/archderm.1988.01670080012007
Crossref Full Text | Google Scholar
6. Whitworth, JM, Thomas, I, Peltz, SA, Sullivan, BC, Wolf, AH, and Cytryn, AS. Vancomycin-induced linear IgA bullous dermatosis (LABD). J Am Acad Dermatol. (1996) 34:890–1. doi: 10.1016/s0190-9622(96)90073-0
Crossref Full Text | Google Scholar
7. Wilson, BD, Beutner, EH, Kumar, V, Chorzelski, TP, and Jablonska, S. Linear IgA bullous dermatosis. An immunologically defined disease. Int J Dermatol. (1985) 24:569–74. doi: 10.1111/j.1365-4362.1985.tb05853.x
PubMed Abstract | Crossref Full Text | Google Scholar
8. Bernard, P, Vaillant, L, Labeille, B, Bedane, C, Arbeille, B, Denoeux, JP, et al. Incidence and distribution of subepidermal autoimmune bullous skin diseases in three French regions. Arch Dermatol. (1995) 131:48–52. doi: 10.1001/archderm.131.1.48
PubMed Abstract | Crossref Full Text | Google Scholar
10. Nanda, A, Dvorak, R, Al-Sabah, H, and Alsaleh, QA. Linear IgA bullous disease of childhood: an experience from Kuwait. Pediatr Dermatol. (2006) 23:443–7. doi: 10.1111/j.1525-1470.2006.00279.x
PubMed Abstract | Crossref Full Text | Google Scholar
11. Zillikens, D, Wever, S, Roth, A, Weidenthaler-Barth, B, Hashimoto, T, and Bröcker, EB. Incidence of autoimmune subepidermal blistering dermatoses in a region of Central Germany. Arch Dermatol. (1995) 131:957–8. doi: 10.1001/archderm.131.8.957
PubMed Abstract | Crossref Full Text | Google Scholar
13. Lammer, J, Hein, R, Roenneberg, S, Biedermann, T, and Volz, T. Drug-induced linear IgA bullous dermatosis: a case report and review of the literature. Acta Derm Venereol. (2019) 99:508–15. doi: 10.2340/00015555-3154
Crossref Full Text | Google Scholar
14. Starzyk, T, Nikakis, J, and Ross, R. Drug-induced linear IgA bullous dermatosis with extensive mucosal involvement. JAAD Case Rep. (2024) 52:77–9. doi: 10.1016/j.jdcr.2024.07.021
Crossref Full Text | Google Scholar
15. Kakar, R, Paugh, H, and Jaworsky, C. Linear IgA bullous disease presenting as toxic epidermal necrolysis: a case report and review of the literature. Dermatology. (2013) 227:209–13. doi: 10.1159/000353584
Crossref Full Text | Google Scholar
16. Collier, PM, and Wojnarowska, F. Drug-induced linear immunoglobulin a disease. Clin Dermatol. (1993) 11:529–33. doi: 10.1016/0738-081x(93)90161-5
Crossref Full Text | Google Scholar
17. Palmer, RA, Ogg, G, Allen, J, Banerjee, A, Ryatt, KS, Ratnavel, R, et al. Vancomycin-induced linear IgA disease with autoantibodies to BP180 and LAD285. Br J Dermatol. (2001) 145:816–20. doi: 10.1046/j.1365-2133.2001.04492.x
Crossref Full Text | Google Scholar
18. Jones, DH, Todd, M, and Craig, TJ. Early diagnosis is key in vancomycin-induced linear IgA bullous dermatosis and Stevens-Johnson syndrome. J Am Osteopath Assoc. (2004) 104:157–63.
PubMed Abstract | Google Scholar
19. Adler, NR, McLean, CA, Aung, AK, and Goh, MS. Piperacillin-tazobactam-induced linear IgA bullous dermatosis presenting clinically as Stevens-Johnson syndrome/toxic epidermal necrolysis overlap. Clin Exp Dermatol. (2017) 42:299–302. doi: 10.1111/ced.13030
Crossref Full Text | Google Scholar
20. Chanal, J, Ingen-Housz-Oro, S, Ortonne, N, Duong, TA, Thomas, M, Valeyrie-Allanore, L, et al. Linear IgA bullous dermatosis: comparison between the drug-induced and spontaneous forms. Br J Dermatol. (2013) 169:1041–8. doi: 10.1111/bjd.12488
Crossref Full Text | Google Scholar
21. Tran, D, Kossard, S, and Shumack, S. Phenytoin-induced linear IgA dermatosis mimicking toxic epidermal necrolysis. Australas J Dermatol. (2003) 44:284–6. doi: 10.1046/j.1440-0960.2003.00011.x
Crossref Full Text | Google Scholar
22. Waldman, MA, Black, DR, and Callen, JP. Vancomycin-induced linear IgA bullous disease presenting as toxic epidermal necrolysis. Clin Exp Dermatol. (2004) 29:633–6. doi: 10.1111/j.1365-2230.2004.01649.x
Crossref Full Text | Google Scholar
23. Huang, J, Su, A, Yang, J, Zhuang, W, and Li, Z. Post-marketing safety concerns of Teprotumumab: a real-world pharmacovigilance assessment. J Clin Endocrinol Metab. (2024) 110:159–65. doi: 10.1210/clinem/dgae417
Crossref Full Text | Google Scholar
24. Mao, X, Zhang, R, Liang, X, Liu, F, Dai, Y, Wang, M, et al. A pharmacovigilance study of FDA adverse events for sugammadex. J Clin Anesth. (2024) 97:111509. doi: 10.1016/j.jclinane.2024.111509
Crossref Full Text | Google Scholar
26. Coto-Segura, P, Fernández-Prada, M, Mir-Bonafé, M, García-García, B, González-Iglesias, I, Alonso-Penanes, P, et al. Vesiculobullous skin reactions induced by COVID-19 mRNA vaccine: report of four cases and review of the literature. Clin Exp Dermatol. (2022) 47:141–3. doi: 10.1111/ced.14835
Crossref Full Text | Google Scholar
27. Hali, F, Kerouach, A, Alatawna, H, Chiheb, S, and Lakhdar, H. Linear IgA bullous dermatosis following Oxford AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. (2022) 47:611–3. doi: 10.1111/ced.15007
PubMed Abstract | Crossref Full Text | Google Scholar
28. Han, J, Russo, G, Stratman, S, Psomadakis, CE, Rigo, R, Owji, S, et al. Toxic epidermal necrolysis-like linear IgA bullous dermatosis after third Moderna COVID-19 vaccine in the setting of oral terbinafine. JAAD Case Rep. (2022) 24:101–4. doi: 10.1016/j.jdcr.2022.04.021
PubMed Abstract | Crossref Full Text | Google Scholar
29. Nahm, WJ, Juarez, M, Wu, J, and Kim, RH. Eosinophil-rich linear IgA bullous dermatosis induced by mRNA COVID-19 booster vaccine. J Cutan Pathol. (2023) 50:24–8. doi: 10.1111/cup.14305
Crossref Full Text | Google Scholar
30. Allen, J, and Wojnarowska, F. Linear IgA disease: the IgA and IgG response to dermal antigens demonstrates a chiefly IgA response to LAD285 and a dermal 180-kDa protein. Br J Dermatol. (2003) 149:1055–8. doi: 10.1111/j.1365-2133.2003.05647.x
Crossref Full Text | Google Scholar
31. Martin, RJ, and Lamprey, PM. Early development of adipose cell lipogenesis and glycerol utilization in Zucker obese rats. Proc Soc Exp Biol Med. (1975) 149:35–9. doi: 10.3181/00379727-149-38738
Crossref Full Text | Google Scholar
32. Paul, C, Wolkenstein, P, Prost, C, Caux, F, Rostoker, G, Heller, M, et al. Drug-induced linear IgA disease: target antigens are heterogeneous. Br J Dermatol. (1997) 136:406–11. doi: 10.1111/j.1365-2133.1997.tb14955.x
Crossref Full Text | Google Scholar
33. Zone, JJ, Pazderka Smith, E, Powell, D, Taylor, TB, Smith, JB, and Meyer, LJ. Antigenic specificity of antibodies from patients with linear basement membrane deposition of IgA. Dermatology. (1994) 189:64–6. doi: 10.1159/000246933
Crossref Full Text | Google Scholar
34. Brière, F, Bridon, JM, Chevet, D, Souillet, G, Bienvenu, F, Guret, C, et al. Interleukin 10 induces B lymphocytes from IgA-deficient patients to secrete IgA. J Clin Invest. (1994) 94:97–104. doi: 10.1172/jci117354
Crossref Full Text | Google Scholar
35. Yawalkar, N, Reimers, A, Hari, Y, Hunziker, T, Gerber, H, Müller, U, et al. Drug-induced linear IgA bullous dermatosis associated with ceftriaxone- and metronidazole-specific T cells. Dermatology. (1999) 199:25–30. doi: 10.1159/000018173
Crossref Full Text | Google Scholar
36. Ho, YH, Chiu, YW, and Liu, HN. Piperacillin-Tazobactam-induced linear IgA bullous dermatosis supported by a T-cell activation assay. Ann Dermatol. (2018) 30:588–91. doi: 10.5021/ad.2018.30.5.588
PubMed Abstract | Crossref Full Text | Google Scholar
37. Porębski, G, Czarnobilska, E, and Bosak, M. Cytotoxic-based assays in delayed drug hypersensitivity reactions induced by antiepileptic drugs. Pol Arch Med Wewn. (2015) 125:823–34. doi: 10.20452/pamw.3160
PubMed Abstract | Crossref Full Text | Google Scholar
38. Schrijvers, R, Gilissen, L, Chiriac, AM, and Demoly, P. Pathogenesis and diagnosis of delayed-type drug hypersensitivity reactions, from bedside to bench and back. Clin Transl Allergy. (2015) 5:31. doi: 10.1186/s13601-015-0073-8
Crossref Full Text | Google Scholar
39. Cerottini, JP, Ricci, C, Guggisberg, D, and Panizzon, RG. Drug-induced linear IgA bullous dermatosis probably induced by furosemide. J Am Acad Dermatol. (1999) 41:103–5. doi: 10.1016/s0190-9622(99)70414-7
PubMed Abstract | Crossref Full Text | Google Scholar
41. Quispe-Gárate, LA, Espinoza-Escudero, RB, Salas-Rivera, C, and Sánchez-Félix, G. Drug-induced linear IgA bullous dermatosis in an oncologic patient. Cureus. (2023) 15:e49185. doi: 10.7759/cureus.49185
Crossref Full Text | Google Scholar
43. Ho, JC, Ng, PL, Tan, SH, and Giam, YC. Childhood linear IgA bullous disease triggered by amoxicillin-clavulanic acid. Pediatr Dermatol. (2007) 24:E40–3. doi: 10.1111/j.1525-1470.2007.00438.x
PubMed Abstract | Crossref Full Text | Google Scholar
44. Panasiti, V, Rossi, M, Devirgiliis, V, Curzio, M, Bottoni, U, and Calvieri, S. Amoxicillin-clavulanic acid-induced linear immunoglobulin a bullous dermatosis: case report and review of the literature. Int J Dermatol. (2009) 48:1006–10. doi: 10.1111/j.1365-4632.2009.04104.x
PubMed Abstract | Crossref Full Text | Google Scholar
45. Santos-Juanes, J, Coto Hernández, R, Trapiella, L, Caminal, L, Sánchez del Río, J, and Soto, J. Amoxicillin-associated linear IgA bullous dermatosis. J Eur Acad Dermatol Venereol. (2007) 21:992–3. doi: 10.1111/j.1468-3083.2006.02066.x
留言 (0)